Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos Latinoamericanos de Nutrición
versión impresa ISSN 0004-0622versión On-line ISSN 2309-5806
ALAN v.54 n.1 Caracas mar. 2004
Effects of different thermal treatments and storage on the proximate composition and protein quality in canned tuna
García-Arias, M.T, Navarro, M.P., García-Linares, M.C.
Instituto de Ciencia y Tecnología de los Alimentos, Universidad de León, León, Spain - Instituto de Nutrición y Bromatología. Facultad de Farmacia, Universidad Complutense, Ciudad Universitaria, Madrid, Spain.
SUMMARY. The purpose of this project was to study the modifications in nutrient composition, amino acid content, and protein quality of white tuna preserves after each of the thermal treatments involved in the canning process. Also the influence that a three years storage period at room temperature has on the nutritional quality of canned tuna was studied. The biological assays used for the study of the protein utilization were carried out on Wistar rats, fed on semi-synthetic diets for 12 days varying only the protein source, casein or tuna provided as follows: raw, cooked in brine, steamed, sterilized tuna, and canned tuna stored for three years. The sterilization process and storage time led to a great increase in the lipid content of the canned tuna and to a porcentual decrease in protein, and moisture content. Amino acid composition of canned and cooked tuna did not show great modifications compared to raw tuna. Neither protein digestibility nor biological value of the cooked, canned, and stored tuna showed any deterioration. The protein quality of white tuna meat preserves has been compared with preserves made up of red and white tuna meat.
Key words: Canned tuna, protein quality, storage time, cooking, sterilization, amino acid, nutrient content.
Efecto de diferentes tratamientos térmicos y del almacenamiento sobre el contenido en macronutrientes y la calidad proteica de atún en conserva.
RESUMEN: El objetivo de este trabajo fue estudiar las modificaciones en el contenido en nutrientes, aminoácidos y la calidad proteínica de conservas de atún blanco después de cada uno de los tratamientos térmicos incluidos en el proceso de enlatado. Así como estudiar la influencia que el almacenamiento durante tres años a temperatura ambiente ejerce sobre la calidad nutricional del atún en lata. Los ensayos biológicos empleados para estudiar la utilización proteínica se llevaron a cabo con ratas Wistar, alimentadas con dietas semisintéticas durante 12 días variando solamente la fuente proteínica, caseína o atún, proporcionado de la siguiente manera: crudo, cocinado en salmuera, cocinado al vapor, esterilizado y enlatado almacenado durante tres años. El proceso de esterilización y el tiempo de almacenamiento producen un incremento en el contenido de lípidos del atún enlatado y un descenso porcentual en el contenido de proteína y humedad. El perfil aminoacídico del atún enlatado y cocinado no mostró grandes modificaciones comparado con el atún crudo. Ni la digestibilidad proteínica ni el valor biológico del atún cocinado, enlatado y almacenado mostró ninguna alteración. La calidad proteínica de las conservas de carne blanca de atún fue comparada con la calidad proteínica de las conservas elaboradas con carne roja y blanca de atún.
Palabras clave: Atún enlatado, calidad proteínica, tiempo de almacenamiento, cocinado, esterilización, aminoácidos, contenido en nutrientes.
Recibido: 28-06-2002 Aceptado: 10-12-2003
INTRODUCTION
White tuna, Thunnus alalunga,, represents a very interesting species of fish from both the nutritional and economic points of view. This is primarily because of the high prices it reaches on the market. Most of the tuna caught is destined for the production of preserves, therefore it is important to study the different stages of the canning process with the objective of optimizing the nutritional quality of the final product.
There are two edible parts in white tuna fish: white and red meat, which each present different nutrient composition (1, 2). Muscle has a very low fat content compared with the high proportion of fat contained in the belly flap. So, depending on the part of the tuna that is canned, the reactions produced in the fish to preparation and storing may vary, modifying hereby the nutritional value of the final product. This is important because each manufacturer cans different parts of the tuna fish, according to consumer preference and/or economic factors (3).
Fish protein damage can be produced during thermal processes, cooking and sterilization, by reactions between amino acids and oxidation products of the fish lipids, which are produced during the processes mentioned above (4, 5, 6). These interactions could continue during storage of the canned product which has not lost the capacity of reaction. So, since canned foods are stored during variable time periods before their consumption, it would be interesting to know if nutritional deterioration occurs, and if so, when this begins, in order to establish an expiration date for canned tuna. Therefore, the purpose of this project was to study:
a) The modifications in: nutrient composition, amino acid content and nutritional quality of protein of white tuna meat after each of the thermal processes involved in preparing canned tuna-steaming or cooking in brine and sterilization.
b) The influence that a three year storage period at room temperature has on the nutritional quality of canned tuna.
These results were compared with those obtained by García-Arias et al (7) in preserves of the same tuna species in which both white and red meat were canned.
MATERIALS AND METHODS
Materials
Twelve tuna Thunnus alalunga weighing between 4.6 and 5.0 kg were caught by a commercial tuna vessel near the point 43ºN and 27ºW during June 1998. The tuna was kept in boxes and transported on ice over 10 days. After arrival, the fish were frozen at -40ºC and stored at -18ºC for 4 months. The different stages of sample preparation describes as follows took place in the pilot plant of the Instituto de Investigaciones Marinas de Vigo (CSIC).
Processing
The twelve whole thawed tuna were, beheaded, eviscerated, washed, and randomly divided into two groups. Both groups were cooked until a final backbone temperature of 65ºC. One of them was cooked in brine (130 g salt/l water) and the other one was steamed (102-103ºC). Afterwards, tuna was cooled at 14ºC for about 5 hours and skin, bones, backbone and red meat were removed from every cooked fish. Approximately 3 kg of each product were set aside and used as samples, that represents 3 kg of steamed sample (S) and 3 kg of cooked in brine sample (B).
The rest of the fish, approximately 20 kg of white meat of each kind of cooked tuna, was canned. In the case of steamed tuna, 80 g of white meat together with 35 ml of soybean oil and 1.6 g of sea salt were put into each can (OL-120 shape), obtaining an amount of 250 cans, and in the case of cooked in brine tuna, 80 g of white meat mixed with 35 ml of soybean oil were put into each can, obtaining an equal number of cans. Both kinds of cans were closed and sterilized at 115ºC for 90 min. The cans were left to "age" for 30 days, for the cover oil and salt to be distributed equally and absorbed by the solid content. After this period of time, a mixture of the content of 12 cans of each modality was made (samples SCO and BCO). The rest of the tins were stored for three years at room temperature (approximately 20ºC). After this period of time, a mixture of the content of 12 cans of each kind was also made (samples SC3 and BC3).
Finally an amount of 5 kg of raw white tuna meat in pieces were chosen at random from different fishes and different parts of their bodies and were used as a reference sample (R).
Each one of the samples was correspondingly lyophilized and later stored at -20ºC until they were analyzed and used as a protein source in the animal assays.
Keys:
R: Raw tuna; S: Steamed tuna; B: Tuna Cooked in Brine;SCO: Steamed Canned Tuna; BCO: Tuna Cooked in Brine and Canned; SC3: Canned Steamed tuna stored for 3 years; BC3: Tuna cooked in Brine and Canned, stored for 3 years.
Analytical techniques
Proximate analysis
Homogeneous mixtures of 3-5 g were dried at 100ºC to constant weight by standard methods (8) for moisture determination. Total protein was calculated from Kjeldahl procedure using a 6.25 conversion factor. Total lipid was extracted from lyophilized samples with petroleum ether 40-60º in an extracting Unit Soxtec System 1040 Tecator. Ether extract was gravimetically evaluated. Samples for ash were dried according to methods outlined in AOAC (8) using a muffle furnace at 550ºC to constant weight.
Amino acid analysis
Amino acids were analysed after hydrolysis (6M HCl) by ion-exchange chromatography (Chromakon 500) using nor-valine as an internal standard (9). Since methionine and cysteine are partially broken down by acid hydrolysis, they were oxidized to cysteic acid and methionine sulphone prior to hydrolysis (10).
Apparent digestibility and protein quality
100 Wistar weanling rats weighing 40±0.5 g were used for balance assays. Each group of 10 rats (5 male and 5 female) was placed in individual metabolism cages in an environmentally controlled room maintained at 20-22ºC, with a 12h light-12h dark cycle and 55-70% humidity.
Isocaloric and semi-synthetic diets, according to the recommendations of the National Research Council (11), were prepared with the following theoretical content: 39.5% starch (Central Ibérica de Drogas, S.A. Madrid), 39.5% sugar (Confisa, S.A. Madrid), 7.5% fat (soybean oil), 5% non-nutritive fiber (Central Ibérica de Drogas, S.A. Madrid), 3.34% mineral mixture (E. Merck Darmstadt) and 0.12% vitamin mixture (Roche).
It must be taken into account that when raw and processed tuna were used as a protein source, fat was supplied simultaneously into the diet. Thus, in our study, that amount of fat supplied by tuna was reduced from the oil added to the diet. The diets composition are shown in Table 1.
Macronutrient composition in diets of rat assays (g/ 100g)
| Protein Source* | Moisture | Protein | Lipid | Ash |
| Casein + DL Methionine | 6.41±0.03 | 9.7±0.1 | 8.0±0.1 | 2.8±0.1 |
| R | 5.72±0.03 | 10.0±0.1 | 7.8±0.1 | 3.4±0.1 |
| S | 5.63±0.05 | 10.0±0.1 | 7.8±0.2 | 3.2±0.1 |
| B | 5.81±0.06 | 10.0±0.1 | 7.7±0.2 | 3.3±0.1 |
| Casein + DL Methionine | 7.00±0.06 | 10.1±0.1 | 7.3±0.3 | 3.6±0.1 |
| SCO | 6.91±0.08 | 10.1±0.1 | 8.5±0.1 | 3.6±0.1 |
| BCO | 7.00±0.09 | 9.6±0.1 | 8.3±0.1 | 3.3±0.1 |
| Casein + DL Methionine | 5.99±0.06 | 10.4±0.1 | 8.1±0.2 | 3.0±0.2 |
| SC3 | 5.19±0.02 | 10.0±0.1 | 7.5±0.3 | 2.9±0.1 |
| BC3 | 4.85±0.01 | 10.3±0.1 | 8.5±0.2 | 2.9±0.2 |
Values are mean ± standard deviations for four samples
*For abbreviations, see Material and Methods.
The balances were conduced in three different stages using different balanced groups of rats.
* First stage, three groups were fed on diets with the protein (10%) provided from raw, steamed, and cooked in brine tuna. The fourth group was fed on diet in which casein + DL methionine (0.2% of the diet) provided the reference protein.
* Second stage, two groups of rats were fed on diets with the protein (10%) provided from SCO and BCO. Casein + DL methionine was again used as reference protein.
*Third stage, after three years storage, new nitrogen balances took place using the samples SC3 and BC3. Casein + DL methionine was again used as reference protein.
The assay involved a preliminary five day adaptation period during which solid intake and body weight changes were monitored, followed by a second period lasting seven days in which nitrogen balances were carried out. Animals were fed and allowed to drink "ad lib". In order to evaluate nitrogen balances, food intake was monitored during the last week of the trial and urine and faeces were collected in 1 week pools. Urine was collected on 0.5 M HCl, and later, filtered and diluited. Faeces were dried, weighed and homogenized. Diets, faeces, and urine were analysed for nitrogen content using the Kjeldahl procedure.
The following indices were calculated: absorbed nitrogen (NA)= ingested nitrogen (NI)-fecal N; retained nitrogen (NR)= NA- urinary nitrogen; digestibility (DC)= NA/NI *100; biological value (BV)= NR/NA * 100 and net protein utilization (NPU)= NR/NI * 100.
The values were analyzed by the one-way analysis of variance (ANOVA). The Scheffe test to compare means was used when a significant variation was highlighted by ANOVA. The significance of the results was established at P<0.05 level.
RESULTS AND DISCUSSION
Nutrient composition
Macronutrients
Moisture, protein, fat, and ash contents in the edible part of the white meat of raw tuna (R) are within the range indicated for tunas (12). The value of protein is among the highest (27.33%) and the value of fat among the lowest (1.90%) (Table2).
Moisture, protein, lipid and ash content for raw, cooked and canned white meat tuna.
| White Meat Tuna | Moisture | Protein Lipid | Ash | Protein | Lipid | Ash
| |
| R | 70.0±0.2 | 27.3±0.5 | 1.9±0.2 | 1.5±0.1 | 91.4±1.8 | 6.4±0.8 | 5.0±0.1 |
| S | 64.0±0.1 | 31.4±0.1 | 4.7±0.1 | 1.2±0.0 | 87.6±0.4 | 13.0±0.3 | 3.4±0.0 |
| SCO | 59.6±1.2* | 32.9±0.4* | 7.4±0.2* | 2.5±0.1* | 80.2±1.0* | 18.3±0.4* | 6.2±0.2* |
| SC3 | 56.6±0.2*° | 32.9±0.1* | 8.4±0.2*° | 2.9±0.0*° | 75.9±0.3*° | 19.3±0.4*° | 6.6±0.1*° |
| B | 64.0±0.1 | 33.0±0.2 | 3.3±0.1 | 1.4±0.0 | 91.7±0.6 | 9.2±0.2 | 3.9±0.0 |
| BCO | 60.1±0.3* | 33.2±0.6 | 7.2±0.1* | 1.3±0.1 | 83.4±1.6* | 18.0±0.3* | 3.3±0.2* |
| BC3 | 55.7±1.5*° | 33.6±0.1* | 9.4±0.1*° | 1.8±0.1*° | 76.0±0.2*° | 21.3±1.9*° | 4.2±0.1*° |
Values are mean ± standard deviations for four samples.
For abbreviations, see material and methods.
Significant differences ( P<0.05), relative to R.
*Significant differences ( P<0.05), relative to S or B respectively.
Significant differences ( P<0.05), relative to S, SCO or SC3 respectively.
°Significant differences ( P<0.05), relative to SCO or BCO respectively
After steaming (S) or cooking in brine (B), moisture decreased by approximately 6%, as has been described in other fish like sardine (13), mussel (14), and other tuna species (15, 16). This decrease made the protein and fat contents increase when expressed as g/100 g edible portion, with a greater protein increase in B and a greater fat increase in S.
By expressing the values in dry matter, we shall see that there was actually a protein loss of 4% in steaming, which was not a result of being cooked in brine. This can be explained by the high saline concentration of brine. Due to this protein percentage stability, the percentage of fat in B was less than that corresponding to S (Table 2).
The quantity of ash descended after cooking raw tuna in both ways. In S, this decrease was less because of the incorporation of Na from the brine.
As is well Known, sterilization involves an exchange of substances between food and coating liquid consisting in an interchange between the water of the fish and the coating oil (5, 17). This effect seems clear in our experiment, as both contents, water and fat, resulted to be inversely correlated (r = -0.9464) (P<0.01). This led to a great increase in the lipid content of the canned tuna and thus to a percentual decrease in protein content if the figures are expressed in dry matter.
In SCO, because of the addition of salt to the cans, the percentage of ash elevated.
When both preserves were stored for three years (BC3 and SC3), water was again lost and fat increased percentually compared to recently canned tuna (SCO and BCO). The percentage of protein expressed in dry matter decreased to reach, in both cases, the same quantity (75.90%) (Table 2).
Amino acids
The amino acid composition of the white tuna meat did not show great variation from other fish species. After cooking in either of the ways practiced, the amino acid profile did not vary much, although valine and tyrosine increased significantly in both samples (Table 3). Once the product is canned, these differences disappeared and only a significant increase in valine in SCO was noted (Table 3).
Amino acid composition of raw, cooked and canned white meat tuna (g/16 g N)
| AMINO ACID | R | S | SCO | SC3 | B | BCO | BC3 |
| ASP | 9.61±0.91 | 9.38±0.40 | 9.62±0.20 | 9.71±0.38 | 8.96±0.36 | 9.17±0.35 | 9.23±0.34 |
| THR | 4.57±0.61 | 4.20±0.22 | 4.22±0.15 | 4.20±0.22 | 3.83±0.24 | 4.10±0.18 | 4.10±0.26 |
| SER | 3.94±0.43 | 3.65±0.19 | 3.59±0.13 | 3.50±0.18 | 3.45±0.14 | 3.59±0.16 | 3.40±0.15 |
| GLU | 12.89±0.18 | 13.38±0.56 | 13.46±0.33 | 14.41±0.55 | 12.98±0.42 | 13.02±0.59 | 13.05±0.41 |
| PRO | 4.19±0.27 | 3.91±0.47 | 3.77±0.63 | 3.85±0.53 | 3.60±0.51 | 3.48±0.40 | 3.54±0.49 |
| GLY | 4.59±0.26 | 4.80±0.34 | 4.95±0.15 | 4.83±0.25 | 4.49±0.05 | 4.72±0.15 | 4.60±0.17 |
| ALA | 5.19±0.33 | 5.59±0.32 | 5.60±0.09 | 5.40±0.20 | 5.47±0.11 | 5.40±0.14 | 5.42±0.12 |
| VAL | 4.87±0.18 | 5.91±0.20 | 6.12±0.08 | 6.00±0.17 | 5.74±0.20 | 5.85±0.27 | 5.80±0.23 |
| ILE | 4.54±0.26 | 5.28±0.23 | 5.62±0.15 | 5.52±0.16 | 4.99±0.18 | 5.36±0.18 | 5.20±0.21 |
| LEU | 7.36±0.56 | 8.02±0.32 | 8.31±0.16 | 8.50±0.28 | 7.77±0.23 | 8.12±0.32 | 7.95±0.30 |
| TYR | 3.09±0.10 | 3.87±0.10 | 4.04±0.14 | 4.01±0.12 | 3.91±0.10 | 3.44±0.13 | 3.56±0.14 |
| PHE | 3.97±0.29 | 4.20±0.20 | 4.37±0.37 | 4.25±0.23 | 4.04±0.13 | 4.20±0.21 | 4.11±0.24 |
| LYS | 8.32±0.51 | 8.85±0.79 | 8.81±0.36 | 8.81±0.49 | 8.87±0.77 | 9.00±0.36 | 8.75±0.56 |
| HIS | 5.71±0.40 | 5.41±0.42 | 5.07±0.25 | 5.12±0.39 | 5.28±0.61 | 5.30±0.22 | 5.20±0.33 |
| ARG | 6.27±0.36 | 7.17±0.41 | 5.85±0.17 | 5.90±0.21 | 5.53±0.24 | 5.54±0.36 | 5.34±0.29 |
| MET | 3.56±0.11 | 3.63±0.23 | 3.54±0.18 | 3.30±0.20 | 2.80±0.19 | 3.68±0.20 | 3.24±0.17 |
| CYS | 1.44±0.09 | 1.44±0.02 | 1.63±0.10 | 1.30±0.10 | 1.60±0.03 | 1.58±0.01 | 1.35±0.08 |
Values are mean ± standard deviations for four samples.
* For abbreviations, see material and methods.
Significant differences ( P<0.05), relative to R.
The results lead one to believe that, in our case, the time and temperature conditions used in processing were not drastic enough to produce great variation in any amino acid as described by Steiner et al (18) and Seet & Brown (19), but that, as occurs in mild thermal treatments, the amino acidic profile was maintained (20).
Protein Quality
The protein quality study showed that the quality of protein in white tuna meat is comparable to the casein DL methionine quality (Table 4).
Digestive and metabolic utilization of protein of raw, cooked and canned white meat tuna.
| Protein | ||||||||||||
| Source* | Ingested N | Faecal N | Urinary N | Absorbed N | Retained N | Apparent | ||||||
| DC |
| BV |
| NPU | ||||||||
| Casein+DL methionine | 146.1±4.9 | 12.3±0.5 | 25.9±1.6 | 133.7±4.8 | 107.8±5.1 | 91.4±0.4 | 80.3±1.4 | 73.5±1.3 | ||||
| R | 143.7±6.7 | 12.0±0.5 | 30.5±4.8 | 131.6±6.5 | 101.0±7.5 | 91.5±0.4 | 76.6±3.6 | 70.1±3.3 | ||||
| S | 147.3±3.9 | 14.8±0.7 | 21.6±2.6 | 132.5±3.9 | 110.8±4.6 | 89.9±0.5 | 83.5±2.0 | 75.0±1.9 | ||||
| B | 147.4±7.6 | 13.6±0.7 | 28.9±2.9 | 133.7±7.6 | 104.7±6.7 | 90.4±0.8 | 78.2±2.0 | 70.7±1.7 | ||||
| Casein+DL methionine | 158.4±5.4 | 15.6±1.3 | 26.3±1.1 | 142.7±4.6 | 116.3±4.6 | 90.2±0.6 | 81.2±1.1 | 73.3±1.1 | ||||
| SCO | 137.2±5.7 | 13.9±1.7 | 18.3±1.4 | 123.2±5.2 | 104.9±4.9 | 89.8±0.9 | 85.0±1.0 | 76.4±1.2 | ||||
| BCO | 126.9±4.8 | 13.4±0.7 | 16.2±1.4 | 113.5±4.4 | 97.2±4.2 | 89.4±0.4 | 85.6±1.2 | 76.5±1.2 | ||||
| Casein+DL methionine | 157.9±5.2 | 13.7±1.5 | 12.9±0.9 | 145.4±6.6 | 132.7±7.4 | 91.2±1.2 | 91.0±1.2 | 83.1±1.9 | ||||
| SC3 | 148.0±3.9 | 16.4±0.5 | 12.5±3.2 | 130.9±4.5 | 118.4±4.0 | 88.8±0.5 | 90.5±0.8 | 80.4±0.9 | ||||
| BC3 | 153.4±5.3 | 17.3±1.8 | 13.6±1.8 | 138.2±5.1 | 124.5±4.6 | 88.9±1.1 | 90.2±1.3 | 80.2±1.5 | ||||
DC: Digestibility Coeficient; BV: Biological Value; NPU: Net Protein Utilization
Values of ten animals each group expressed as mean ± SEM
* For abbreviations, see Material and Methods. No significant differences.
The cooking process with or without salt did not change the coefficients of apparent digestibility (ADC), apparent biological value (ABV), nor apparent net protein utilization (ANPU). This proves that since moderate heat treatments did not alter the amino acid content, they did not modify the nutritional value of the protein either, as noted by Finot (21).
After the sterilization of the canned tuna, the protein digestibility did not vary, nor did the biological value, so the net protein utilization of the canned tuna was high and very near that of the protein reference according to Varela et al (22); and Hellendorn et al (23).
When these tuna preserves were stored at room temperature for three years, the digestibility and biological value of their protein showed no deterioration (Table 4).
The stability of these results can be comparatively seen in Figure 1. The coefficients ADC, ABV and ANPU of each raw and processed tuna sample have been recalculated, assigning a value of 100 to the casein reference.
This protein stability after storage seems to contrast with the results obtained from an earlier project in our laboratory, in which we studied the nutritional value of canned tuna of the same species but made up of red and white meat, therefore with a higher fat content (24.8 g to 100 g dry matter). Storage of this type of canned tuna for 3 years produced in the coefficients AVB and ANPU a significant decrease of approximately 16% (7). We think that the different conduct of the tuna samples used by García-Arias et al., (7) and those used in the present study is due to the difference in fat and hemic iron contents. Red meat is richer in fat and iron than white, and therefore reaction among the oxidation products of lipids catalized by hemic iron (12, 24, 25) occurred sooner in the storing of mixed red and white meat.
Reduce coefficients of digestibility /ADC), biological value (ABV), and net protein utilization (ANPU), with respect to the vlue of 100 assigned to the casein reference
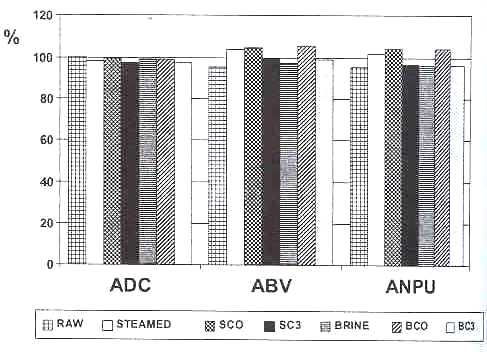
By comparing the results obtained in both studies, we may conclude that protein quality deterioration in canned tuna during storage is greater when the canned product contains a higher percentage of red meat, and therefore, fat. Thus we suggest that one of the factors to take into consideration in order to establish an expiration date for canned tuna with reference to its protein quality would be the fat content of the sample that is canned.
ACKNOWLEDGEMENTS
Authors wish to acknowledge most gratefully the "Instituto de Investigaciones Marinas de Vigo" (Spain). Financial support of this work by the Spanish CAICYT (Project Nº516) and CICYT (Project ALI 88-025) is also gratefully acknowledged.
REFERENCES [ Links ]
2. Aubourg S, Sotelo CG, Gallardo JM. Zonal distribution of fatty acids in albacore (Thunnus alalunga) triglycerides and their changes during cooking. J Agr Food Chem 1990; 38: 255-257. [ Links ]
3. Carril A. Technological developments in tuna canning and procesing. Infofish-Int 2002; 4: 53-56. [ Links ]
4. Medina I, Sacchi R, Aubourg SP. A 1-3C-NMR study of lipid alterations during fish canning: Effect of filling medium. J Sci Food Agr 1995; 69: 445-450. [ Links ]
5. Aubourg S, Gallardo JM, Medina I. Changes in lipids during different sterilization conditions in canning albacore (Thunnus alalunga) in oil. Int J Food Sci Technol 1997; 32: 427-431. [ Links ]
6. Yeun-Suk-Gu, Hyoung-Sik-Yoon, Douck-Choun-Park, Cheong-Il-JI, Tae-Yong-Cho, Myung-Chan-Kim, Sung-Jae-You, Saeng-Gyu-Yeo, Seon-Bong-Kim. Effects of muscle types and cooling on discoloration ofcanned skipjack. Fish Sci 2001; 67: 1145-1150. [ Links ]
7. García-Arias MT, Navarro MP, Castrillón AM. Estudio de las variaciones en macronutrientes y valor nutritivo de la proteína de una conserva de atún a los tres años de su preparación. Arch Latinoam Nutr 1991; 40: 1. [ Links ]
8. AOAC. Methods of Analysis for Nutritional Labeling. Association of Official Analytical Chemists 1993, Washington, DC. [ Links ]
9. Gehrke CW, Wall LL, Absheer JS, Kaiser FE, Zumwalt RW. Focus: Amino acid analysis (sample preparation for chromatography of amino acids: Acid hydrolysis of proteins). JAOCS 1985; 68: 811-820. [ Links ]
10. Moore S, Stein WH. Chromatographic determination of amino acids by the use of automatic recording equipment. In: Methods in Enzymology, S.P. Colowick and N.O. Kaplan (eds). 1963. pp. 819-824. New York: Academic Press. [ Links ]
11. National Research Council. Nutrient Requirements of Laboratory Animals. 1995, 4th Revised Edn. National, Academy Press, Washington, DC. [ Links ]
12. Pigott GM, Tucker BW. Seafood. Effects of technology on nutrition. 1990, Marcel Dekker, Inc. (ed). pp 66-84. New York and Basel. [ Links ]
13. Beamonte A, Castrillon AM. Variaciones en el contenido de triptófano en sardina (Sardina pilchardus) originadas por los procesos térmicos culinarios. Papel de la grasa. Grasas y Aceites 1989; 40: 194-198. [ Links ]
14. Lema ML, Navarro P, Mataix FJ, Varela G. Influencia de los procesos de cocción y desecación a distinta temperatura sobre el valor nutritivo de la proteína de mejillón (Mytilus edulis). Arch Latinoam Nutr 1986; 36: 495-504. [ Links ]
15. Pirazzoli P, Anbroggi F, Incerti I. Conserve di tonno all'olio: variazione della composizione in funzione del tipo di cottura e influenza della temperatura di magazzinaggio sulla maturazione. Ind Conserve 1980; 55: 279-285. [ Links ]
16. Jane Wyatt C, Ronan K. Effects of processing on the sodium: potassium and calcium: phosphorus content in foods. J Agr Food Chem 1983; 31: 415-420. [ Links ]
17. Mermelstein NH. Seafood processing. Food Technol 2000; 54: 66-74. [ Links ]
18. Steiner M, Asiedu D, Njaa LR. Effect of local processing methods (cooking, frying and smoking) on three fish species from Ghana. Part 11. Amino acids and protein quality. Food Chem 1991; 41: 227-236. [ Links ]
19. Seet ST, Duane Brown,W. Nutritional quality of raw, precooked and canned albacore tuna (Thunnus alalunga). J Food Sci 1983; 48: 288-289. [ Links ]
20. Castrillón AM, Navarro MP, García-Arias MT. Changes in tuna protein quality after canning. J Food Sci 1997; 61: 1250-1253. [ Links ]
21. Finot PA. Effects of processing and storage on the nutritional value of food proteins. In: Food proteins and their Applications. 1997, Damodaran S, Paraf A (eds) Marcel Dekker Inc, New York. pp. 551-577. [ Links ]
22. Varela G, Pujol A, Moreiras-Varela O. Sobre el valor biológico de la proteína de las sardinas frescas y enlatadas. Anal Bromatol 1963; 15: 117-125. [ Links ]
23. Hellendoorn EW, De Groot AP, Slum P. Effect of sterilization and three years storage on the nutritive value of canned prepared meals. Voeding 1996; 30: 44-63. [ Links ]
24. O'Brien J, Morrissey PA. Metal ion complexation by products of the maillard reaction. Food Chem 1997; 58: 17-27. [ Links ]
25. Alzagtat AA, Alli I. Protein-Lipid interactions in food systems: a review. Int. J Food Sci Nutr 2002; 53: 249-260. [ Links ]














