INTRODUCTION
Venomous snakebite is a worldwide problem, especially in tropical and subtropical geographical regions. There are more than five million snakebite accidents annually worldwide; the members of the Viperidae family 1 produce the most common snakebites that take place in the American continent (~ 98%).
Traditional antivenom production is based on purified antibodies extracted from hyperimmunized horses’ plasma 2. In recent years, several authors report that bird’s (mainly chicken) antibodies produced against snake venoms, presented venom effective neutralization activities 2,3. However, despite their potential considering their body size and egg-laying advantages, ostriches (Struthio camelus) have not been previously tested for snake anti-venom production. Clinical assays will be need to assess their security as an antidote to human victims of ophitoxemia and further experimentation addressing IgY-based antivenoms safety and effectiveness is required.
MATERIALS AND METHODS
Reagents
Polyvalent anti-ophidic serum (PAOS) (Biotecfar C.A, Universidad Central de Venezuela, Pharmacy Faculty, Caracas, República Bolivariana de Venezuela). Sodium chloride, sodium citrate, sodium azide, tris, hydrochloric acid, sodium hydroxide, Coomassie blue, acrylamide, bis-acrylamide, ammonium per-sulphate, glacial acetic acid, temed, glycerol, sodium bicarbonate, caprylic acid (Sigma-Aldrich, Missouri, USA). Saran wrap (S.C. Johnson & Son, Inc, USA). Complete Freund’s and incomplete Freund’s adjuvants (GIBCO, USA). Antibody goat anti-avian (included anti-ostrich) (Laboratories ABCAM, USA). Peroxidase substrate (TMB)(Vector Lab, USA). 30 kDa cassette filtration unit (Vivaflow 50 R, Sartorius, Germany). Filter (Sartorious Laboratories, Germany). Microtitration plates (Corning® ELISA microplates, USA). Automatic ELISA reader (Bio-Tek Laboratories, USA). Mini-Protean II system (Bio-Rad Laboratories, USA). Trans Blot SD system (Bio-Rad Laboratories, USA). Molecular mass standards for SDS-PAGE were from Bio-Rad Laboratories Ltd (California, USA).
Ostriches
Two female healthy adult ostriches (Struthio camelus) were obtained from a local ostrich farm (Villa de Cura town, Aragua state, República Bolivariana de Venezuela). They were housed under standard environment (humidity, lighting and temperature) and fed ad libitum with standard ostrich diet and potable water (Fig. 1).
Venom
A pool of venoms from common rattlesnake (Crotalus durissus cumanensis) (0.15 mg/mL), Uracoan rattlesnake (Crotalus vegrandis) (0.03 mg/mL), Guayana rattlesnake (Crotalus durissus ruruima) (0.02 mg/mL) and black rattlesnake (Crotalus pifanorum) (0.012mg/mL) were obtained by milking the snakes and then crystallized under vacuum in a desiccator containing CaCl2 as a desiccant and maintained at 4°C until use (Fig. 1).
Ethical statement
Skilled staff prepared all the experimental methods relating to the use of live animals. These methods were permitted by the Institute of Anatomy Ethical Committee of the Universidad Central de Venezuela under assurance number (Protocol N° 190619) and followed the norms obtained from the Guidelines for the Care and Use of Laboratory Animals, published by the USA National Institute of Health 4.
Immunization procedure
Two female ostriches were injected intramuscularly intro the bird’s anterior thigh muscles on day 0 with the Crot/pool of the Venezuelan rattlesnake venoms (1µg/kg body weight) emulsified with an equivalent volume of complete Freund’s adjuvant. The primary booster dose was administered two weeks later in an incomplete Freund’s adjuvant. Successively, the second and third boosters emulsified with an equivalent volume of incomplete Freund’s adjuvant were administered at 45-day intervals in order to sustain high antibody titers. Blood was collected through the wing vein, arranged to obtain the serum. Sera were separated by centrifugation (1500 G for 15 min). Pre-immune serum and egg yolk replicas collected from ostriches were used as negative controls. They were stored in a freezer at −20°C, until used.
Purification of antibodies from egg yolk
Isolation of IgY from egg yolk was performed with a modified method 5. Briefly, once the egg shell was opened, the yolk was softly detached from the white of the egg, washing it with abundant distilled water, until all the white egg disappeared. Then, the yolk was punctured with a syringe and all its contents were extracted and mixed completely with a five-fold dilution with PBS pH 7.4 and slow addition of caprylic acid (CA), then the pH was adjusted to 5.0 with 10 M hydrochloric acid. Concisely, the CA was slowly added dropwise at an approximate rate of 0.6 mL/min, until a final concentration of 6% (v/v) was obtained.
After the first step of the process, the sample was filtered through a 15 µm and then 0.45 µm filter.
The preparation was kept to room temperature, and afterward ammonium sulfate at 30% (W/V) concentration was added, keeping it under constant stirring for one hour at room temperature and then filtered through a 2-µm filter. In order to eliminate the ammonium sulfate, a tangential filtration process was applied using a cassette of 30 kDa. The sample was resuspended in saline solution and kept under stirring for one hour- Subsequently, filtered through a 2-µm filter to remove remaining solids and a diafiltration process was started until eight diafiltration volumes were completed. When the ammonium sulfate was removed, the washed sediment was dissolved in 200 mL saline pH 6.3.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of IgY
Purified IgY under non-reduced and reduced conditions were electrophoresed with a MINIPROTEAN II (BioRad, USA) chamber. SDS-PAGE was performed using 12% gels. Wide range molecular weight markers (Bio-Rad) were run in parallel and gels were stained with Comassie blue (National Diagnostic, USA).
Determination of antibody titers by an indirect ELISA
The immunoglobulins titers in serum and egg yolk of immunized ostriches were tested by enzyme-linked immunosorbent assay (ELISA). Titer was considered as the concentration of an antibody, as determined by finding the highest dilution at which it was still able to cause recognition of the antigen. The uncomplicated way to do this is as follows: selecting the highest responding serum and another that shows low response. Do readings for serial dilutions as we have done (Fig. 3). Looking for one dilution were both sera are in the steepest part of the curve, are clearly different (generally a 1:1000 - 1:4000 dilutions will do it) and the OD450nm of the most responding serum is nearly 1.0. Then it is possible to define the titer as the OD450nm for each serum at the dilution obtained before.
All ELISA incubations were carried out at 24-26°C. Briefly, aliquots (100 µL) of the Crot/pool venom (1 µg/mL PBS) were pipetted into the wells of the micro-titration plates in overlay phosphate buffer saline (PBS) pH 7.4 that were protected with saran wrap and stored overnight at 4°C. The wells content was aspirated, and washed three times with washing buffer (PBS, pH 7.4 containing 0.05% Tween-20), the wells were then overflowed with blocking buffer (skimmed milk 2% in PBS-T) and left for one hour. After the aspiration of blocking buffer, the ostrich sera or purified IgY immunoglobulins samples were diluted properly in blocking buffer and 100 µL added to the wells before incubating at 37°C for one hour. Later, the plates were washed three times with washing buffer and incubated with goat anti-avian (included anti-ostrich) IgY peroxidase (1:10.000) at 37°C for one hour. The contents of the wells were subsequently aspirated, the wells washed three times with PBS-0.05% Tween 20 (PBS-TW), and 100 µL of peroxidase substrate (TMB)(Vector Lab, USA) was added to each well. The plates were maintained in the dark, at room temperature for 20 min for the progress of dye. The reaction was stopped with 50 µL of sulfuric acid 1 M. The absorbance of solutions at X = 450 nm was determined after the addition of substrate by an automatic ELISA reader.
Immunoblot analysis
Recognition of IgY antibodies was performed by western blot according to the modified method 6 (n=3). Briefly, to determine the specificity of the immunoglobulins against Crot/pool snake venoms, the anti-bodies were tested with a total of six venoms Crotalus durissus cumanensis, Crotalus vegrandis, Crotalus durissus ruruima, and Crotalus pifanorum venoms.
These venoms were electrophoresed on a 10% SDS gel using a Mini-Protean II system at 150V (Bio-Rad PowerPac Basic) for one hour. Then, were transferred onto a 0.2 μm nitrocellulose membrane (Millipore) using a Trans Blot SD system at 100 mA for one hour. After blocking the membrane for one hour at room temperature with 5% skimmed dry milk in PBS buffer pH 7.4 containing 0.05% (w/v) Tween 20, the nitrocellulose membrane was incubated under stirring for one hour with anti-Crot/pool ostrich IgY antivenom diluted to 1:1000 in PBS-Tween 20. After the rinses, the secondary antibody goat anti-avian IgY (coupled to horseradish peroxidase) diluted 1:20000 in PBS-Tween 20 was complemented. Finally, blots electrophoretic bands identified by Crot/pool ostrich IgY antivenom were colorimetric visualized using the peroxidase substrate (TMB), and the image was analyzed.
Lethality Dose (LD50)
Five groups of five NIH female mice (Mus musculus) for Crot/pool venom were maintained in plastic boxes (Tecniplast, Italy) and observed throughout the quarantine period and experiments. The endpoint of lethality of the mice was established after 48 hr. The venom was suspended in 0.85% saline at the maximum test dose per mouse. Serial dilutions of 2-fold using saline solution were prepared to obtain four extra concentrations. All solutions throughout the experiment were kept at 0°C and warmed to 37°C before being injected into mice. The lethal toxicity was determined by injecting 0.2 mL of venom (containing dosages ranging between 38.0 to 11.6 μg/mouse) into the peritoneum of 18-20 g female NIH mice. The injections were dispensed using a 1-mL syringe fitted with a 25-gauge, 0.5-inch needle. Saline as normal controls were used. The lethal dose fifty (LD50) was calculated following the Spearman-Kärber (n = 3 ± SD) method 7. The estimated LD50 was then used for testing median effective dose fifty (ED50/2 LD50).
Antivenom neutralization test: median effective dose fifty (ED50/2 LD50) assays of yielded antibodies (anti-Crot/pool ostrich IgY antivenom neutralizing lethal toxic activity of Crot/pool venom)
The median effective dose value (ED50) of the anti-Crot/pool ostrich IgY antivenom from ostrich egg yolk was measured for analysis of quantitative and categorical data, with the aid of the Prism 8 program (Graphpad, USA). Five groups of five female NIH mice (18-20 g) were confronted with a combination of serial dilutions of a certain amount of anti-Crot/pool venoms (1.488, 1.395, 1.321, 1.145, 0.843), containing constant concentration of Crot/pool venom (33 μg). 2LD50: (LD50=16.5 μg/mouse x 2 = 33 μg venom/20 g mouse). The Ostrich IgY anti-Crot/pool venom/ Crot/pool venom combinations were pre-incubated for 30 min at 37°C, then was intravenously injected into mice for each dose. Negative control mice were injected with two LD50 of venom alone. The neutralizing potency reproduces the ratio of mL of anti-Crot/pool venom /mg of Crot/pool venom or mg of antivenom/mg of venom. The control group was also injected with venom pre-incubated with normal ostrich serum.
RESULTS
Immune response
Two female ostriches were immunized as described in Materials and Methods. Detectable specific IgY anti-venom responses were not observed in sera until 15 days from the initial dose, by the time of the first booster-dose. This secondary response was sustained thereafter by successive booster injections given at 45-days pauses.
IgY purification
At a pH of 5-6 most of the immunoglobulins were recovered and the caprylic acid precipitation leads to a maximum recovery of IgY, with minimum contaminating proteins. After ammonium sulfate purification, the obtained sample under reduction conditions gave two mains electrophoretic IgY bands of ∼65 and ∼20 kDa. Otherwise, under non-reducing conditions a IgY single band of ∼175 kDa was observed (Fig. 2). The average recovery of IgY from a single egg yolk was ~ 3800 mg (N=5).

Fig. 2 IgY tested under native and reduction conditions. (1) Molecular mass markers. (2) IgY (native conditions); (3) Under reducing conditions HC: Heavy chain; DHC: Detritus heavy chain; LC: Light chain; VH: Variable fraction of heavy chain; VL: Portion variable light chain; CL: Constant fraction of light chain.
Immune-specific recognition of crotalic venom pool by IgY
The anti- Crot/pool venom-specific activity of IgY in partially purified preparations was assayed by a home-designed indirect ELISA as described in Materials and Methods. The purification procedure resulted in venom-specific IgY recognition of Crot/pool venoms (Fig. 3). Microtiter plates with preimmune serum showed no binding.
Specificity of Crot/pool antivenom (IgY) to Crotalus venoms via Western blot
The identity of the Crot/pool venom proteins was confirmed with the Crot/pool antivenom (IgY) by Western-blot, identifying most of the fraction venoms used in the present work (Fig. 4). As a reference, the polyvalent antivenom of equine origin produced by BIOTECFAR C.A (Caracas, Venezuela) was used.
Antivenom effective dose (ED50)
In order to estimate the ED50 of the final Crot/pool antivenom (IgY), different amounts of this antivenom were preincubated with 2LD50 (33 μg) of a Crot/venom pool, as described in Materials and Methods. An ED50 of 19.66 mg was calculated for the Crot/pool antivenom, as the minimum amount of purified IgY preparation able to protect 50% of the mice population. There were no survivals in the control group (Table 1, Fig. 5).
Table 1 Determination of the survive percentage of mice after the injection of 2LD50 of Crot/pool venom with different concentrations of ostrich IgY. Evaluation of the neutralizing capacity of anti-crotalic IgY antibodies.
LD50: Lethal dose fifty; Vol: Volume.
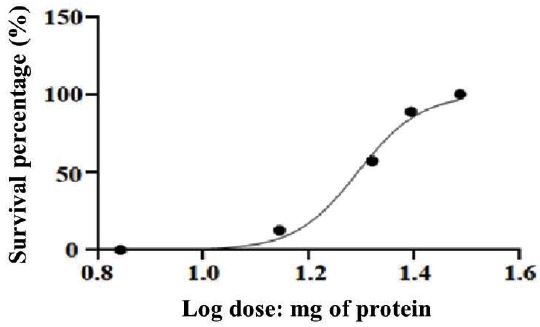
Fig. 5 Curve of Log doses of mg of protein with the percentage of survival animals. An ED50 of 19.66 mg Crot/pool antivenom (IgY) was calculated from the represented data as the mass of IgY that was able to protect at least 50% of the mice population against a 2LD50 (33 μg) challenge of Crot/pool venom. In the control group there were no survivals. (%): percentage. (Log): logarithm.
DISCUSSION
Snakebite accidents, categorized as a neglected tropical disease by the World Health Organization (WHO) is responsible for nearly 50.000 deaths annually, mostly in Third World countries 8.
In the current work specific anti-Crotalus snake venoms antibodies were obtained by an immunization schedule in female ostriches (Struthio camelus). The eggs from bird species have demonstrated being a desirable basis for the production of antibodies, without invasive methods, which present a predominant class of IgY immunoglobulin 3,9,10. This IgY has been used in diagnosis, research, and immunotherapy 11-13. Furthermore, bird’s immunoglobulins production poses several benefits over mammalian antibodies with respect to the antigenic specificity and low manufacturing expenses 3,12. It was possible to obtain about 2-4 g of IgY per ostrich egg. Hence, almost 400 g of IgY can be purified from only one ostrich per year. For that reason, ostrich eggs could represent an exceptional source of immunoglobulins for antivenom production.
A great amount of cross-reactive and neutralizing antibodies (IgY) were produced in the egg yolk of ostriches (Struthio camelus) by immunizing with the venom of four Crotalus (Crot/pool) using a simple and inexpensive method. Knowledge of venom variations permits the selection of suitable specimens for production of more effective antivenoms and biological substances.
The anti- Crot/pool venom immunoglobulins were carried out from egg yolk by the McLaren RD et al. 5 method and given a single pure electrophoretic IgY band of 174 kDa (native conditions) and 65/20 kDa (under reduced conditions) in the SDS-PAGE.
Neutralization experiments demonstrated a high neutralization capacity of the anti- Crot/pool preparation, as a preincubated mixture of both purified antivenom (19.66 mg) with two LD50 dose of Crot/pool venom (33 μg) protected 50% of the mice.
It has been showed since the early nineties (1990) that chicken egg yolk and their IgY immunoglobulins were able to neutralize scorpion and rattlesnake venoms, confirmed by in vivo experiments performed in rodents 13. Comparing hens with ostriches, the ostrich is one of the most primitive living avian suggesting that the diverse features of the bird Ig genes appeared very early during the divergence of the avian species and are thus common by most, if not all, bird’s species 14.
Antibodies are competent of explicitly recognizing a wide diversity of antigens with different affinities. Affinity, simultaneously with avidity is closely related to sensitivity, which is an experimentally measurable value in terms of antibody titer. Affinity is the concentration of the antigen that is needed to occupy the binding sites, of half of the antibody molecules present in an antibody solution, while avidity is the universal summation of the affinities of various antibodies (bivalent, multivalent and of different isotypes) fixed to all places to all available epitopes, considering conformational and valence aspects of the antibody 15. Authors 3 have developed successful anti-coral snake venom IgY antibodies, which were carried out in chicken egg yolks and their neutralizing action was similarly presented in mice by in vivo counteraction experiments. These antivenom immunoglobulins neutralized the toxic and lethal consequences of venom and, accordingly, could function to treat coral snake envenomed victims. Similarly, authors 16 have also produced hen antibodies against Scolopendra gigantea toxins with high neutralization titers and antivenom for the treatment of scolopendrism 17.
The present experimental work refers to the production of anti-(Crot/pool) anti-venom in ostrich egg yolk and its ability in deactivating the lethal consequences of the above mentioned Crotalus venoms. Immunization of ostriches with Crot/pool venom provoked a characteristic primary humoral response of low antibody titer in the ostrich sera and the egg yolks at 15 days, followed for a higher secondary response. The immunoglobulins titres amplified after the second booster and the intensities were sustained by continuing boosters. Ostrich eggs kept at 5°C during a year exhibited no substantial reduction in the antibody titers.
The caprylic acid 18,19 method showed that under controlled pH conditions, at a constricted pH range of 5.3-6.3 and low ionic strength of acidified water simplified the separation of IgY, after the yolk lipids aggregation, producing a clear IgY enriched supernatant, which was confirmed by SDS- PAGE analysis. This technique has been used for experimental purposes 18, but actually is used in the production of horse antivenoms 19, with novel fractionation approaches for antivenom production with the purpose of achieving antivenoms of higher purity, which would stimulate less allergic reactions in snake bitten patients treated with this product.
In the current work, it has been established by an immunoblot and/or ELISA assays that the anti-(Crot/pool) antivenom IgY recognized and responded to Crot/pool venom proteins. Immunoblot analysis revealed not only the specific binding of the antivenom but also dose-dependent blocking of antivenom by venom proteins.
The outcomes of inhibition studies show a specific neutralizing capacity of venom activity in the experimental anti-(Crot/ pool) IgY antivenom. This ending activity was regularly dose dependent, presenting ample inhibition at a concentration of 2LD50 (33µg) of Crot/pool venom, being indicative of specific binding of the IgY antibodies to venom proteins to which they were developed and their capacity to block the lethal effects of Crot/pool venoms. These types of immuno-neutralization help reverse toxicity and define the kinetics of toxins and antibodies.
Until now, traditional treatment of snakebite accidents is based on the use of antivenoms from horse’s origin. Nevertheless, equine serum in theory could activate complement cascade and initiate acute hypersensitivity reactions in patients formerly sensitized to horse serum proteins 20. The immunoglobulin IgY has the capacity of eliminating this possible side effect since it does not react as the mammalian IgG performs and it does not activate the mammalian complement factors 9. For that reason, the horse serum mammalian IgG activating mammalian complement factors 20 does not permit physicians to give out much larger doses of horse antivenom.
Regarding recent developments of chicken antivenoms, Latin American authors 17 have characterized IgY antivenoms capable of neutralizing the lethal activity of B. alternatus snake venom, at a preclinical level. An antivenom, as an alternative to the conventional antivenom production with egg yolk antibodies (IgY-technology) was proposed. Similarly, to the results presented in the current work, antivenom efficacy assays were carried out by them and after successive immunizations, levels of specific IgY reached a maximum that was maintained throughout the observation period; IgY antivenoms obtained after several immunizations neutralized 35.65 µg of B. alternatus venom per mg of antivenom. Other authors 21 proposed that birds were excellent hosts for the production of neutralization antibodies at low cost. These antibodies could be applied in the development of diagnostic kits or as an alternative for snakebite envenomation handling in the immediate future.
In conclusion, the specificity and specific activity of the antibody were scrutinized by western blotting and confirmed the presence of highly specific antibodies to Crot/pool venoms in the treated ostrich egg yolk. Ostrich’s antivenom can represent an excellent alternative for producing high amounts of antivenoms at very low costs 22. Thus, they could be a very good option to treat these accidents in countries with low economic resources where the ophitoxemia is a collective health problem. Therefore, the cleanliness, efficiency, and simplicity of producing antivenoms in ostriches, and the inability of these antibodies (IgY) to bind to the human complement formulates an interesting alternative to other antivenoms produced in mammals. These findings point out that ostrich egg antibody can be helpful as a therapeutic instrument to treat snakebites in humans, cattle and domestic animals.
In addition, these results open a therapeutic field, for the manufacturing of other antivenoms against the broad spectrum of toxins and also as a probable diagnostic tool.












 uBio
uBio 






