Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos Latinoamericanos de Nutrición
versión impresa ISSN 0004-0622versión On-line ISSN 2309-5806
ALAN v.51 n.1 supl.1 Caracas mar. 2001
The absorption and metabolism 01 iron amino acid chelate
H. DeWayne Ashmead
Albion Laboratories, Inc. Clearfield, Utah U.S.A.
SUMMARY.
This paper summarizes several studies which describe significant increases in the intestinal absorption of iron from iron amino acid chelate compared to inorganic iron salts. While these increased uptakes of iron from the amino acid chelate into mucosal tissue are highly significant, this paper also demonstrates that there is a mechanism in the mucosal tissue which controls the quantity of iron from the amino acid chelate that is transferred to the plasma. For example, the higher the hemoglobin value, the less iron transferred. When considered together these studies demonstrate that iron amino acid chelate is both a safe and effective source of iron for treatment of iron deficiencies.
Key words: Ferrochel, absorption mechanism, iron deficiency anemia, iron deficiency, hemoglobin.
RESUMEN.
Absorción y metabolismo del hierro aminoquelado. Este artículo resume algunos artículos que describen incrementos significativos en la absorción del hierro aminoquelado comparado con la absorción de sales inorgánicas de hierro. También se demuestra la existencia de un mecanismo en la mucosa intestinal que controla la cantidad de hierro aminoquelado que es transferida al plasma. Por ejemplo, a mayor nivel de hemoglobina, menor cantidad de hierro es transferida al plasma. Considerados en la luz de los estudios discutidos, se demuestra que el hierro aminoquelado es una thente segura y efectiva para el tratamiento de la deficiencia de hierro y la anemia ferropriva.
Palabras clave: Ferrochel, mecanismo de absorción, anemia ferropriva, deficiencia de hierro, hemoglobina.
Iron, as a therapeutic agent, was first documented about 2735, B.C., when it was declared by the Chinese Emperor, Shen Nung, as a cure for "anemia."(l). The ancient Greeks, Romans, Byzantines, and Arabs began consuming iron to reverse symptoms of anemia about 1500 B.C.(2). In the sixteenth century, Monarde proposed a relationship between iron and blood (3). Lemery and Geoffroy subsequently demonstrated the presence of iron in the erythrocytes (4). In the 1700's, Sydenham employed iron as a specific therapy for "chlorosis," a term he used to describe iron deficiencies. Symptoms of this malady included pallor, edema, muscular weakness, headache, rapid pulse, dyspnea, prolonged sleep, and cessation of the menses. Although iron became an accepted treatment for chlorosis, it was not until 1886, that Zinoffsky discovered that equine hemoglobin crystals actually contained 0.335% iron. Subsequently, in 1893, Stockman demonstrated that ingestion of iron increased hemoglobin in women (5). In parallel research, Menghini reported that administration of oral doses of iron augmented total red blood cells (4).
Iron is not only an essential component of erythrocyte hemoglobin for the transfer of oxygen and carbon dioxide, but it also has an important function or functions in every other cell of the body. For example, iron in either the heme or nonheme form plays important roles in cellular metabolism and growth due to its enzymatic involvement in energy production and DNA synthesis. Some other equally essential but frequently overlooked functions include catalyzing the conversion of carotene into vitamin A, the synthesis of purines into nucleic acid, the synthesis of carnitine to transport fatty acids, synthesis of collagen, its participation in antibodies, and the metal's involvement in detoxication of drugs in the liver (6). Ponka, et al., have postulated that, since iron is a cofactor in numerous cellular enzymatic reactions, it is essential for the evolution of aerobic life on earth (7).
An adult male body contains about 50 mg Fe/kg of body weight, whereas a female adult body contains about 35 mg Fe/kg (8). Over two thirds of this iron is concentrated in the blood, primarily in hemoglobin. About 3% of the heme iron resides in the muscles as myoglobin. The remainder of the functional iron is found in specific enzymes of every living cell due to the essentiality of iron for cellular respiration (6).
Iron is found in abundance on our planet and, relative to the nutritive amount required by man, is theoretically plentiful. In spite of this natural abundance, iron deficiency anemia is still the largest single nutrient disease affecting the world today (9-12). It is estimated that more than 500 million people throughout the world suffer from severe iron deficiency anemia (13). In some geographical areas, as high as 58% of the young healthy women have been found to be iron deficient, with the percentages of deficiency being even higher during pregnancy (14).
This worldwide deficiency is, in part, a result of diet. The availability of iron from many foods is very low. Generally speaking, no more than 5% of vegetable iron is absorbed, and while the absorption of iron from meat, poultry, and fish may be somewhat higher, significant worldwide consumption of animal proteins is limited to the more affluent (15). Consumption of certain foods, such as coffee or tea, will generally reduce iron absorption. The phenols in the tea, for example, bind dietary iron and render it insoluble. Phosphates, phytates, and bran will inhibit iron absorption, similarly (16,17). Conversely, organic acids, such as ascorbic acid, (16) amino acids, (6) or meat protein, (18) will generally enhance absorption of iron. Because of these and many other dietary factors, the variance in iron uptake may be as great as tenfold (19).
Besides diet, an individual's iron status is also related to his or her needs. Age and the sex will affect iron requirements and potential deficiency. Other individual differences include physical activity ranging from sufficient exercise to a sedentary lifestyle, lactations, etc. (16).
The general health of an individual, as well as the drugs he or she is consuming, is a third factor affecting iron status. The use of oral contraceptives, aspirin, antacids, anti-inflamatories, anticoagulants, and steroids may all increase the risk of iron deficiency. Diseases of the gastrointestinal tract, including cancer, hemorrhoids, or gynecological diseases, such as intrauterine fibrosis, will result in a greater risk of iron deficiency due to increased iron requirements and/or an increased inability to efficiently absorb iron (20). Illness associated with a fever may also reduce iron utilization, even if the iron is absorbed. This was shown in an experiment on the nutritional response to infection. Within hours after inoculation, iron levels began to decline in the plasma and accumulate as hemosiderin, primarily in the liver. Throughout the period of infection, this iron remained sequestered. Its incorporation into new hemoglobin was blocked throughout the infection and absorbed iron was sequestered in body storage depots (21).
Finally, the social-economic habits of a population including customs, religious practices, attitudes, etc., may affect the iron status of the members of the group. Included in this category is the living environment of the group (20).
Because of the global magnitude of iron deficiency, and because a dietary supplement is unavailable to the majority of the population, many governments have mandated iron fortification of basic foods (22,23). Frequently, however, in order to retain palatability and retard oxidation of certain food components, the iron salts selected for fortification have 'had low solubility. Low solubility generally equates to low bioavailability (24,25). For example, ferrous fumerate, ferrous glycine sulfate, ferrous sulfate, ferrous citrate, ferrous tartrate, ferrous pyrophosphate, ferric coline citrate, ferric sulfate, ferric citrate, and ferric ethylenediaminetetraacetate showed decreasing degrees of iron absorption, respectively, with the ferric compounds being absorbed at less than half of the first three ferrous sources (26). Increasing iron levels in a meal does not proportionately increase iron absorption. The mean absorption of nonheme iron has been reported to decrease from 18% to 6.4% as the nonheme iron content of the meals was increased from 1.52 mg to 5.72 mg.(27). Although 6.4% of 5.72 mg is higher than 18% of 1.52 mg (0.37 mg versus 0.27 mg), the amount of increased absorption is not proportional to the increased dose. It is, in fact, greatly suppressed. Increasing doses of unprotected iron is, thus, far more likely to elicit toxicity, than significantly increasing absorbed iron.
In order to enhance iron bioavailability and still avoid interactions with food ingredients, chelating iron with amino acids has been employed. Chelation was first described in 1893, when Wemer wrote, "If we think of the metal ion as the center of the whole system, then we can most simply place the molecules bound to it at the corners of an octahedron"(28). In 1920, Morgan and Drew applied the name, "chelate" to the molecule described by Wemer (29).
Not all chelates have the same biological consequences. Chelates can be produced that have little or no nutritional value. A nutritionally viable iron amino acid chelate must have a stability constant that is higher than the potential formation constants which would result if the iron were chelatecd or complexed to the food ligands found in the stomach and intestines (30). This is necessary for the original chelate to remain intact in the gastrointestinal tract prior to absorption. If the chelate dissociates in the gut, it has no more value than ionized iron from a soluble salt. The stability constant should also be high enough to allow the chelate to cross the intestinal cell membrane into the cytoplasm, and yet be low enough that the cytoplasmic ligands are capable of removing the iron from the absorbed amino acid chelate by complexing with the absorbed iron. In this fashion, the rate of delivery to the target tissue or enzyme from the mucosal cell is controlled (31).
A stability constant indicates logarithmically the affinity of a metal to bond with a ligand. A ligand with a potentially higher stability or formation constant will displace a metal from a ligand with a lower stability constant. The strength of a stability constant is dictated by the number of members in the chelate rings (5- and 6- member rings being most stable), the number of rings, formed with a single cation, the basic bonding strength of the ligand(s), the size and charge of the ligand(s), the metal being chelated, the resonance effect of the chelate ring, and the steric orientation of the rings (32). In vitro and in vivo studies have demonstrated that the Albion® iron amino acid chelate developed via patented technology and described in this paper appears sufficiently strong to pass through the acid pH of the stomach into the duodenum and still provide reasonable protection of the iron from unwanted chemical reactions with dietary phosphates, phytates, fiber, etc. (33). Radioactive isotope studies have demonstrated that once the iron amino acid chelate has been absorbed into the mucosal tissue, its stability constant is low enough to allow hydrolyzation of the chelate with subsequent transport of the iron to tissues (34).
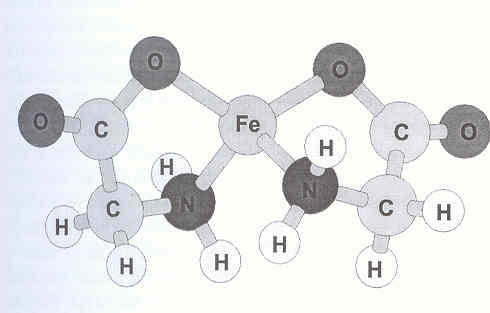
To form such an iron amino acid chelate, each amino acid or ligand, must furnish at least two reactive moieties to combine with the iron atom. The carboxyl group (COOH) of the ligand forms an ionic bond with the cation, whereas the alpha-amino group (NH2) donates an electron pair back into a vacant d-orbital of the metal ion, thus forming a coordinate covalent bond (35). Each amino acid ligand forms two bonds with the iron ion to produce a stable 5-member ring with the iron atom being the closing member of the ring (36). The two ligands tend to orient themselves in the least sterically hindered conformation possible and are tied into the metal at uniform tetrahedral angles (Figure 1) (37,38). This configuration provides a degree of protection to the iron by limiting its reactivity with dietary components. The same ligands that protect the cation from reacting with dietary substances also reduce the potential for gastric irritability of the iron on the mucosal lining because the cation is less likely prevented from coming in contact with the mucosal lining by the amino acid ligands.
Iron amino acid chelate composed of 2 glucine molecules to 1 iron atom drawn to depict the tetrahedral relationship of the bonds of carbon, nitrogen, and the iron and the resulting perpendicular orientation of the two ring structures. This figure is based on computer generated models resulting from X-ray diffraction analysis of metal amino acid chelate crystals
Because an iron amino acid chelate is a different form of iron molecule compared to a non-heme salt, the absorption characteristics of an iron amino acid chelate are also different, as demonstrated in a study involving 10 adult mate volunteers. Each was given 1.5 m Ci 59FeSO4 and 3.0 m Ci 55Fe amino acid chelate simultaneously in 100g of dry cornmeal which was red as a breakfast porridge. Neither isotope source of iron affected the absorption of the other. Absorption of iron from FeSO4 was 1.34% compared to 8.68% for the chelates. The difference was highly significant (p<0.0001). The investigator concluded, "there was no exchange between the 59FeSO4 and Ferrochel [55iron amino acid chelate] in the intestinal pool or before entering the mucosal cell. If the FeSO4 and the [iron amino acid] chelate exchanged iron between themselves, the same proportion of label would be absorbed from both compounds. However, [iron amino acid chelate] is consistently absorbed about 5.3 times more than FeSO4, and this is not modified by the simultaneous mixing with FeSO4. The [iron amino acid chelate] is probably entering the mucosal cell as a chelate. Although no subjects in this experiment were iron deficient, the results also suggest that iron absorption from the [chelate] and ferrous sulfate was regulated similarly by iron status" (24).
For an iron amino acid chelate to be absorbed into the mucosal tissue, it must be a low molecular weight chelate. Kratzer and Vohra cite the upper limit for this intact absorption of chelates as being 1,000 daltons (31). Tiffin reported that the amino acid chelate must have a molecular weight of less than 1,500 daltons if it is to be absorbed in humans. The iron amino acid chelate described in Figure 1 has a molecular weight of less then 800 daltons (40).
Generally, more iron can be absorbed from the gastrointestinal tract into the mucosal cell as a chelate than as a salt because, as demonstrated above, the cate of uptake for the two sources of iron is different (34). Once absorbed into the mucosal tissue, the body regulates how much is transferred to the plasma, thus preventing iron overload and toxicity. This regulation is seen in a double blind cross-over study in which seven (7) adult males and five (5) females ingested 18 mg Fe/day as ferrous sulfate for seven (7) days, had a washout period of seven (7) days, and then received 18 mg Fe/day as an amino acid chelate for seven (7) days. Mean absorption of the iron amino acid chelate was 59% greater than that of the ferrous sulfate source, as determined by fecal analysis (47). The percent of increased absorption of the iron amino acid chelate compared to FeSO4 was highly significant (p<0.001) in males (7.24%±2.2% vs. 12.7%±3.6%) and significant (p = 0.02) in females (13.8%±1.8% vs. 18.2%±3.7%) (42). Regression analysis demonstrated that both iron compounds followed the same absorption pattern and were possibly subject to the same type of regulation (R2 = 0.9411 with slope = 0.8445 and intercept = 7.6)(41).
This process of absorbing the iron amino acid chelate into the mucosal cell can be described as R0+Fe-C Fe C (R)0 Fe-C (R)I Fe+C+Ri, where C is the ligand, and R is the receptor inside (i) or outside (o) of the cell membrane. If C is metabolized in the cytoplasm, or if the pH is changed while Fe is still attached to C, then the iron uptake into the cell is irreversible (24,30).
The hydrolysis of absorbed iron amino acid chelate within mucosal tissue is clearly demonstrated in a double isotope in vitro study (34). In this study" replicated five times, jejunal tissue was removed from Sprague-Dawley rats, washed and everted. The ends were tied off and the intestines were placed in mucosal solution baths. Serosal solution was injected into the everted intestines. 59Iron chelated to 14C-lysine was injected into the mucosal solution bathing each everted intestine. The ratio of iron to Iysine in the mucosal solution was 1 :2.7. Samples of the serosal solution were removed hourly for five hours, and each sample assayed for 59Fe and 14C. The mean ratio of iron to lysine in the serosal solution dropped from what it had been in the mucosal solution to 1: 1.56 ± 0.65. At the end of the five hours, the everted intestines were also assayed for 59Fe and 14C, after being washed to remove external contamination. The mean ratio of iron to lysine in the mucosal tissue was higher than what it had been in the mucosal solution: 1:3.05 ± 0.049.
This study demonstrated that there was a significant (p <0.05) hydrolysis of the iron amino acid chelate as it passed from the mucosal solution through the mucosal tissue before delivery of either the iron or amino acid to the serosal solution (42). More Iysine was retained in the mucosal tissue than iron, indicating that some of the 59Fe was separated from the lysine after absorption into the tissue, and that iron was transferred by other means to the serosal solution without being bonded to the lysine. Less Iysine was found in the serosal solution than in the mucosal solution, also indicating hydrolysis of some of the amino acid chelate (34).
When hydrolysis of the amino acid chelate occurs within the mucosal cell, then the movement of the metal out of the mucosal cells and into body tissues is metabolically regulated similarly to the regulation of iron absorbed into mucosal cells from salts or from other food sources. If there were no regulatory mechanism in the mucosal cells to control the transfer of iron to the plasma, regardless of the source, there would immediately be iron over-loading and subsequent toxicity from iron ingestion, regardless of whether the iron originated from food or from an amino acid chelate.
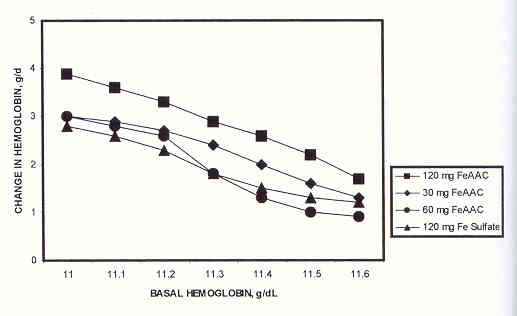
This similarity in regulation of iron transferred to the plasma from iron amino acid chelate or FeSO4 is seen in a dose-response study in which 100 anemic adolescents received either 120 mg of iron as FeSO4 (n=27), (or 120 mg of iron (n=26), 60 mg of iron (n=21), or 30 mg of iron (n=26) as iron amino acid chelate, daily, for 28 days (43). Each source and quantity of iron was equally effective (p<0.01) in restoring the anemic adolescents' hemoglobin levels to normal (<11 to <13.5 g/dL), implying the greater bioavailability of the iron amino acid chelate compared to ferrous sulfate. When the changes in hemoglobin were plotted, however, the slopes showing these changes after treatment with the FeSO4 or with different dose levels of the iron amino acid chelate were essentially the same, regardless of the quantity of iron administered (Figure 2). In each case, as hemoglobin levels increased, the uptake of iron, regardless of the source, decreased (44). These similar data suggest that all of the absorbed iron sources were regulated similarly by the body after absorption.
Line graphs showing that the absorption of iron from three dosage levels of amino acid chelate or from ferrous sulfate is directly related to the hemoglobin level
The transfer of absorbed iron from the mucosal cells to the plasma is controlled by the body's iron need. After cellular hydrolysis, the iron is first delivered to plasma transferrin in the mucosal cell, if the body has an immediate need for iron. Each transferrin molecule can bind to two atoms of iron. When approximately 33% of the transferrin is saturated with iron, it will not pick up any more iron. The remaining ferrous iron is oxidized to ferric iron. This iron remains complexed to ferritin within the mucosal cells until transferrin calls for more iron. The iron is then released from the ferritin through reduction of the ferric iron to ferrous iron and is subsequently bonded to transferrin in the blood (6).
The mucosal cells aggressively conserve iron that is not immediately required by the body, the iron being "trapped" in the mucosal cell. This could potentially result in toxicity within the mucosal tissue if the iron remained there indefinitely. Nature has, however, devised a plan to remove this non-transferred iron from the intestinal tissue. Mucosal cells migrate up the intestinal villus from the crypt, the site of their formation, to the tip of the villus, replacing older cells as they move towards the villus tip. After three to four days, these cells (and their iron, if it has not been transferred to the plasma) will reach the top of the villus where they will be sloughed off and excreted in the feces (6).
If all iron absorbed into mucosal tissue is generally handled and regulated similarly, what, if any, are the advantages of ingesting the iron in the form of an amino acid chelate? There are two basic advantages. The first is that the amino acid chelated iron is not as reactive with food ingredients, which leaves more of the iron potentially available for absorption. The second advantage is that the iron amino acid chelate is absorbed into mucosal cells in greater quantities, due, in part, to entering into fewer absorptive inhibiting reactions in the gut. The greater amount of absorbed iron is of great importance when iron deficiency or anemia exists. In these cases, more iron is made available for repletion of the iron need. These advantages are examined in greater detail below.
When dietary iron is ingested with phytic acid, bran, etc., the formation of an insoluble precipitate may leave the iron unavailable for absorption (45). In those cases, the iron is simply eliminated in the feces as part of a phytate, oxalate, phosphate, etc. There is less likelihood of insoluble compounds forming when that iron is ingested as an amino acid chelate because this form of iron is shielded by the amino acid ligands of the chelate and is made electrically neutral by the charge balancing effects of the chelating ligands. To illustrate, an experiment was devised in which cookies containing known iron absorption inhibitors (bran fiber, phytates, phosphates, phenols, and tannins) and 30 mg of iron as an amino acid chelate were red to 10 anemic children. Blood samples from each child before and after treatment were obtained by venipuncture and assayed for hemoglobin. Each child then received one of the above described cookies per day, for 30 days. There was a significant (p<0.01) increase in the mean hemoglobin level of the group as the hemoglobin rose over the 30 day study from 8 g/dL to 10.1 g/dL, even though the iron amino acid chelate was combined with a multitude of dietary iron absorption inhibitors (41).
The same characteristics that reduce the reactivity of the iron amino acid chelate in food also reduce its oxidative effect on other food ingredients, such as vitamins (25,46). When the iron amino acid chelate was stored with vitamins A, D3, tocopherol, K, ascorbic acid, niacinamide, biotin, folic acid, and pyroxidine at 20°C, the degradation of these vitamins was significantly less (p<0.01) at 180 days, than when stored with iron sulfate under the same conditions. At a temperature of 37°C, the differences between the two sources of iron on vitamin oxidation were even more significant (p<0.001). The iron sulfate form stimulated more rapid oxidation and inactivation of the vitamins compared to the iron amino acid chelate.
Besides being less affected by dietary absorption inhibitors, the absorption of the iron amino acid chelate is not inhibited by the presence of other minerals in the diet (47-49). For example, normally, ionic iron and ionic copper are mutually antagonistic in the small intestine where a high dietary level of either will reduce the absorption of the other (50-55). This antagonism can be manifested as a decrease in hemoglobin values (56).
The lack of copper and iron antagonism as amino acid chelates was seen in a double blind study involving 30 healthy nonanemic adult volunteers divided into three groups (5 males and 5 females/group). Blood samples were obtained by venipuncture at the commencement and conclusion of the study, and assayed for hemoglobin (57). One group each received 30 mg Fe/day. The second group each received 9.9 mg Cu/day. The third group each received 30 mg Fe and 9.9 mg Cu per day. Both the iron and copper were chelated with amino acids. Daily supplementation continued for 90 days. Changes in hemoglobin were analyzed statistically. As seen in Table 1, when the copper and iron were ingested as amino acid chelates, there were no significant changes in hemoglobin, leading to the conclusion that there had been no antagonistic competition between the iron and copper amino acid chelates during intestinal absorption, due to both metals being in the form of amino acid chelates.
Mean 90 day changes in hemoglobin (g/dL±S.D) following Fe and Cu amino acid chelate supplementation
|
| Group A 30 mg Fe/day) | Group B (9,9 mg Cu/Day) | Group C 30 mg Fe, 9,9 mg Cu/day) |
| Before supplementation After supplementation |
13,90±0,85ª 14,50±0,85ª | 16,03±0,90ª 16,73±0,94ª | 15,30±1,61ª 15,29±1,02ª |
ª Means not significant (p>0,10)
The second major advantage of iron amino acid chelates over iron salts is that the absorption of the chelates into mucosal tissue is greater, even under controlled conditions (58). In an in vitro study, replicated three (3) times, rat jejunal segments were randomized, washed, and then exposed for 120 seconds to identical gastric solutions containing iron from either a sulfate, an oxide, a carbonate, or from iron amino acid chelate. The control tissue was not exposed to any iron source. After exposure, each jejunal segment was washed thrice to remove external unabsorbed iron and then assayed by atomic absorption spectroscopy for the quantity of iron in the mucosal tissue. The mean net absorption of iron into the mucosal tissue (after subtracting the amount of iron in the control tissues) ranged from 4.7 to 7.2 times more for the amino acid chelate than from the inorganic salts (Figure 3).
Mucosal tissue uptake of iron after 120 seconds of exposure to iron from an amino acid chelate, carbonate, sulfate, or oxide mixed in gastric solution. The absorption represents the mean quantity of three (3) replications

Although excess iron in the mucosal tissue will be lost as the mucosal cells migrate to the tips of the intestinal villi, nevertheless, greater deposition of iron into the mucosal tissue potentially allows for a quicker recovery from iron deficiency. This is seen in a study in which twenty fasted 2-day-old anemic pigs (10 per group) were administered a single oral dose of 10 m g of 59Fe as either FeCI2 or as an amino acid chelate (59). The 59Fe was in the form of 59FeCI2. This 59FeCI2 was divided into two parts, and one part used to make the iron amino acid chelate. Beginning one hour after dosing with either the FeCI2 or iron amino acid chelate and continuing hourly for five hours, blood samples were obtained from each pig, centrifuged, and fue red blood cells washed and then assayed for 59Fe incorporation. Significantly more (p<0.05) iron from the amino acid chelate was found in the red blood cells compared to the chloride (Figure 4) (42).
Mean five hour incorporacion of 59Fe from iron amino acid chelate or ferrous chloride into blood cells of 20 (10/group) two-day old pigs

At the end of 20 hours, all of the above pigs in both groups were sacrificed. Whole blood, livers, spleens, and bone marrows obtained at 20 hours were assayed for 59Fe. The 20 hour blood samples were also assayed for hemoglobin. The data summarized in Table 2 demonstrate that 59Fe tissue deposition from the amino acid chelate was significantly greater in the whole blood (p <0.01) and red bone marrow (p<0.01). There was significantly less (p<0.10) 59Fe from the amino acid chelate in the liver, suggesting less toxicity. These data also indicated that more iron was absorbed from the chelate in the 20 hour period and was immediately available for hemoglobin production, which was also significantly higher (p<0.05) (42).
A possible reason for the lower 59Fe liver levels observed in Table 2 is suggested from a study conducted by Fairweather-Tait, et al (60}They fed two groups of 16 each weanling male Wistar rats a diet containing marginal levels of iron for four weeks and then added 30 mg Fe/kg as ferrous sulfate or iron amino acid chelate to the food for 4 weeks. At the end of the second four week period all of the animals were sacrificed and their livers removed for iron analysis by atomic absorption spectroscopy. Their blood was also analyzed for hemoglobin and packed cell volume. The mean hemoglobin concentration was significantly higher in the group red the iron amino acid chelate (p<0.001), but there was no difference in the packed cell volume. These investigators concluded that most of the absorbed iron from the chelate was immediately incorporated into the 13% increase in hemoglobin which they observed, rather than being stored in the liver. They also noted, that the amount of iron absorbed from the chelate source must have been far greater than was reflected in the increased hemoglobin in order to meet the anabolic needs of the rapidly growing animals.
A similar response to the iron amino acid chelate can be seen in anemic human subjects. A group of 40 infants (Hb < 11 g/dL) were paired for age, sex, weight, and hemoglobin. After random assignment to 1 of 2 groups, each was given 5 mg Fe/kg body weight/day as FeSO4 or as iron amino acid chelate for 28 days. Both treatments significantly increased (p<0.001) hemoglobin levels, but only the chelate significantly increased (p<0.005) serum ferritin. Calculated bioavailability of the iron amino acid chelate was 75.0% compared to 27.8% for FeSO4. As suggested by Fairweather- Tait, et al. above, this study also demonstrated that the greater bioavailability of the iron amino acid chelate allows for the rapid incorporation of iron into hemoglobin first, followed by a quicker repletion of depleted iron pools (i.e. serum ferritin) than is possible with FeSO4(61).
Multiple regression analyses of the hemoglobin changes in the above 40 infants demonstrated that those changes were dependent upon the form of iron administered as well as the hemoglobin level. Hemoglobin increases were greatest when consuming iron amino acid chelate, but the higher the hemoglobin value, the lower was the iron absorption, regardless of the iron source. This suggested that in people with normal iron levels, there is little potential danger of iron overloading or subsequent toxicity when consuming foods fortified with nutritionally appropriate amounts of either FeSO4 or iron amino acid chelate.
After first satisfying hemoglobin requirements, the greater bioavailability of the iron amino acid chelate allows for a more rapid repletion of the tissue iron stores. This is seen in a study in which two groups (N= 15/group) of gestating albino rats were fasted for 24 hours and then orally administered 4.4 m Ci 59Fe as a single dose as either 59Fe amino acid chelate or 59FeCI2 mixed in 25 m L of water (62). Approximately 72 hours after dosing, or one day before expected parturition, all animals were sacrificed and their tissues and fetuses removed, dried and assayed for 59Fe. Table 3 summarizes the results and demonstrates significant increases in iron deposition in certain tissues
Mean ± S.D. hemoglobin levels and corrected counts per minute of 59Fe incorporated into pig tissues 20 hours after oral dosing
|
| Whole Bloond (59Fe ccpm/ml) | Liver (59Fe ccpm/mg) | Spleen (59Fe ccpm/mg) | Red Bone Marrow (59Fe ccpm/mg) | Hemoblobin G/dL |
| Fe AAC FeCI2 %Increase or decrease Chelate vs. CI2 | 402,7±90,7 292,3±61,2 +37,8b | 3,3±5,4 4,7±2,1 -29,8ª | 8,5±2,5 7,7±1,9 +10,4 | 16,7±5,6 10,9±6,1 +53,3c | 11,7±2,7 10,5±1,8 +11,4 |
ªp<0,10 bp<0,05 cp<0,01
Mean 59Fe incorporation into tissues (ccpm/g dried tissue) from gestatin rats and their fetuses
| Tissue | FeCI2 | Fe Amino Acid Chelate | % Increase AAC FeCI2 |
| Uterus Liver Kidney Spleen Heart Lung Fetus | 3,333 8,167 567 134 3,367 1,367 16 | 4,926 9,675 950 325 1,425 2,925 46 | 48 18 68 143b 328b 114ª 188c |
ªp<0,05 ; bp<0,01 ; cp<0,005
In spite of the highly significant total increase in iron deposition into the fetuses from the chelate, no analysis of individual fetal tissue was conducted in the above study, due to the size of the fetuses. Nevertheless, by combining the data from the two non-isotope studies in pigs, a pattern of iron deposition from the maternal blood into the fetus can be demonstrated. In the first study, Brady. et al.., sacrificed one piglet at birth from each of 12 sows (6 control and 6 treated). Each mother had been red 85 mg of supplemental Fe daily in the feed as iron amino acid chelate or FeSO4 for four weeks before expected parturition. At birth, the piglets farrowed from sows consuming the iron amino acid chelate had 34.9% more iron in their livers, 8.5% more iron in their spleens, and 3.2% more iron in their skeletal muscles. Hemoglobin levels were also 11.3% higher (63).
In the second pig study, Yamamoto, et al., also gave gestating sows 60 mg of supplemental Fe daily in the feed as either iron amino acid chelate, or ferrous fumarate, commencing four (4) weeks before expected parturition (64). At parturition, the newborn piglets from sows red iron amino acid chelate had significantly greater (p<0.01) mean hemoglobin levels compared to piglets from sows receiving ferrous fumarate. Their mean hematocrit values were also significantly higher (p<0.01), as were the mean increases in serum ferritin concentration (p<0.001).
When the data from these two pig studies are combined, it can be seen that greater placental transfer of iron from the mother to the fetus occurs when the iron is ingested by the pregnant mother as an amino acid chelate, and that greater amounts of iron are delivered to the fetus via maternal blood which are subsequently stored in the iron pool s of the fetus for use after parturition. After birth the serum ferritin levels dropped dramatically in both groups as the piglets began producing hemoglobin to support their rapid growth rates. The chelate group, however, had serum ferritin values that were significantly (p<0.05) above the level of the fumarate group. At four (4) weeks of age, the mean hemoglobin concentration in the chelate group was 14.9% higher (p<0.01) than in the fumarate group. Neither group of piglets in these two (2) studies received supplemental iron after parturation. The first study showed that more iron was stored in the piglets farrowed from mothers ingesting iron amino acid chelate than equivalent amounts of iron from salts. This potentially allowed for greater hemoglobin production after birth without the need for supplemental dietary iron, which the second study demonstrated.
In summary, the absorption of the iron as an amino acid chelate has been shown to be greater than iron absorption from salts, presumably due to less chemical reactions that can potentially interfere with iron absorption. The iron amino acid chelate is well absorbed, whereas the iron from the salt source may or may not be. Once absorbed into the mucosal tissue, the chelate is hydrolyzed, and the release of the iron into the plasma and the rest of the body tissues and organs is regulated similarly to that of any other source of iron. The amount of iron transferred to the body from the mucosal tissue is directly related to the body's iron needs. Greater need results in greater transfer. The advantage of the iron amino acid chelate over other sources of supplemental iron is that its greater bioavailability into the mucosal tissue cells results in more iron being quickly and safely delivered to target tissues of the body in times of need. This potentially allows for smaller doses of supplemental iron being required to achieve physiological results, which can also result in fewer gastric complaints and reduce risks of iron toxicity and iron overload. As the above data have demonstrated, iron deficiencies are less likely to occur when ingesting even small amounts of iron amino acid chelate because of its greater bioavailability.
REFERENCES
1. Mehler CC. History of Medicine. NY, McGraw-HiII, 1947,p.177. [ Links ]
2. Bryan CP. The Papyrun Ebers. NY, D. Appleton and Co., 1931, p.156. [ Links ]
3. Monarde M. Joyful newes out of the newe founde worlde. 1500 (Trans.) NY, A. Knopf, 1925, p. 135. [ Links ]
4. Herchberg S. La Carence en Fer en Nutrition Humaine. París, Editions Medicals Intemationales, 1988, p. 3. [ Links ]
5. Combs, GF. Iron in Poultry Nutrition. En: Iron in Animal and Poultry Nutrition. HR Conrad, DR Zimerman, & GF Combs. West Des Moines, IA, NFIA, 1980, p. 2. [ Links ]
6. Guthrie HA. Introductory Nutrition. St. Louis, MO, Times Mirror, 1989, p. 290-293. [ Links ]
7. Ponka P, HM Schulman & RC Woodworth. (Eds.) Iron Transport and Storage. Boca Raton, FL, CRC Press, 1990, Preface. [ Links ]
8. Beutler E. Iron. En: Modem Nutrition in Health and Disease. RS Goodhart & ME Shils. (Eds.) Philadelphia, PA, Lea & Febiger, 1980, p. 324. [ Links ]
9. Charlton RW and TH Bothwell. Definition, prevalence and prevention of iron deficiency. Clin Haematol 1982;11:309. [ Links ]
10. Demaeyer EM. Epidemiologic, traitement et prevention de la carence ferriprive. Rev Epiden et Sante Publ. 1980;28:235. [ Links ]
11. Herchberg S & C Rousud. La carence en fer. Cab Nutr Dict, 1981;16:219. [ Links ]
12. World Health Organization. Control of nutritional anemia with special reference to iron deficiency. Technical Report No. 580. Geneva, WHO, 1975. [ Links ]
13. Finch CA & H Huebers. Perspectives in iron metabolismo N Engl J Med 1982;306:1520-1528. [ Links ]
14. Beutler E. Iron. En: Modem Nutrition in Health and Disease. RS Goodhart & ME Shils. (Eds.) Philadelphia, PA, Lea & Febiger, 1980, p. 339. 1 [ Links ]
15. Layrisse M & C Martinez- Torres. Absorption of iron from foods. Prog Hematol 1971;7:37. I [ Links ]
16. Gillooly M, TH Bothwell, JD Torrance, AP MacPhail, DP Derman, WR Bezwoda, W Mills, RW Charlton & F Mayet. The effects of organic acids, phytates, and polyphenols on the absorption of iron from vegetables. Br J Nutr 1983;49:331-342. [ Links ]
17. Monsen ER, L Hallberg, M Layrisse, DM Hegsted, JD Cook, M Mertz & CA Finch. Estimation of available dietary iron Am J Clin Nutr 1978;31:134-141. [ Links ]
18. Cook JD & ER Monsen. Food iron absorption in human subjects. III Comparison of the effect of animal proteins on nonheme iron absorption. Am J Clin Nutr 1976;29:859-867. [ Links ]
19. Hallberg L & L Rossander. Improvements of iron nutrition in developing countries: comparison of adding meat, soy protein, ascorbic acid, citric acid, and ferrous sulphate on iron absorption from a simple Latin American-type of meal. Am J Clin Nutr 1984;39:577. [ Links ]
20. Fairbanks FV. Iron in medicine and nutrition. En: Modern Nutrition in Health and Disease. ME Shils, JA Olson & M Shike. (Eds.) Philadelphia, PA, Lea and Febiger, VI, 1994, p. 186. [ Links ]
21. Beisel WR. Magnitude of the host nutritional responses to infection. Am J Clin Nutr 1977;30: 1236-1247. [ Links ]
22. Morton I. How mineral deficient are we? Paper presented at Booker Conference, London, England, 1976. [ Links ]
23. Ashmead HD, EJ Rapp & JJ Name. Uso de amino acidos quelatos na fortificaco de alimentos. Proc O Seminario Brasilero de Alimentos Enriquecidos, 1994, p. 32-37. [ Links ]
24. Allen LH. Properties of iron amino acid chelate as iron fortificants for maize. In: Proceedings International Conference on Human Nutrition. Salt Lake City, UT, Albion Laboratories, Inc., 1998, p. 96-108. [ Links ]
25. Marchetti M, HD Ashmead, N Tossani, S Marchetti, & SD Ashmead. Comparison of the rates of vitamin degradation when mixed with sulfates or amino acid chelates. J Food Comp Analy. In Press. [ Links ]
26. Brise H & L Hallberg. Iron absorption studies II. Acta Med Scandinav, 1962;171 (Supp 376):1-73. [ Links ]
27. Bezwoda WR, et al. The relative dietary importance of heme and nonheme iron S Afr Med J 1983;64:552. [ Links ]
28. Werner A. Beitrag Zur Konstitution Anaorganischer Verbindungen. Z, Anorg. U Aligem. Chem 1893;3:267. [ Links ]
29. Morgan G & H Drew. Research on residual affinity and coordination. II Acetylacetones of selenium and telluriumn. J Chem Soc 1920;117:1456. [ Links ]
30. Williarns RJP. An introduction to the nature of iron transport and storage. En: Iron Transport and Storage. Ponka, P, HM Schulman & RC Woodworth. (Eds.) Boca Raton, FL, CRC Press, 1990, p.I-15. [ Links ]
31. Kratzer FH & P Vohra. Chelates in Nutrition. Boca Raton, FL, CRC Press, 1986, p. [ Links ]
32. Chaberek S & AE Martell. Organic Sequestering Agents. NY, John Wiley & Sons, 1959. [ Links ]
33. Jensen N. Biological Assimilation of Metals. US Patent 4,167,564,11 September, 1979. [ Links ]
34. Ashmead HD, DJ Graff& HH Ashmead. Intestinal Absorption of Metal long and Chelates. Springfield, IL, Charles C Thomas, 1985, p. 171-232. [ Links ]
35. Bailar JC, T Moeller, J Kleinberg, CO Guss, ME Castellion & C Metz. Chernistry. Orlando, FL, Acadernic Press, 1984, p. 223. [ Links ]
36. Bell CF. Principles and Application of Metal Chelation. Oxford, Clarendon Press, 1977, p. 17-18. [ Links ]
37. Rogers K & J Lancaster. Reports on electron paramagnetic resonance spectra of Albion Metal Amino Acid Chelates. Albion Laboratories, Inc. Personal Communication. [ Links ]
38. Dalley NK. Report on X-Ray Diffraction Crystallography of Albion Zinc Amino Acid Chelate. Albion Laboratories, Inc., Unpublished Research Report. [ Links ]
39. Tiffin LO. Translocation of micronutrients in plants. En: Micronutrients in Agriculture. RC Dinauer (Ed.) Madison, WI, Soil Science Society of America, Inc., 1972, p.199-228. [ Links ]
40. Johnson B. Albion Laboratory Chelates, Physical Characteristics versus Product K. Unpublished Research Report. [ Links ]
41. Pineda O. Effectiveness of Ferrochel (Iron Amino Acid Chelate) in the treatment of iron deficiency and iron deficiency anemia. Proc Arab Republic of Egypt Ministry of Supply and Internal Trade Conference on Food Fortification for a Better Heaith. Cairo,16 September 1995. [ Links ]
42. Ashmead S. Statistical analysis of previously published data. Albion Laboratories, Inc., Unpublished Research Report. [ Links ]
43. Pineda O, HD Ashmead, JM Perez & CP Lemus. Effectiveness of iron amino acid chelate on the treatment of iron deficiency anemia in adolescents. J Appl Nutr 1994;26:2-13. [ Links ]
44. Pineda O. Regulation and Toxicity of Ferrochel. Unpublished Report, 1996. [ Links ]
45. Layrisse M. Iron bioavailability from a breakfast enriched with iron bis-glycinate chelate. Effect of phytate and polyphenols on iron absorption in humans. Submitted for publication. [ Links ]
46. Marchetti M, N Tossani, & S Marchetti. Degradazione delle vitamins A,E,B1,C, e k3 negli integratori zootecnici in funzione del tipo di oligoelementi presenti. Zool Nutr Anim 1995;21:67-73. [ Links ]
47. Mervyn L.The clinical significance of chelated minerals as nutritional supplements in medical practices. Proc of Malaysian Medicai Assoc, 1978. Later published as: Human therapeutic applications of amino acid chelates. En: Proceedings International Conference on Human Nutrition, Salt Lake City, UT, Albion Laboratories, Inc., 1995. [ Links ]
48. Lowe JA, J Wiseman & DJA Cole. Zinc sources influence zinc retention in hair and hair growth in the dog. J Nutr 1994;124:2575S-2576S. [ Links ]
49. DiSilvestro RA, J Marten, & M Skehan. Effects of copper supplementation on ceruloplasmin and copper-zinc superoxide dismutase in free-living rheumatoid arthritis patients. J Am Coll Nutr 1992; 11: 177 -180. [ Links ]
50. Cuser WO & A Resurrecion. En: Influence of copper, zinc, and protein on biological response to dietary iron. En: Nutritional Bioavailability of Iron. Washington, DC, American Chemical Society, 1982, p. 97. [ Links ]
51. Greger JL, ML Storey, JL Stahl, ME Cook, SE Gentry-Roberts & JC Lynds. Zinc, iron and copper interactions in humans, rats, and chicks. En: Trace Elements in Man and Animals. LS Hurley, CL Keen, B Lonnerdal & RB Rucker. (Eds.) NY, Plenum Press, 1988, p. 231-232. [ Links ]
52. Howell JM & JM Gaiothoma. Copper in Animals and Man. Boca Raton, FL, CRC Press, 1987, VI, p. 90. [ Links ]
53. O'Dell, B. Copper and zinc in poultry nutrition. En: Copper and Zinc in Poultry, Swine and Ruminant Nutrition. B. O'Dell, ER Miller & WJ Miller. West Des Moines, IA, NFIA, 1979, p.5. [ Links ]
54. Bezkorovainy A. Biochemistry of Nonheme Iron, 1980, p. 5. [ Links ]
55. El-Shobaki FA & W Rummel. Binding of copper to mucosal transferrin and inhibition of intestinal iron absorption in rats. Res Exp Med 1979;174:187-195. [ Links ]
56. Grassman E & M Kirchgessner. On the metabolic availability of absorbed copper and iron. En: Trace Element Metabolism in Man and Animals. Canberra City, Australia Academy of Science, 1981, p. 593. [ Links ]
57. Ashmead HD. A lack of competition in the absorption of Albion iron and copper amino acid chelates. Clearfield, UT, Albion Laboratories, Inc., Prepared for publication. [ Links ]
58. Graff D, HD Ashmead & C Hartley. Absorption of minerals compared with chelates made from various protein sources into rat jejunal slices, in vitro. Paper presented at Utah Academy Arts, Letter, Sciences, April 1970. Later published in :Intestinal Absorption of Metal lons and Chelates. HD Ashmead, DJ Graff & HH Ashmead. Springfield, IL, Charles C Thomas, 1985, p. 118-125. [ Links ]
59. Ashmead HD & RB Jeppsen. Enhanced tissue metabolism of minerals chelated to amino acids. Proc. Bioavailability 93, Ettlingen, Germany V2, 1993, p. 63. [ Links ]
60. Fairweather- Tait SJ, TE Fox, SG Wharf & NA Ghani. A preliminary study of the bioavailability of iron- and zinc- glycine chelates. Food Add Contam 1992;9:97-101. [ Links ]
61. Pineda O & HD Ashmead. Treatment of iron deficiency anemia in infants and young children with bis-glycine iron chelate or ferrous sulfate. Submitted for publication. [ Links ]
62. Graff D & HH Ashmead. Transplacental movement of iron59 chelated with amino acids versus iron59 in inorganic form in synchronously impregnated rats. Paper presented at Nutrition Conference, Cancun Mexico, November, 1977. [ Links ]
63. Brady PS, ER Miller, PK Ku, FF Green & DE Ullrey. Evaluation of an amino acid iron chelate hematinic. Report of Swine Research, 1975, p. 4-6. [ Links ]
64. Yamamoto M, Y Yoshino & K Migawaki. A comparative study of iron proteinate and ferrous fumerate on pig performance. En: Chelated Mineral Nutrition in Plants, Animals and Man. Ashmead, D, (Ed.) Springfield, MO, Charles C Thomas, 1982, p. 210-222. [ Links ]














