Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Latinoamericana de Metalurgia y Materiales
versión impresa ISSN 0255-6952
Rev. LatinAm. Metal. Mater. v.30 n.1 Caracas jun. 2010
Synthesis and characterization of Fe3O4 magnetic nanofluid
Javier A. Lopez1*, Ferney González2, Flavio A. Bonilla3, Gustavo Zambrano1, Maria E. Gómez1
1: Thin Films Group, Universidad del Valle, Cali, Colombia.
2: Chemistry Analytical Laboratory, Universidad del Valle, Cali, Colombia..
3: Asylum Research. Nanomechanics and tribology, Santa Barbara, United States.
* E-mail: javierlo21@gmail.com
Publicado On-Line el 14-Jun-2010 Disponible en: www.rlmm.org
Trabajo presentado en el congreso X Iberoamericano de Metalurgia y Materiales (X IBEROMET)celebrado en Cartagena, Colombia, del 13 al 17 de Octubre de 2008; y se seleccionó para ser remitido a la RLMM para su arbitraje reglamentario y publicación.
Abstract
Ferrofluids are colloidal systems composed of single domain of magnetic nanoparticles dispersed in a liquid carrier. In the present work, Fe3O4 magnetic ferrite nanoparticles were synthesized by chemical coprecipitation method, and were coated with oleic acid as surfactant agent. Magnetic properties of nanoparticles in ferrofluids were investigated with the aid of a vibrating sample magnetometer (VSM) at room temperature. Superparamagnetic behavior, characteristic of magnetic nanoparticles, was determined from the hysteresis loop of M vs. H measurements. The sample as powder was characterized by means of X-ray diffraction. XRD pattern result shows the presence of the most intense peak corresponds to the (311) crystallographic orientation of the spinel phase of Fe3O4 magnetic nanoparticles. The mean size of the nanoparticles was determined from the X-ray diffraction pattern by using the Scherrer approximation. The particle size was calculated to be 9.64 nm. Atomic Force Microscopy was used to visualize the morphology of nanoparticles and to measure their diameter. The AFM method showed an average nanoparticles diameter of DN = 15.3 nm. FTIR absorption spectroscopy was used to confirm the formation of Fe–O bonds, allowing to identify the inverse ferrites spinel structure, as well as, the presence of other chemical substances adsorbed on the surface of particles.
Keywords: Ferrofluids, nanoparticles, coprecipitation chemical synthesis, spinel structure
Resumen
Los ferrofluidos son sistemas coloidales compuestos de nanopartículas magnéticas con mono-dominios magnéticos, dispersas en un líquido portador. En el presente trabajo, nanopartículas magnéticas de ferrita (Fe3O4) se sintetizaron por el método de co-precipitación química, y se recubrieron con ácido oleico como agente surfactante. Las propiedades magnéticas de las nanopartículas en el ferrofluido fueron investigadas por medio de un magnetómetro de muestra vibrante (VSM) a temperatura ambiente, mostrando un comportamiento superparamagnético, característico de estas nanopartículas magnéticas. Esto se determinó a partir de la curva de histéresis de M vs H. La muestra en polvo se caracterizó por medio de Difracción de Rayos X. El patrón DRX resultante muestra la presencia del pico más intenso correspondiente a la orientación cristalográfica (311) de la fase espinel para las nanopartículas magnéticas de Fe3O4. El tamaño medio de las nanopartículas se determinó a partir del patrón de difracción de rayos X utilizando la aproximación de Scherrer, siendo este tamaño medio de 9,64 nm. La microscopía de fuerza atómica, se utilizó para visualizar la morfología de las nanopartículas y para determinar su diámetro. Las medidas de AFM muestran un diámetro promedio para las nanopartículas de DN = 15,3 nm La espectroscopia de absorción FTIR se utilizó para confirmar la formación de los enlaces Fe-O, permitiendo identificar la estructura tipo espinela de la ferrita, así como la presencia de otras sustancias químicas adsorbidas en la superficie de las partículas.
Palabras Claves: Ferrofluidos, nanopartículas, síntesis por coprecipitación química, estructura espinel.
Recibido: Nov-2008; Revisado: 28-Nov-2009; Aceptado: 22-Nov-2009
1. INTRODUCTION
Ferrofluids (FF) or magnetic nanofluids are stable magnetic colloids systems composed of nanoparticles of single-magnetic domain with a mean diameter of around 10 nm, dispersed in an organic or inorganic carrier fluids (aqueous or nonaqueous). Recently, FF or magnetic nanofluids have been the subject of much interest because of their unusual optical, electronic, and magnetic properties [1-3], which can be changed by applying an external magnetic field. The special feature of FF is the combination of normal liquid behavior with superparamagnetic properties. They are said to be superparamagnetic, meaning that they are attracted by a magnetic field but retain no residual magnetism after the field is removed. Due to their small size and superparamagnetic behavior, magnetic ferrofluids can be viewed as a new class of magnetic materials with potential technological applications in biomedicine and nanotechnology. Nowadays, they are promising materials for cancer diagnosis and therapy [4-8].
There are various methods to prepare the magnetite nanoparticles of nanometer size range, due to their immense technological applications. In the present work, surfacted and ionic aqueous Fe3O4 magnetic nanofluids have been synthesized and characterized. Magnetic properties of nanoparticles in ferrofluids and their mean crystallite size in powder, were investigated through a vibrating sample magnetometer (VSM) and X-ray diffraction (XRD) by using the Scherrer approximation, respectively. Scanning probe microscopy (SPM) was used in two ways: atomic force microscopy (AFM) to determine the morphology of conglomerates of nanoparticles and to measure their diameter; and magnetic force microscope (MFM) to visualize the magnetic domains of Fe3O4 nanoparticles. The IR spectra were used to confirm the formation of bonds, related to metal in the octahedric and tetrahedric sites, allowing to identify the ferrites spinel structure.
2. EXPERIMENTAL DETAILS
Chemical coprecipitation is probably the most common method to prepare magnetic nanoparticles. In this method, it is usually necessary to start from a mixture of ferrous and ferric salts suspended in an aqueous alkaline medium. Then, the solution is subjected to different procedures such as decantation, magnetic separation, centrifugation, and dilution. In order to avoid the agglomeration of magnetic nanoparticles in ferrofluids, they are usually covered with a shell of an appropriate material (sterical stabilization). According to the coating, the FF are classified into two groups: surfacted ferrofluid (SSF), if the coating is a surfactant molecule, and ionic ferrofluid (IFF), if it is water or other type of electric shell. The preparation of surfacted and ionic aqueous ferrofluids based on spinel ferrite nanoparticles has been reported by Massart et al. [9,10]. Synthesis of magnetite (Fe3O4) nanoparticles has been of great interest, especially in the form of nanofluids.
2.1. Synthesis of Fe3O4 nanoparticles
The synthesis of nanofluids has two main steps: the preparation of nano-sized magnetic particles and the subsequent dispersion-stabilization of the nanoparticles in various non-polar and polar carrier liquids (i. e. water and kerosene). The magnetic nanofluids based on magnetite Fe3O4 and mineral oils or organic solvents are prepared by coprecipitation technique from aqueous salt solutions Fe (II) and Fe (III) in alkaline medium and suspended in the carrier liquid [11-13]. The magnetite obtained is stabilized by a surfactant. Oleic acid is usually used as surfactant, which forms the waterproofing shell around the magnetite nanoparticles. The treatment of the magnetite by oleic acid is a very important stage of the magnetic nanofluids preparation, because the size and physical properties of nanoparticles depend of preparation parameters, such as, reaction temperature, pH of the suspension, initial molar concentration, and others.
2.2. Materials
To prepare the ferrofluid, FeCl3 6H2O (97 %) and FeCl2 4H2O (99 %) and sodium hydroxide (NaOH) from MerckTM were used. HPLC grade Oleic acid (C18H34 O2) was used as surfactant. All the materials were reagent grade and used without further purification. Distilled, deionized water was used as a solvent.
2.3. Procedure
Nanofluid sample was prepared by the coprecipitation technique. The synthesis of Fe3O4 magnetic nanoparticles (MNP) is based in the mixture of FeCl3 6H2O and FeCl2 4H2O salts in the molar ratio 1:2. The reaction in this process is as follows:
2Fe3+ + Fe2+ + 4OH- ® Fe3O4 + 4H+
After mixing 1.0 mL of 2M FeCl2 4H2O and 4.0 mL of 1M FeCl3 6H2O in the same solution, 25 mL of a 1.0 M NaOH solution was added dropwise, under vigorous stirring. Then, the precipitation process occurred immediately, promoting a color change in the solution to dark-black, characteristic of the magnetite. In order to neutralize the anionic charges on the nanoparticles surface, 0.5 mL of 2M hydrochloric acid (HCl) was used.
The pH of the solution was constantly monitored when the NaOH solution was added to produce the precipitation of the Fe3O4 ferrofluid nanoparticles. The reactants were magnetically stirred during the precipitation, and the pH level was reduced from approximately 12 to 7 – 8 by a washing with deionized water. Then, the sample was dispersed in kerosene (1 mL) and deionized water, and stirred for 2 h at 30°C. Finally, an amount of 5 mL of oleic acid was added to the solution as a surfactant and coating material [14]. The precipitated liquid was brought to a reaction temperature of 80°C, in order to evaporate the liquid, and then, it was stirred for 1 h and cooled to room temperature. To obtain sodium and chlorine free particles, the precipitate was washed twice with deionized and distilled water, as a solvent, in order to prevent the presence of impurities in the final product and, later on, with ethanol, to remove the excess surfactant from the solution. To isolate the supernatant liquid (ethanol plus water), the beaker contents were then centrifuged for 15 min at 4000 rpm. The supernatant liquid was then decanted, and centrifuged until only thick black precipitate remained. A part of volume of this precipitate was dried at 100 °C for 10 h and grinded into a fine powder to perform the XRD and FTIR analysis. The other part of colloid was used to carry out the magnetic and AFM measurements [15, 16].
3. RESULTS AND DISCUSSION
3.1. Structural and particle characterization
The precipitated fine particles were characterized by XRD for structural determination and estimation of crystallite size. The X-ray powder diffraction pattern of the sample was recorded on a Bruker D8 Advance diffractometer using CuKa (1.5406 Å) radiation at room temperature in the range of 10 to 80° in the 2q scale, with a scanning speed of 0.02°/s and a step time of 3 s. Generally, XRD can be used to characterize the crystallinity of nanoparticles, as well as the average nanoparticle diameter. All the peaks of XRD patterns were analyzed and indexed using ICDD data base, comparing with magnetite standards [17,18]. The lattice constant a was found to be 8.310 Å, which was compared with the lattice parameter for the magnetite of 8.39 Å. Finally, the analysis of the diffraction pattern showed the formation in the sample of a cubic spinel structure, due to the strongest reflection that proceeds from the (311) plane (Fig. 1), characteristic of such a phase [19]. The peaks indexed as planes (220), (311), (400), (422), (511) and (440) corresponded to a cubic unit cell, characteristic of a cubic spinel structure [20,21]. Therefore, it was confirmed that the crystalline structure of obtained magnetite nanoparticles, agreed with the structure of an inverse spinel type oxide. Crystallite size measurements were determined from the full-width at half maximum (FWHM) of the strongest reflection of the (311) peak, using the Scherrer approximation, which assumes the small crystallite size to be the cause of line broadening (Equation 1):
Here, DN is the crystallite mean size, k is a shape function for which a value of 0.9 is used, l is the wavelength of the radiation, b the fullwidth at halfmaximum (FWHM) in radians in the 2q scale, and q the Bragg angle. The crystal size calculated were (9.64 ± 0.13) nm. Due to the broad diffraction pattern lines, it can be said that particles have size about of nanometers; therefore, the nanofluids can be conveniently prepared by making use of particles in this size range.
3.2. Magnetic measurements
Magnetic characterization of the nanoparticles was done using vibrating sample magnetometer (VSM) in the physical property measurements system (PPMS) of Quantum Design, at room temperature, with a magnetic field in the range of -5000 to 5000 Oe, where parameters as saturation magnetization (Ms), and coercive field (Hc) were evaluated. The behavior of nanofluids is mainly determined by their magnetic properties.
Ferrofluids usually show a superparamagnetic behavior, since each of the particles can be treated as a thermally agitated permanent magnet in the carrier liquid. In the presence of a magnetic field H, the magnetic moment (m) of the particles will try to align with the magnetic field direction, leading to a macroscopic magnetization of the liquid. The magnetization M of the liquid behaves like the magnetization of a paramagnetic system, furthermore, this type of materials often show hysteresis loops (M–H curves), explained by an irreversibility in the magnetization process related to the pinning of magnetic domain walls at impurities or grain boundaries within the material. Another possible cause of such a behavior is the magnetic anisotropy of the crystalline lattice. The shape of these loops is determined, in part, by particle size. Even at smaller sizes (around tenths of nanometer or less), superparamagnetic behavior can be seen [22], where the magnetic moment of the particle as a whole is free to fluctuate in response to thermal energy, while the individual atomic moments maintain their ordered state relative to each other.
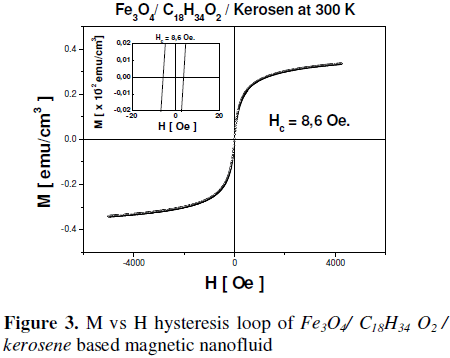
This leads to the low anhysteretic behavior show in the M–H curve for the magnetite sample based in water or kerosene like carrier liquid, respectively (Fig. 2 and 3). The obtained saturation magnetization (MS) and the coercive field (Hc) at room temperature were 0.067 emu/cm3 and 16.7 Oe (Fig. 2) for the Fe3O4 sample water based magnetic nanofluid, and 0.335 emu / cm3 and 8.6 Oe (fig. 3), for the Fe3O4 sample kerosene based, respectively.
When magnetic nanoparticles are suspended in kerosene, and coated with oleic acid as surfactant agent, the difference in the magnetic properties of particles can be attributed to a weak structuration favored by the combined action of interfacial (Van der Waals or acid–base), magnetic attractions between the particles immersed in the carrier liquid and the steric repulsion produced between particles, which avoids the formation of conglomerates. Therefore, they can be considered like single– domain nanoparticles showing a superparamagnetic behavior [23, 24]. Finally, the recorded hysteresis loops shows that the particles are superparamagnetic at room temperature, which is characteristic of a soft ferromagnetic material such as magnetite.
3.3. AFM measurements
AFM investigation provides qualitative and quantitative information on the diameter and height of the scanned particles. The equipment used to visualize the morphology of nanoparticles was an Asylum Research MFP-3D Atomic Force Microscope in both AC non-contact mode in air and magnetic mode (MFM) operating at room temperature. An Asylum Research ASYMFM cantilever and tip were used. This cantilever was made up of silicon, coated with a 50nm CoCr layer. The cantilever had the following characteristics: coercivity in the range of 300-450 Oe, spring constant 1-2 N/m, resonant frequency of 55 to 90 KHz and a tip radius less than 20 nm.
AFM measurements have been carried out on dried samples deposited on mica and hard disk substrate (useful immobilizing individual particles). They have been repeated on different sites of the deposited sample, prepared in the same conditions of room temperature and ambient atmosphere. The analysis of magnetic particles consisted in the observation of the surface of Fe3O4 nanoparticles. The mean diameter from the height, measured by AFM is larger than the mean size of the nanoparticles measured by XRD. This can be explained because the analysis is statistical and some particles are, in fact, conglomerates, which may result in apparent particles with relatively larger height. The existence of such agglomerates implies that an improvement in the preparation of the samples is required, because the attractive electric and magnetic forces are not totally balanced by the steric repulsion conferred by the oleic acid coating molecules.
The agglomeration of Fe3O4 nanoparticles is evident in the topography image, figure 4a, obtained by means of AFM. Figure 4b, represents the best curve of the particle size histogram using the lognormal distribution function. It shows that the most probable particle diameter of conglomerates was of DN = (15.3 ± 0.2) nm, with a coefficient of variation of 1.3% [25-27]. Figure 4c shows a 2D MFM image, where we can see the magnetic domains of Fe3O4 nanoparticles conglomerates with a random direction. The samples were magnetized by an external field oriented vertically to the mica substrate plane prior to the measurement. In this case, the interpretation of magnetic domains shows that a dark zone represents a repulsive force of domains, while a bright zone is an attractive force.
3.4. FTIR Spectral measurements.
Fourier Transform Infrared Spectroscopy (FTIR) spectra was performed to the dried sample of magnetite using a FTIR – Shimadzu 8400 spectrophotometer in wave range of 3500 - 400 cm-1 with a resolution of 4 cm-1. The dried sample was placed on a silicon substrate transparent to infrared, and spectra were measured according to the transmittance method.
FTIR spectrum in figure 5 shows that the H-O-H bending vibration at about 1000 - 1600 cm-1, typical of the H2O molecule, is less intense. Additionally, the second absorption band, between 900-1000 cm-1, corresponds to bending vibration associated to the O – H bond. The O–H in plane and out of plane bonds appears at 1583.45 – 1481.23 and 935.41 – 838.98 cm-1, respectively [28]. For strong hydrogen bridges its maximum lies at about 900-1000 cm-1. These first two bands correspond to the hydroxyl groups attached by the hydrogen bonds in the iron oxide surface, as well as the water molecules chemically adsorbed to the magnetic particle surfaces. In the spectrum showed (fig. 5), the sample exhibits two intense peaks, in 582 and 640 cm-1 bands, that are due to the stretching vibration mode associated to the metal-oxygen absorption band (Fe – O bonds in the crystalline lattice of Fe3O4) [29]. They are characteristically pronounced for all spinel structures and for ferrites in particular. This occurs because, in these regions, the contributions from the stretching vibration bands related to metal in the octahedral and tetrahedral sites of the oxide structure are found. Moreover, the FTIR spectrum shows an absorption band at 1706 cm-1, which presents the stretching vibration of the carboxyl group (C = O), associated to the oleic acid molecule, adsorbed on to the surface of the crystallites. Summarizing, magnetite nanoparticles have crystalline structure of inverse spinel type, and FTIR absorption spectroscopy allowed identifying characteristic features of the spinel structure, as well as a presence of certain types of chemical substances adsorbed on the surface of nanoparticles [30-31].
4. CONCLUSIONS
In the present study, the average crystallite size (DN) of the synthesized ferrofluid, was estimated through XRD analysis by calculation of the FWHM value. This size was found to be 9.64 nm, making the produced ferrofluid, at a first glance, suitable for technological applications. Additionally, AFM measurements allowed obtaining information about the sample surface morphology, indicating a difference in DN associated to conglomeration of Fe3O4 cristallites. XRD analysis also showed that magnetite nanoparticles produced had, in fact, a crystalline structure of inverse spinel. FTIR absorption spectroscopy confirmed this feature as well. Summarizing, the Fe3O4 nanofluids prepared in this work, through the coprecipitation method, showed superparamagnetic behavior at room temperature, documented by the hysteresis loop recorded. Therefore, magnetite nanoparticles in the ferrofluid can be regarded as a soft material due to their low coercive field.
5. ACKNOWLEDGEMENTS
This work was supported by COLCIENCIAS (Colombia), the Excellence Center for Novel Materials – CENM and UNIVALLE, under Projects contracts 1106-05-17612, 239-05, 043-2005 and 7703. 6.
REFERENCES
1. Bahadur D, Giri J, Bibhuti B N, Sriharsha T, Pradhan P, Prasad N K, Barick K C and Ambashta R D. Pramana - J. Phys. 2005, 65 (4): 663 – 679. [ Links ]
2. Perez-Castillejos R, Plaza J. A, Esteve J, Losantos P, Acero M. C, Cane C, Serra-Mestres F. Sensors and Actuators 2000, 84:176-180. [ Links ]
3. Piso M.I. Journal of Magnetism and Magnetic Materials 1999, 201 (1): 380-384. [ Links ]
4. Scherer C, and Figueiredo Neto A. M. Brazilian Journal of Physics. 2005, 35 (3A): 718-727. [ Links ]
5. Gupta A. K, Gupta M. Biomaterials. 2005, 26: 3995 – 4021. [ Links ]
6. Salata OV. Journal of Nanobiotechnology. 2004 [On-Line], 2(3): 1 – 6. (Cited 21-June-2008) This article is available from: http://www.jnanobiotechnology.com/content/2/1/3. [ Links ]
7. Jordan A, Scholz R, Maier-Hau K, Johannsen M, Wust P, Nadobny J, Schirra H, Schmidt H, Deger S, Loening S, Lanksch W, Felix Roland. Journal of Magnetism and Magnetic Materials. 2001, 225: 118 – 126. [ Links ]
8. Jordan A, Scholz R, Wust P, Fähling H, Felix Roland. Journal of Magnetism and Magnetic Materials. 1999, 201: 413 – 419. [ Links ]
9. Massart R. IEEE Trans. Magn. 1981. 17: 1247 - 1248. [ Links ]
10. Tourinho F. Franck R. and Massart R. J. Mater. Sci. 1990, 25 (7): 3249 - 3254. [ Links ]
11. Kholmetskiia A.L, Vorobyovab S.A,T, Lesnikovichb A.I, Mushinskiib V.V, Sobalb N.S. Materials Letters. (2005), 59: 1993-1996. [ Links ]
12. Râcuciu M, Creangâ D. E, Câlugâru Gh. Jounral of Optoelectronics and Advanced Materials. 2005, 7(6) 2859 - 2864. [ Links ]
13. Hong R.Y, Zhang S.Z, Han Y.P, Li H.Z, Ding J, Zheng Y. Powder Technology. 2006, 170: 1 –11. [ Links ]
14. García-Cerda L.A., Rodríguez-Fernández O.S., Betancourt-Galindo R., Saldívar-Guerrero R. Superficies y Vacío. 2003 16(1): 28-31. [ Links ]
15. Martínez-Mera I, Espinosa-Pesqueira M.E, Pérez-Hernández R, Arenas-Alatorre J. Materials Letters. 2007, 61: 4447 – 4451. [ Links ]
16. Wang X, Zhang C, Wang X, Gu H. Applied Surface Science. 2007 253: 7516 –7521. [ Links ]
17. Bragg W.L. Nature. 1915, 95: 561. [ Links ]
18. Ayala-Valenzuela O, Matutes-Aquino J, Betancourt-Galindo R, Garcia-Cerda L.A, Rodriíguez F. O, Fannin P.C, Giannitsis A.T. Journal of Magnetism and Magnetic Materials. 2005, 294: e37–e41. [ Links ]
19. Kim Y. Il, Kim D, Lee C.S. Physica B. 2003, 337: 42 – 51. [ Links ]
20. Vaidyanathan G., Sendhilnathan S, Arulmurugan R. Journal of Magnetism and Magnetic Materials. 2007 313: 293 – 299. [ Links ]
21. Betancourt-Galindo R, Rodríguez-Fernández O.S. Superficies y Vacío 17 (1): 38 - 41. [ Links ]
22. Pankhurst Q A, Connolly J, Jones S K, Dobson J. J. Phys. D: Appl. Phys. 2003, 36: R167 – R181. [ Links ]
23. Peia W, Kumadaa H, Natusmea T, Saitoa H, Ishio S. Journal of Magnetism and Magnetic Materials. 2007, 310: 2375–2377. [ Links ]
24. López-López M.T, Durán J.D.G, Delgado A.V, González-Caballero F. Journal of Colloid and Interface Science. 2005, 291: 144 – 151. [ Links ]
25. Apetroaie N, Roca A, Creanga D. E. Journal of Optoelectronics and Advanced Materials. 2005, 7(6): 2865 – 2868. [ Links ]
26. Lacava L. M, B. Lacava M, Azevedo R. B, Lacava Z. G. M, Buske N, Tronconi A. L, Morais P. C. Journal of Magnetism and Magnetic Materials. 2001, 225: 79 - 83. [ Links ]
27. Butter K, Philipse A.P, Vroege G.J. Journal of Magnetism and Magnetic Materials. 2002, 252:101 – 103. [ Links ]
28. Ling Z; Rong He, Hong-Chen G. Applied Surface Science. 2006, 253(5): 2611-2617. [ Links ]
29. Ahn Y, Choi E.J, Kim E.H, Rev. Adv. Mater. Sci. 2003, 5: 477. [ Links ]
30. Farmer V.C, The Infrared Spectra Of Minerals Ed. By V.C. Farmer Mineralogical Society, London, 1974 p.18. [ Links ]
31. Farmer V. C. Phil. Trans. R. Soc. Lond. A. 1982; 305: 609-619. [ Links ]