Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos Latinoamericanos de Nutrición
versión impresa ISSN 0004-0622versión On-line ISSN 2309-5806
ALAN v.58 n.2 Caracas jun. 2008
Chemical composition and fatty acid profile of rhea (Rhea americana) meat
Pedro Fernando Romanelli, Elizeu Trabuco, Andréia Borges Scriboni, Jesuí Vergílio Visentainer, Nilson Evelázio de Souza
UNESP, Campus de São José do Rio Preto, Depto de Engenharia e Tecnologia de Alimentos, rua Cristóvão, São José do Rio Preto/SP. Chemistry Department,, Universidade Estadual de Maringá, Maringá, Paraná State, Brazil
Summary.
The purpose of this work was to determine the proximate composition and fatty acid profiles of the Gastrocnemius pars interna intramuscular fat (IMF) of rhea (Rhea americana) thighs. The birds were bred in captivity, fed with balanced feed (Nutriavestruz Crescimento – Purina) and kept in a pen with grass ad lib. The birds of both sexes used in the research weighed 23 kg on average and were aged about twelve (12) months old. They were subjected to hydric diet (12 h) before slaughtering by electric shock. The rhea meat showed an average moisture, protein, ash and total lipid contents of 74.1%, 22.8%, 1.5% and 1.6%, respectively. It was noticed the predominance of monounsaturated fatty acids (MUFA) in intramuscular fat (IMF), 42.3% and a high percentage of polyunsaturated fatty acids (PUFA), 29.7%.. The fatty acids found in higher proportion in rhea IMF were: 18:2n-6 (24.33%), 18:1n-9 (19.25%), 16:0 (13.70%), 22:1n9 (11.40%), 18:0 (10.66%), 15:1n-10 (8.62%), 24:1n-9 (2.90%) and 20:4n-6 (1.72%). The PUFA/SFA and n-6/n-3 ratios were 1.06 and 31.30, respectively. The consumption of rhea meat is a healthy alternative to red meat as it points to a lower susceptibility to cardiovascular diseases caused by the high consumption of fat comparatively to the consumption of meat from most domesticated animals.
Key words: Ratite, Rhea americana, fatty acid profile, rhea.
Resumen.
Composición química y perfil de ácidos grasos de la carne de ñandú (Rhea americana). El objetivo de este trabajo fue determinar la composición aproximada y el perfil de ácidos grasos en la carne de la parte intramuscular IMF) interna de la pierna de ñandú (Rhea americana). Las aves fueron criadas en cautiverio, alimentadas con ración balanceada (Nutriavestruz Crescimento – Purina) y mantenidas en corral con pasto ad lib. La edad media de las aves de los dos sexos usados en la investigación fue de 12 meses y el peso medio de 23 kg. Fueron sometidas a dieta hídrica por 12 horas antes de matarlas por aplicación de descarga eléctrica. Los resultados de contenido medio de humedad, proteína, cenizas y lípidos totales fue de 74.1%, 22.8%, 1.5% y 1.6%, respectivamente. Fue posible notar una predominancia de ácidos grasos monoinsaturados (MUFA) en la gordura intramuscular (IMF) ), 42.3% y un alto porcentaje de ácidos grasos poli-insaturados (PUFA), 29.7%. Los ácidos grasos encontrados en mayor proporción en rhea IMF fueron: 18:2n 6 (24.33%), 18:1n 9 (19.25%), 16:0 (13.70%), 22:1n9 (11.40%), 18:0 (10.66%), 15:1n 10 (8.62%), 24:1n 9 (2.90%) y 20:4n 6 (1.72%). La PUFA/SFA (ácidos grasos saturados) y relación n-6/ n-3 fue 1.06 y 31.30, respectivamente. El consumo de carne de ñandú es una alternativa saludable frente a la carne roja pues su consumo presenta una menor susceptibilidad a enfermedades cardiovasculares causadas por alto consumo de grasas cuando comparado con el consumo de carne de animales domesticados.
Palabras clave: Aves corredoras, Rhea americana, Ácidos grasos, ñandú.
INTRODUCTION
Wild animals may become renewable sources of largely profitable products and contribute to food production at compatible operational costs. Normally, they produce meats with low total lipid contents and cholesterol besides high proportions of polyunsaturated fatty acids, comparatively to domesticated animals (1-4). The consumption of ratite meats such as ostrich (Strutio Camelus Australis), rhea (Rhea americana), and emu (Dromiceius novaehollandiae) stands out among those of wild animals. Ratite meat, in contrast to beef, is free of parasites transmittable to the consumer (5). In the last years, the interest in raising ratites for meat, leather, and oil production has grown. Rhea (Rhea americana) is the only species native to Brazil and practically there are no studies on its technological use. Rhea meat is red, has low fat content (@1.3%), high protein content (@23.5%) and significant amounts of minerals rich in iron in comparison to those of other species (6). The apparent fat of rhea has been cited as an important therapeutic product and as having a high skin penetration capacity and a large concentration of polyunsaturated fatty acids. In the United States and in some European countries, rhea oil is already studied as a nutritional supplement, an analgesic, a cicatrizant and as a cosmetic (5). The Omega 3 (n-3) and Omega 6 (n-6) fatty acids human dietary requirements are still debatable. Uauy et al., (7) observed that the Japan Society for Lipids Nutrition recommends dietary n-6:n-3 ratios of 4:1 for healthy adults and 2:1 for the prevention of chronic elderly diseases, while the World Health Organization (8) recommends 3:1 and 4:1, respectively. As no information on Brazilian rhea fat has been found in literature and based on the references to its pharmacological properties (therapeutic and cosmetic), we sought to characterize the fat of ratites for information and technical/scientific knowledge, which will favor future nutritional and pharmacological research.
MATERIALS AND METHODS
Sampling
Raw Material: Male and Female Rhea americana (common rhea) birds (registration at IBAMA nº 1/35/93/830-7) raised under nutritional management in confinement system from six to twelve months of age were used. During confinement, the birds feed on balanced commercial feed (Nutriavestruz® Crescimento from Purina) ad lib and three types of forage available in the pen. Rheas were slaughtered at the age of 12 months and average weight of 23 kg after 12-h hydric diet. The birds were grouped in lots for slaughtering: one lot with two birds and three lots with three birds each. Lot grouping was necessary to allow the work on the slaughter day and the analysis procedure afterwards. The birds were stunned with electric shock (110 V, 1 min) with an adapted electrode placed on the neck and another on the cloaca. After stunning, the birds were weighed and hung by the feet. Next, the birds were bled, manually plucked, eviscerated and the carcass was completely washed with plenty of water and the parts were separated (thigh, drumstick, etc.) from the carcass. Intramuscular fat (IMF) samples were taken from eight rheas at random. IMF samples were taken from every rhea. Each sample was submitted to lipid extraction and derivatization in triplicate (n=24). Meat samples for IFM analysis were taken from the Gastrocnemius pars interna (inner steak), located on the bird thigh. The muscles were ground, homogenized, separated in plastic bags, labeled and freeze stored in N2 atmosphere at -18 °C for later analysis.
Analytical methods
Moisture and ash contents were determined gravimetrically by desiccation at 105 °C and by incineration in oven at 600 °C, respectively. Crude protein was obtained by the Kjeldahl method (9). Total lipids were extracted from muscle tissue using the Bligh and Dyer (10) method. The fatty acid methyl esters (FAME) were prepared by methylation of the total lipids (TL), as described by Joseph and Ackman, (11). Fatty acid esters were separated in a gas chromatograph 14-A (Shimadzu, Japan) equipped with a fused silica capillary column CP- cyanopropyl (Select fame Varian - CP 7420) (100 m x 0.25 mm i.d. x ?0.25 μm film) and flame ionization detector. The operation parameters were as follows: detector temperature, 240 °C; injection port temperature, 220 °C; column temperature, 165 °C for 18 min, programmed to increase at 4 °C/ min up to 235 °C, with final holding time of 24 min. The gas flow rates used were 1.0 mL/min, carrier gas (H2), 30 mL/ min make-up gas (N2), and 30 and 300 mL/min flame gases (H2 and synthetic air, respectively). The sample splitting rate was 1:50 and samples (1 μL) were injected in triplicate. Peak areas were determined by Varian Star.
For fatty acid identification, retention times were compared with those of standard methyl esters (Sigma, St. Louis, MO).Equivalent Chain-Length values (ECL) were determined in gas chromatograph 14-A (Shimadzu, Japan) equipped with a fused silica capillary column (DB-WAX 20M, 30m x 0.25 mm i.d. x ?0.25 μm film), and 200°C/isotherm, values for all the 23 methyl esters were compared with standard used by Strànsky et al. (12) and Thompson (13). The observed values were also in line with the certified values reference material (RM 8415) distributed by the National Institute of Standards and Technology (NIST). Repeatability tests were performed injecting a pattern and a sample consecutively six times in a day. Reproducibility tests were also carried out, injecting the pattern and the sample twice a day for 3 days, under the same experimental conditions. Significant differences (P<0.05) were not found between the results obtained in either of the tests.
RESULTS
The proximate chemical composition of the Gastrocnemius pars interna from rhea is shown in Table 1. Similars values were obtained by one Hofman et al. (14) for ostrich Musculus iliofibularis and Girardi et al., (2005) for capybara (Hydrochaeris hydrochaeris) in Loin (76.7%; 74.4%), (21.6%;20.9%), (1.2%; 1.2%) and (2.0%; 1.8%), respectively. Sales et al. (6) found values of 1.2% and 1.3% for fat of Greater and Lesser Rhea.
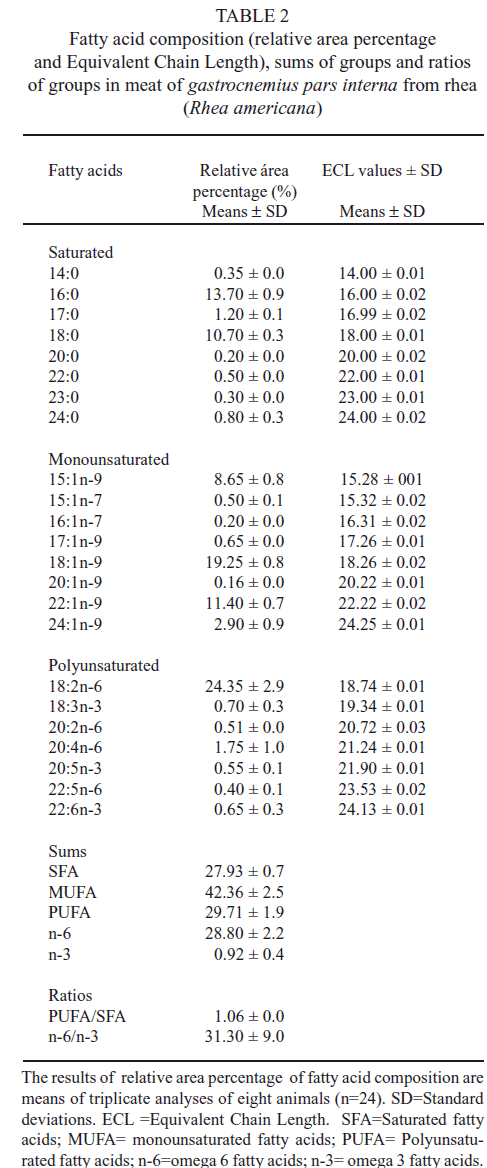
It was detected twenty-three fatty acids (FA), as shown in Table 2. It was observed that the amount of essential fatty acids of the n-6 family such as 18:2n-6 (LA) and 20:4n-6 (AA) is very significant as they make up about one fourth of total FA.
DISCUSSION
These data agree with those presented by Martino & Takahashi (16), who reported the predominance of these FA in terrestrial animal tissues, mainly of 18:2n-6 (LA). In this respect, Mancini-Filho and Chemin (17) corroborated that human beings do not synthesize these essential FA. As they are associated to the synthesis of vital substances, they must be obtained through the diet. The presence of some n-3 FA such as 18:3n-3 (LNA), 20:5n-3 (EPA), and 22:6n-3 (DHA) in foods, even in small amounts, stands out as they are considered primordial for the maintenance of biological membranes, the retina, the cerebral cortex, nervous tissues and for anti-inflammatory and other actions in the human body (18).Campos et al. (19) concluded that the proportion of long chain MUFA in relation to short chain MUFA must be 3:1 for a beneficial effect. The proportion found for these MUFA was 2:1. The PUFA/SFA ratio agrees with the recommendations for human consumption. According to Enser et al. (20), a ratio higher than 0.45 is beneficial for health maintenance. Although the n-6 and n-3 ratio shown in Table 2 is distinct from that found by Simopoulos et al. (21), it agrees with the value obtained by Longo et al. (22).Sales et al. (6) described the FA profile of IMF of two species of rhea: Common (Rhea americana) and Darwin (Pterocnemia pennata). In that work, the samples were made up of five different thigh and drumstick muscles: (iliofibularis, iliotibialis lateralis, femorotibialis medius, iliotibialis cranialis, and gastrocnemius pars externa). They used common rhea from farms in the Patagonia region of the Argentinean Pampas and five Darwin rheass from Patagonia. The birds, aged 11-12 months old, were raised in extensive system on cornmeal- and alfalfa-enriched diets. The rheass were slaughtered in the farm itself. No slaughtering technical details are given. The qualitative and quantitative variations in FA found in the two cases may be related to differences in operational procedures between the present work and that of Sales et al. (6). It is worth noting that in the present work, it was also detected other 16 FA not mentioned in literature besides those indicated by Sales et al.(6): 14:0, 17:0, 20:0, 22:0, 23:0, 24:0, 15:1n-9, 15:1n-7, 16:1n-7, 17:1n-9, 20:1n-9, 22:1n-9, 24:1n-9, 20:2n-6, 22:5n-6 and 22:6n-3. French et al. (23) emphasized that there were changes in FA composition in fat due to diet. Thus, the amount of PUFA may be increased by slaughtering animals with low fat deposition (24). However, Marmer et al. (25) and De Smet et al. (24) reported that the change in the FA profile of animals is also related to the increase in body fat. The larger the amount of body fat is, the larger the ratio SFA/PUFA is. It the present work, it was analyzed IMF samples from a single muscle, while in Sales et al. (6), and data were obtained from samples made up of five muscles. Webb et al. (26) showed that variations of FA profile for different cuts occur. Ruiz et al. (27) also confirmed the existence of differences in the distribution of lipids in animal muscles. If we compare the FA profile of IMF of three wild species, rhea (Rhea americana), studied here, ostrich (Struthio camelus), in Gastrocnemius pars interna (6), and capybara (Hydrochaeris hydrochaeris), in Loin and Ham (15), it is observed that the PUFA values of the three species are rather significant. The concentrations of 18:2n-6 (LA) in ostrich and capybara are very close, while those of 18:3n-3 (LNA) are very distinct in the three species. The direct comparison of the concentrations and the presence of FA with nutritional value (n-6 and n-3 type) is affected by the fact that these data may vary with age, muscle segment, sex, feed (nutritional management), and pasture management for each species. These results show that wild animal muscles may have large nutritional importance due to the presence of PUFA in considerable concentrations. Regarding meat consumption, the nutritional context must be taken into consideration.FA results for 20:4n-6 (AA) and 20:5n-3 (EPA) were the highest for capybara, followed by those of ostrich and ema. However, ema meat is richer in 22:6n-3 (DHA), an essential FA whose presence in IMF adds to its medicinal value, along with its traditional nutritional values (28). It was also observed that the SFA concentration in rhea is lower, which is in agreement with its nutritional evaluation in literature (6). This fact adds to the nutritional quality of rhea meat. The ostrich and rhea species have eight FA in common. FA 16:0, 18:0, and 18:2n-6 have very close concentrations, which may be attributed to the type of food and the digestive physiology of these animals (1-3). In relation to the PUFA values with large nutritional importance, it was observed that rhea has the largest concentration of 18:2n-6 (LA). These values agree with those of Sales (6), who found that rhea meat is rich in type n-6 PUFA.
CONCLUSIONS
The intramuscular fat profile of rhea meat presented twenty-three fatty acids. The total content of saturated, monounsaturated, and polyunsaturated fatty acids, n- 6, and n-3 were 27.93%, 42.36%, 29.71%, 28.80%, and 0.92%, respectively. The PUFA/SFA and n-6/n-3 ratios were 1.06 and 31.30. The consumption of rhea meat is a healthy alternative to red meat as it points to a lower susceptibility to cardiovascular diseases caused by the high consumption of fat comparatively to the consumption of meat from most domesticated animals.
REFERENCES
1. Crawford MA, Casperd MN, Sinclair AJ. The long chain metabolites of linoleic and linolenic acids and liver and brain in herbivores and carnivores. Comp Biochem Phys–part B, 1976; 54B: 395-401. [ Links ]
2. Sinclair AJ, Slattery WJ, Odea K. The analysis of polyunsaturated fatty acid in meat by capillary gas-liquid chromatography. J Sci Food Agric 1982; 33: 771-776. [ Links ]
3. Naughton JM, Odea K, Sinclair AJ. Animal foods in traditional aboriginal diets: polyunsaturated and low in fat. Lipids 1986; 21: 684-690. [ Links ]
4. Sinclair AJ, Odea K. Fats in Human diets though history: is the western diet out of step? In: Wood, J. D., Fisher, A. V. Reducing fat in meat animals. London: Elsevier, 1990. p. 1-47. [ Links ]
5. Giannoni ML. Viabilidade da exploração de ratitas em São Paulo. Biológico 1998; 1: 91-96. [ Links ]
6. Sales J, Navarro JL, Martella MB, Lizurume ME, Manero A, Bellis L, Garcia PT. Cholesterol content and fatty acid composition of rhea meat. Meat Sci 1999; 53: 73-75. [ Links ]
7. Uauy R, Mena P, Valenzuela A. Essential fatty acids as determinants of lipids requirements infants, children and adults. European J Clin Nutr 1999; 53: 66-77. [ Links ]
8. FAO/WHO. Report of a joint expert consultation: fats and oils in human nutrition. Food Nutr Paper 1994; 57: 49-55. [ Links ]
9. Cunniff PA. Official methods of Analysis of AOAC international. 6th ed.Arlington: Assoc. off Off. Analytical Chemists. CD-Rom. 1998. [ Links ]
10. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Phys 1959; 37: 911-917. [ Links ]
11. Joseph JD, Ackman RG. Capillary column gas chromatography method for analysis of encapsulated fish oil and fish oil ethyl esters: collaborative study. J AOAC Inter 1992; 75: 488- 506. [ Links ]
12. Stransky K, Jursik T, Vitek A. Standard equivalent chain length values of monoenic and polyenic (methylene interrupted) fatty acids. J High Res Chrom 1997; 30: 143-158. [ Links ]
13. Thompson RH. Simplifying fatty acid analyses in multicomponent foods with a standard set of isothermal GLC conditions coupled with ECL determinations. J Chrom 1996; 34: 495-504. [ Links ]
14. Hoffman LC, Joubert M, Brand TS, Manley M. The effect of dietary fish oil rich in n-3 fatty acids on the organoleptic, fatty acid and physicochemical characteristics of ostrich meat. Meat Sci 2005; 70: 45-53. [ Links ]
15. Girardi F, Cardozo RM, Souza VLF, Moraes GV, Santos CR, Visentainer JV, Zara RF, de Souza NE. Proximate composition and fatty acid profile of semi confined young capybara (Hydrochoerus hydrochaeris hydrochaeris L. 1766) meat. J Food Comp Anal 2005; 18: 647-654. [ Links ]
16. Martino R, Takahashi NS. A importância da adição de lipídios em rações para a aqüicultura. Óleos e Grãos 2001; 58: 32-37. [ Links ]
17. Mancini-Filho J, Chemin S. Implicações nutricionais dos ácidos graxos trans. Óleos e Grãos 1996; 31: 41-45. [ Links ]
18. Schmidt MA. Gorduras inteligentes. Trad. Dirceu H. Pereira. São Paulo – SP. Ed. Roca Ltda , 2000; 231p. [ Links ]
19. Campos FG, Waitzberg DL, Habr-Gama A, Loqullo AF, Noronha IL, Jancar S, Torrinhas RSM, Furst P. Impact of parenteral n-3 fatty acids on experimental acute colitis. Brit J Nutr 2002; 87: S83-S88. [ Links ]
20. Enser M, Hallett K, Hewitt B, Fursey-G AJ, Wood JD. Fatty acid content and composition of English beef, lamb and pork at retail. Meat Science 1996; 42: 443-456. [ Links ]
21. Simopoulos AP, Leaf A, Salem N. Essentially and recommended dietary intakes for omega-6 and omega-3 fatty acids. An Nutr Metab 1999; 43: 127-130. [ Links ]
22. Longo S, Nakasato M, Costa RP, Lottenberg AN, Fisberg M, Quintão E. Alimentação e Ácidos Graxos ω-3 e ω-6. Arquivos Brasileiros de Cardiologia 2001;77: 308-310. [ Links ]
23. French P, Stanton C, Lawless F, ORiordan EG, Monahan FJ, Caffrey PJ, Moloney AP. Fatty acid composition including conjugated linolenic acid, of intramuscular fat from steers offered grazes grass, grass silage, or concentrated based diets. J Anim Sci 2000; 78: 2849-2855. [ Links ]
24. De Smet S, Webb EC, Claeys E, Uytterhaeqen L, Demeyer DI. Effect of dietary energy and protein levels on fatty acid composition of intramuscular fat in doublé-muscled belgain blue bulls. Meat Sci 2000; 56: 73-79. [ Links ]
25. Marmer WM, Maxwell RJ, Williams JE. Effects of dietary regimen and tissue site on bovine fatty acid profiles. J Anim Sci 1984; 59: 109-121. [ Links ]
26. Webb EC, De Smet S, Van Nevel C, Martens B, Demeyer DI, Effects of anatomical location on the composition of fatty acids in double-muscled Belgian Blue cows. Meat Sci 1998; 50: 45-53. [ Links ]
27. Ruiz R, Martin CA, De-Souza NE, Visentainer JV, Prado IN, Matsushita M. Importância dos ácidos graxos de cadeia longa: DHA, EPA e araquidônico presentes na carne. Rev Nac Carne 2004; 338: 72-76. [ Links ]
28. Park EI, Paisley EA, Mangian HJ, Swartz DA, Wu MX, Omorchoe PJ, Behr SR, Visek WJ, Kaput J. Lipid level and type alter stearoyl CoA desaturase mRNA abundance differently in mice with distinct susceptibilities to diet-influenced diseases. J Nutr 1997; 127: 566-573. [ Links ]