Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos Latinoamericanos de Nutrición
versión impresa ISSN 0004-0622versión On-line ISSN 2309-5806
ALAN v.51 n.1 supl.1 Caracas mar. 2001
The chemistry of ferrous bis-glycinate chelate
Stephen D. Ashmead
Albion Laboratories, Inc., Clearfield, Utah U .S.A.
SUMMARY.
In order to produce a ferrous chelate tour criteria 1 must be met: 1) the ligand must contain two functional groups which are capable of entering into covalent and coordinate covalent bonds; 2) a ring structure with the ferrous ion being the closing member of the ring must be created; 3) the chelate must be ( sterically possible; and 4) the chelation reaction must be energetically possible. In addition to the above, a totally nutritionally functional ferrous chelate must meet three further criteria: 1) it must have low molecular weight; 2)its stability constant must be nutritionally functional; and 3) the ligand must be metabolizable by the body. When. ferrous iron is reacted with glycine and forms a bis-glycinate chelate, it meets all of the requirements of being both a chelate and being a totally nutritionally functional chelate.
Key words: Chelation, criteria for chelation, Ferrochel, amino acid chelates.
RESUMEN.
La química del hierro ferroso bis-glicinato quelado. Para producir un hierro ferroso bis-glicinato quelado es necesario llenar los siguientes cuatro criterios: 1) el ligando debe contener dos grupos funcionales capaces de producir uniones covalentes y covalentes coordinadas; 2) debe poder crearse una estructura en la cual el ion ferroso sea el elemento que cierra una estructura en anillo; 3) el quelado debe ser estéricamente posible, y 4) la reacción de quelación debe ser energéticamente posible. Adicionalmente, un quelado ferroso para ser nutricionalmente funcional debe también llenar los siguientes criterios: 1) debe tener un bajo peso molecular; 2) su constante de estabilidad debe ser nutricionalmente funcional, y 3) el ligando debe poder ser metabolizado en el organismo. Cuando el hierro ferroso se reaciona con glicina para formar un bis-glicinato quelado, este llena todos los requerimientos tanto para ser un verdadero quelado como para ser nutricionalmente funcional.
Palabras clave: Quelación, criterios de quelación, Ferrochel, quelados con amino ácidos.
The chemistry of chelation is not novel. As a chemical phenomenon, various iterations of it have been studied for a little more than a century, beginning in the early part of the last decade of the nineteenth century. It was recognized as early as then that certain atoms could have more than one valence state. It was not, however, understood until the theory of chelation was proposed just how these particular atoms could form highly stable compounds.
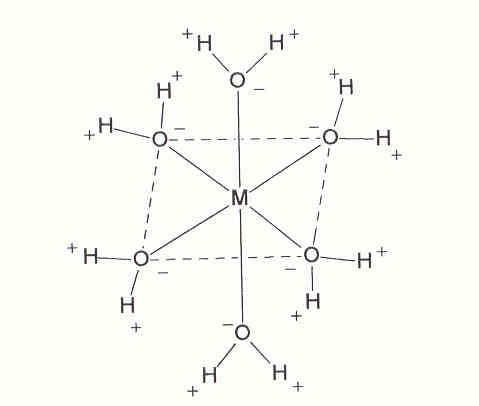
In 1893, Alfred Werner postulated a new molecular structure to describe those stable molecules. He had noted that certain structural entities, which he called "complexes", remained intact through a series of chemical transformations. As a result of those observations, he wrote, "If we think of the metal ion as the center of the whole system, then we can most simply place the molecules bound to it at the corners of an octahedron" (1). His initial concept is illustrated in Figure 1, wherein the soluble metal ion (M) exists in a hydrato-complex bound to a number of water molecules in water with the negative oxygen of the water dipole oriented towards the positive metal. This results in a certain spatial configuration of the donor ligand around the central metal atom.
In the ensuing years, from 1894 through 1914, Werner refined his concepto Ultimately, he concluded that the cation was characterized by two valences. The first of these he termed the "principal valency". This corresponds to the oxidation state, or oxidation number of the metal. Werner's second valency was called the "auxiliary valency". This referred to the number of atoms from the ligand associated with the central metal ion and today is known as the coordination number of the metal (2-7).
A few years later, in 1920, Morgan and Drew applied the term "chelate" to the molecular structure postulated by Werner (8). In order to form a chelate, it was recognized that the ligand must have two points of attachment to the metal ion. It was this caliper-like mode of attachment that led to the use of the Greek word "chele", meaning lobster claw, to describe how the ligand was attached to the metal ion. When the claw, or ligand, held the cation, the metal was restricted in its ability to enter into other chemical reactions. Once chelated, the metal's chemical and physical characteristics changed (9). The metal chelates are coordination compounds in contrast to metal salts where the cation is bound by electrostatic attraction. In a chelate, the ligand donates electrons to the cation. More than one donor atom must come from the ligand so that a heterocyclic ring is formed with the metal being part of that ring. Comprehension of the structure and characteristics of metal chelates has had far reaching consequences in medicine, biology, chemistry, environmental chemistry, and, particularly, nutrition, due to the stability of the metallic molecule formed.
Complexation of a metal by water in an octahedral geometry as described by Werner
The American Association of Feed Control Officials (AAFCO), an organization composed from all 50 individual state (U.S.) chemists and the United States and Canadian Food and Drug Administrations, have officially defined a metal amino acid chelate as "the product resulting from the reaction of a metal ion from a soluble salt with amino acids with a mole ratio of one mole of metal to one to three (preferably two) moles of amino acids to form coordinate covalent bonds. The average weight of the hydrolyzed amino acids must be approximately 150 and the resulting molecular weight of the chelate must not exceed 800" (10). This definition states that the metal and amino acids must be reacted before a chelate can be formed.
Since, by definition, the formation of a chelate requires chemical reactions to occur, four inviolate chemical requirements must be met. If any one of the four criteria is not fulfilled, the resulting molecule will not be a chelate, but simply a metal complex, a salt, or perhaps even an admixture.
The first of these criteria requires that the ligand must possess two functional groups, each capable of donating electrons to bond with the metal ion (11). The elements in the ligand that commonly function as donors are the more electronegative ones in the right hand side of the periodic table, primarily in Group V (12,13). The most important of these ligands contain N or O or both (14). The donor atoms may form a part of either an acidic or a basic functional group. Additionally, approximately 65% of the various types of amino acid sirle chains contain potential metal binding sites such as the sulfuydryls and hydroxyls binding groups. The common backbone of the naturally occurring amino acids contain the α-carboxyl and α-amino groups each of which can bind metal ions (11). In an aqueous environment the α-amino acid exists in the zwitterionic state with both the α-carboxyl and the α-amino groups ionized with opposite charges. Both reactive groups can thus participate in the chelation of the metal ion. The carboxyl group contains an electron that can be shared with the metal ion through a covalent bond. While the amino group has a pair of electrons that can be donated to the metal ion to form a coordinate covalent bond. The amino acid ligand is considered to be bidentate. The amino moiety meets the criteria of Lewis base when joined to the metal in accordance to current chemistry theory.
There are three classes of bidentate ligands: (1) two basic groups, (2) one acidic and one basic group, and (3) two acidic groups. Amino acids fall into the second category of bidentate ligands. Using glycine (NH2CH2COOH) as an example, in water at a pH of between 3 and 9, this ligand exists as the zwitterion, H3N+CH2COO-. The amino acid has lost a proton from the COOH group and is thus capable of chelating a metal ion and forming a five member ring through donation of one or more electrons to the metal (15).
A second prerequisite for chelation to occur requires that the functional groups of the ligand be located so that a ring structure can be formed with the metal atom being the closing member of the ring (12). Logically if the ligand has two donor atoms that must attach to a single metal ion, then a ring structure must be formed. Due to the nature of the elemental constituents of the ring members, this ring, by definition, must be heterocyclic. The formation of a ferrous bis-glycinate chelate is shown in Figure 2 and illustrates this concept.
The formation of ferrous bis-glucinate chelate from two ligands of glycine and a ferrous (+2) iron atom. The resulting chelate rings are five member, heterocyclic rings

Each heterocyclic ring in Figure 2 contains two bonds which extend between the ferrous ion and the glycine ligand in each ring. The first bond between the cation and the anionic, or polar portion, of the ligand is covalent in nature because they share one electron from the carboxyl group and one electron from the ferrous ion. This is the bond between the oxygen from the COO- group, and the Fe++. The second bond is a coordinate covalent bond. In this case, the iron behaves as Lewis acid, and the glycine as a Lewis base. The donation of both electrons from the same atom in the amino group of the ligand to the metal ion establishes the coordinate covalent bond. This donation of electrons will go to the lowest energy orbital of the iron ion that is unfilled, which in this case is a p-orbital (15,16).
The third requirement for chelation to occur necessitates that the potential reaction between the metal ion and the ligand must be sterically possible. The ferrous iron has an atomic radius of between 74 and 77 nanometers (nm) making it one of the larger transition metal ion s (17,18). Nevertheless, it is not a large cation when compared to many other ions, such as the alkaline earth metal ions. The size depends on either high spin or low spin configurations according to the magnitude of the surrounding ligand field. All non-heme iron ions are high spin (20). The larger the metal ion the greater the number of ligands that can surround it and still have contact with the metal ion (17).
The size of the ligand will also affect the stereochemistry of the chelate. While one ligand may be able to attach itself to a metal ion without problem, the addition of a second or third may be prevented by a clash between the first ligand and parts of the second or third when the latter ligands attempt to position themselves properly for attachment (12). In the case of the ferrous bis-glycinate chelate formed from the reaction in Figure 2, only the amino acid backbone is involved in the bonding of the ligand to the ferrous ion. This results in the amino acid backbone assuming a configuration that sterically allows it to function as a ligand without straining the bonds within the amino acid.
Using x-ray diffraction spectrometry, it has been determined that when the two glycine ligands are chelated to the ferrous ion, the ligands orient themselves in the least sterically hindered conformation possible. The covalent and coordinate covalent bonds are tied to the metal at uniform tetrahedral angles (11,21,22). Figure 3 illustrates a ferrous bis-glycinate chelate molecule drawn from a computer generated model that employed thermodynamic algorithms 10 determine the most thermodynamically stable configuration. This figure is consistent with the models previously developed by Pettit and Hefford describing the steric orientation of amino acid ligand in this class of bidentate chelates and with x-ray diffraction studies of known chelates (22,23).
The fourth requirement for chelation to occur is that the potential reaction between the metal ion and ligands must be energetically possible. As noted by Werner, the charge on a metal ion influences its coordination number. If this charge is low, then only a few lone pairs of electrons from a small number of ligands could prevent the bonding of greater numbers of ligands. Where the bonding between the metal and the ligand is primarily covalent, as in the case of an amino acid chelate, the coordination number of the metal is determined by the number of bonding orbitals available on the metal for combination with the ligand orbitals (19). In the case of the ferrous ion, it is satisfied by a bonding of two glycine ligands as demonstrated in Figure 2. If a molar ratio (ligand to iron) of one glycine molecule or less is employed in the reaction, the ferrous ion has the capability of being bonded to some other ligand which may ultimately displace or interfere with chelation by the original glycine ligand. Thus in most situations a ferrous bis-glycinate chelate is relatively stable since the charges on both the ion and ligands are balanced and the molar ratio of ligand to metal is stoichiometrically correcto However in the presence of a strong oxidizer or reducer, the valency of the metal may change.
FIGURE 3
Ferrous bis-glycinate chelate drawn to correctly depict the tetrahedral relationship of the bonds of O, N, C and the metal and the resulting perpendicular orientation of the two ligand ring structures. The figure is based on x-ray diffraction spectrometry of pure metal – bis-glucinate chelate crystals

Not all chelates of iron have equal bioavailability. Chelation does not guarantee mineral absorption from the gut or its subsequent metabolism if absorbed (24). After comparing various iron chelates (EDTA, fructose, citric acid) to ferrous sulfate, Bates, et al. concluded, "Chelation does not, in itself, ensure efficient uptake [of iron]. . . " (25). Rubin et al. reported that an EDTA chelate of iron may bypass the normal absorptive mechanism in the intestines and be absorbed by diffusion, but due to its high stability constant most of that EDTA chelated iron later appears in the urine unmetabolized (26,27). Because some FeEDTA is absorbed intact through the intestine but subsequently deposited in the urine, Kratzer and Vohra report this form of iron cannot be effectively used in the treatment of anemia (28). In order for mineral absorption and metabolism to occur, the chelate must be nutritionally functional. There are several criteria that must be met for a chelate to be classified as a nutritionally functional chelate.
The first of these requirements is that the iron chelate must have a low molecular weight. In 1970, it was suggested that in order for an iron amino acid chelate to cross cell ( membranes intact it must have a molecular weight of less , than 1500 daltons (29). Subsequently, others have reported that small molecular weight chelates facilitate the transfer of iron from serum to tissues (30). Kratzer and Vohra reported ( that in order for a ligand from a chelate to promote metal absorption (which goes beyond simply protecting the metal t ion in the gastrointestinal tract), it must have a molecular weight of under 1000 daltons. They have added that the higher molecular weight metalloproteins, such as ferritin or hemosiderin facilitate storage of the absorbed iron but suggested that these proteinaceous ligands are too large for intact transport of the iron molecule across cell membranes. For the transfer to occur the iron must be removed from the protein complex and bonded to another ligand that has a lower molecular weight (28).
It is relatively easy to calculate the molecular weight of a ferrous bis-g1ycinate chelate. The iron atom has an atomic weight of about 56 daltons. Each glycine ligand has a molecular weight of 75 daltons. Thus using the formula illustrated in Figure 2, the ferrous bis-glycinate chelate has molecular weight of about 204 daltons. This molecular weight has been confirmed in the laboratory (31). This in turn confirms that the molecular weight of the ferrous bis-glycinate chelate is well below the maximum molecular weight of 800 daltons established by AAFCO for a molecule to be classified as an amino acid chelate and well below the postulated absorptive limits (10,29,30).
The second requirement for a nutritionally functional chelate relates to its stability constant. If fue chelate is to be classified as nutritionally functional, it must have a stability constant that is higher than the potential formation constants of the ligands in the intestinal chyme. This higher stability constant of the amino acid chelate prevents the molecule from being destroyed in the gut and allows the chelate to cross the intestinal cell membrane intact with the metal. Once absorbed into the mucosal cell, a nutritionally functional chelate must have a stability constant which is lower than those ligands in the storage systems of the mucosal cells and the transport systems that deliver the iron to target tissues (29). In this way, the chelate can be metabolized (degraded into the ligands and metal) after absorption across the mucosal cell membrane, and still participate in the regulatory pathways of the metal.
The stability constant can be affected by the size of the chelate ring. Ligands, which form saturated five and unsaturated six member rings are the most stable (32). The glycine ligand forms a saturated five member ring with the ferrous ion.
The number of chelate rings that can potentially be formed with one carian will also affect stability. As the number of rings increase, so does stability of the molecule (32). This concept, of course, is limited by sterochemistry and the valence state of the cation. If a ligand is too large to allow the bonding of a second ligand, then chelate stability may be sacrificed. So too, if the valence of the cation exceeds that of the available ligand(s), stability is reduced. The ferrous bis-glycinate has the ideal combination. The +2 charges of the ferrous iron are satisfied with an equal number of glycine ligands that each have a -1 change.
The Lewis basic strength of the ligand will also affect stability. The greater Lewis basicity of the ligand, the more stable the resulting chelate molecule (32). Glycine is an weak Lewis base, overall. This moderates the stability of the bis- glycinate chelate, and while it is more stable than the food ligands, it has a lower stability constant than the iron storage and transport ligands in the body. In a study in which 59FeSO4 and 55Fe bis-glycinate chelate were mixed in a cornmeal porridge and fed to 10 volunteers at breakfast, the absorption of the iron from the chelate was significantly (p<0.00 1) higher (5.3 times) than was iron absorption from the sulfate. There was no exchange of the radiolabelled irons from the ferrous bis-glycinate chelate and ferrous sulfate in the intestinal pool before absorption demonstrating that this bis- glycinate chelate was not affected by food ligands and was absorbed intact into the mucosal cells (33). Once absorbed into the mucosal tissue there is significant (p<0.05) hydrolyzation of the iron amino acid chelate into its individual components: iron and amino acids with the rates of transfer to the serosa from the mucosal tissue being different for each component (34).
The size and charge of the ligands will also affect the stability of the chelate. More stable chelates are formed by smaller ligands than are formed by larger ligands (32). If a large ligand is bonded to the reactive site of a metal ion, then the number of ligands able to chelate the metal ion are restricted due to steric hindrance. As noted above, this will also decrease chelate stability. Stability constants tend to become lower as the bulk of the ligand increases, suggesting a decrease in coordination sites of the ligand to the metal due to stearic hindrance (32). Glycine is the smallest of all of the amino acids. Its size favors the stability of the chelate.
Multidentate ligands, if not stearically hindered, form more stable chelates than do monodentate ligands (32). Glycine is a bidentate ligand, and as shown in Figure 3, when the ferrous ion is chelated with two glycine ligands there is no stearic hindrance to either ligand (35). The stability constant is also affected by the π-bonding strength of the central metal atom (32). It has been shown that, among the transition metal ions, copper has the highest stability. Iron is relatively low: Zn2+ < Cu2+ > Ni2+ > Co2+ > Fe2+ > Mn2+ (36).
The nature of the ligand will also affect the overall stability of the chelate (32). Different ligands have different potential formation constants at the same pH (37). EDTA, for example, will form a much stronger chelate than will glycine, but the ability of the body to extract the iron nutrient from the EDTA chelate is severely limited, whereas in the case of the iron bis-glycinate chelate, it is re1atively easy (26-28,34).
It is estimated that a nutritionally functional chelate must have a stability constant of between 107 and 108, in order for that chelate to survive the environment of the stomach and intestine and still be hydrolyzed within the mucosal cells or other tissues (38). An absorbed iron chelate, if it is functional, must have a bonding constant which is lower than that of transferrin (39). The iron bis-g1ycinate chelate meets the criteria of having the ideal stability constant for a nutritionally functional chelate. It has a stability constant of approximately 107.5 at pH 7 (37).
The final requirement for a totally nutritionally functional chelate is that the ligand must be easily metabolized by the body and also be utilized as a nutrient in addition to the metal contained in it (15). Amino acids are represented throughout mammalian biochemistry and physiology. Thus, a continuous dietary supply of amino acids is essential for the well being of the individual (40). It was previously noted that, after absorption into the mucosal cell, the majority of the iron amino acid chelate is hydrolyzed into its individual components before the iron is transferred to the serosa (34). The small amount of amino acid chelate that escapes this initial hydrolysis is broken apart later in the various other cells of the tissue which receive the absorbed chelate (38). The amino acid portion of the chelate is then free to be metabolized as it normally would if it had been absorbed from the lumen as a free amino acid.
Glycine is considered to be a conditionally essential amino acid. While it is absolutely essential for human nutrition, it is not generally required in the diet because under normal circumstances the body can synthesize it from more complex precursors: The body's ability to synthesize this amino acid from a precursor is, however, limited by the availability of the precursor, which in the case of glycine, is a nonessential amino acid. Furthermore, the synthesis can also be limited and potentially constrained by developmental or pathophysiological factors. For example low birth weight infants lack the ability to synthesize adequate quantities of glycine from more complex amino acids. When this lack of physiological development or pathological interference occurs, and when foods low in glycine, such as cow's milk, are mainly consumed, a glycine deficiency can resu1t (40- 43). Thus, when provided as part of the iron bis-glycinate chelate, the glycine in and of itself has nutritional value to the individual. Unlike EDTA, it is not excreted into the urine unmetabolized.
The ferrous bis-glycinate is a proven chelate. More importantly, however, it is a totally nutritionally functional chelate. By its atomic structure and chemistry it protects the ferrous ion from undesirable chemical reactions in the stomach and intestines that limit iron absorption. Once absorbed into the mucosal cells and to a limited extent, the tissues, it is easily hydrolyzed into its individual components. Each component is then employed by the body as normal metabolism would dictate.
REFERENCES
1. Werner A. Beitrag zur Konstitution Anaorganischer Verbindunge, Z, Anorg U Allgem Chem 1893;3:267. [ Links ]
2. Werner A &A Miolati. Z physik Chem Leipzig, 1894;14:506. [ Links ]
3. Werner A & Z Vilmos. Beitrag zur Konstitution Anaorganischer Verbindunge, A anorg U Allegem Chem 1899;21: 153. [ Links ]
4. Werner A. Ueber Acetylacetonverbindungen des Platins, Ber Deut Chem Ges 1901;34:2584. [ Links ]
5. Werner A. Zer Kenntnis des Asymmetrischen Kobaltatoms V. Ber Deut Chem Ges, 1911;45:121. [ Links ]
6. Werner A. Über spiegelbild-isomerie bei chromverbindungen. III. Ber Deut Chem Ges, 1912;45:3065. [ Links ]
7. Werner A. Zur Kenntnis des Asymmetrischen Kobaltatoms XII. Uber Optische Aktivitat bei Kohlenstoffreien Verbindugen. Ber Deut Chem Ges, 1914;47:3087. [ Links ]
8. Morgan G. & H Drew. Research on residual affinity and co- ordination. II. Acetylacetones of selenium and tellurium. J Chem Soc, 1920;117:1456. [ Links ]
9. Bell CF. Principles and Application of Metal Chelation. Oxford, Clarendon Press, 1977, p. 3. [ Links ]
10. Bachman PM. (Ed.) Official Publication 2000. Oxford, IN, American Association of Feed Control Officials, 2000, p. 257. [ Links ]
11. Glusker JP. Structural Aspects of Metal Liganding to Functional Groups in Proteins. In Anfinsen, CB, JT Edsall, FM Richards & DS Eisenberg, (Eds.) Advances in Protein Chemistry. San Diego, CA, Academic Press,1991, V.42, p. 4. [ Links ]
12. Dwyer FP & DP Mellor. Chelating Agents and Metal Chelates. NY, Academic Press, 1964. [ Links ]
13. Bell CF. Principles and Application of Metal Chelation. Oxford, Clarendon Press, 1977, p. 9. [ Links ]
14. Van Uitert LG & WC Femelius. Coordination compounds. IX. Solution stabilities of the chelate compounds of a number of organic ligands. J Am Chem Soc 1954;76:375. [ Links ]
15. Bell CF. Principles and Application of Metal Chelation. Oxford, Clarendon Press, 1977, p. 11-18. [ Links ]
16. Bailar JC, T Moeller, J Kleinberg, CO Guss, ME Castellion & C Metz. Chemistry. Orlando, FL, Academic Press, 1984, p. 246-253.Ahrens, LH. Ionization Potentials: Some Variations, Implications, and Applications. Oxford, UK, Pergamon Press, 1983. [ Links ]
17. Ahrens RD. Ionization potentials: Some Variations, Implications, and Applications. Oxford, U.K. Pergamon Press. 1983. [ Links ]
18. Shannon RD & CT. Prewitt. Effective ionic radil in oxides and flourides. Acta Crystallogr 1969;25:925-46. [ Links ]
19. Bell CF. Principles and Application of Metal Chelation. Oxford, Clarendon Press, 1977, p. 33-35. [ Links ]
20. Howard JB & DC Rees. Perspectives on Non-Heme Iron Protein Chemistry. In C.B. Anfinsen, J. T. Edsall, F.M. Richards & D.S. Eisenberg, (Eds.) Advances in Protein Chemistry. San Diego, CA, Academic Press, 1991, V.42, p.206. [ Links ]
21. Dalley NK. X-ray diffraction of iron amino acid chelate, Albion Laboratories, Inc., Unpublished Research Report, 1978. [ Links ]
22. Dalley NK. X-ray diffraction crystallography of Albion Zinc Amino Acid Chelate. Albion Laboratories, Inc., Unpublished Research Report, 1991. [ Links ]
23. Pettit LD & RJW Hefford. Steroselectivity in the metal complexes of amino acids and dipeptides. In: Metal Ions in Biological Systems. H. Sigel (Ed.) NY, Marcel Deker, 1979; V.9 p. 173-212. [ Links ]
24. Ashmead H, D Ashmead & N Jensen. Chelation does not guarantee mineral metabolism. J App Nutr 26:5-21, 1974. [ Links ]
25. Bates GW, J Boyer, JC Hegenauer & P Saltman. Facilitation of iron absorption by ferric fructose. Am J Clin Nutr, 1972;25:983. [ Links ]
26. Rubin M., J Houlihan & JV Princiotto. Chelation and Iron Metabolism I; Relative iron binding of chelating agents and siderophilin in serum. Proc Soc Exp Biol and Med 1960;103:663. [ Links ]
27. Rubin M & JV Princiotto. Synthetic amino acid chelating agents and iron metabolism. Ann NY Acad Sci 1960;88:450. [ Links ]
28. Kratzer FH & P Vohra. Chelates in Nutrition. Boca Raton, FL, CRC Press, 1986, p. 42-44. [ Links ]
29. Hofner W. Eisen und manganhaltige Verbindungen im Blutungassafit von Helianthus annuus, Physiol Plant, 1970;23:673-677. [ Links ]
30. Tiffin LO. Translocation of micronutrients in plants. In: Micronutrients in Agriculture. RC Dinauer (Ed.) Madison, WI, Soil Science Society of America, Inc., 1972, p. 207. [ Links ]
31. Johnson B. Molecular weights of several commercial chelates. Albion Laboratories, Inc. Unpublished research, 1974. [ Links ]
32. Kratzer FH & P Vohra. Chelates in Nutrition. Boca Raton, FL, CRC Press, 1986; p. 25-27. [ Links ]
33. Allen LH. Properties of iron amino acid chelate as iron fortificants for maize. In: Proceedings International Conference on Human Nutrition. Salt Lake City, UT, Albion Laboratories, Inc., 1998, p. 96-108. [ Links ]
34. Ashmead HD, DJ Graff & HH Ashmead. Intestinal Absorption of Metal Ions and Chelates. Springfield, IL, Charles C Thomas, 1985, p. 205-212. [ Links ]
35. Jeppsen RB. Organic minerals and their bioavailability on the basis of chemistry. In: Proceedings International Animal Conference. Salt Lake City, UT, Albion Laboratories, Inc., 1997, p. 8. [ Links ]
36. Irving H & RJP Williams. Order of stability of metal complexes. Nature 1948;162:746. [ Links ]
37. Kragten J. Atlas of Metal-ligand Equilibria in Aqueous Solution. Chichester, Ellis Horwood, 1978. [ Links ]
38. Ashmead SD. Metabolism of zinc amino acid chelate: A preliminary study. Masters Thesis, University of Utah, 1994. [ Links ]
39. Williams RJP. An introduction to the nature of iron transport and storage. In: Iron Transport and Storage. P Ponka, HM Schulman & RC Woodworth. (Eds.) Boca Raton, FL, CRC Press, 1990, p.1-15. [ Links ]
40. Reeds PJ & PR Beckett. Protein and amino acids. In: Present Knowledge in Nutrition. EF Ziegler & U Filer (Eds.) Washington, DC, ILSI 1996, p. 67-68. [ Links ]
41. Jaksie T, F Jahoor, PJ Reeds & W Heird. The determination of amino acid synthesis in human neonates with a glucose stable isotope. Surg Forum 1993;44:642-686. [ Links ]
42. Jackson AA, JCL Shaw, A Barber & M Golden. Nitrogen metabolism in pre-term infants red human donor breast milk: The possible essentiality of glycine. Ped Res 1981; 15: 1454- 1461. [ Links ]
43. Davis T, HV Nguyen, R Garcia-Bravo. The amino acid composition of human milk is not unique. J Nutr 1994;124:1128-1134. [ Links ]














