Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos Latinoamericanos de Nutrición
versión impresa ISSN 0004-0622versión On-line ISSN 2309-5806
ALAN v.58 n.1 Caracas mar. 2008
Prevalence of anemia and deficiencies of iron, folic acid and vitamin B12 in an Indigenous community from the Venezuelan Amazon with a high incidence of malaria
Maria Nieves García-Casal, Irene Leets, Carmen Bracho, Mariana Hidalgo, Gilberto Bastidas, Ana Gomez, Ana Peña, Hilda Pérez
Instituto Venezolano de Investigaciones Científicas (IVIC), Centros de: Medicina Experimental y Microbiología y Biología Celular. Caracas, Venezuela. Hospital José Gregorio Hernández de Puerto Ayacucho, Amazonas, Venezuela
SUMMARY.
The objective of this work was to determine the prevalence of anemia and deficiencies of iron, folic acid and vitamin B12 in Betania del Topocho, a Piaroa community from Estado Amazonas, Venezuela, a zone with a high incidence of malaria. The group studied included 184 subjects of all ages that assisted to the local health center for malaria diagnosis. Analysis performed included hematology by coulter counter, ferritin quantification by ELISA with monoclonal antibodies and folic acid and vitamin B12 determinations by an immunoradiometric assay. It was found that the prevalence of anemia was 89.6% and deficiencies of iron, folic acid and vitamin B12 affected 37.1, 70.3 and 12.4% of the population studied, respectively. Plasmodium infection was detected by molecular diagnosis in 53.2% of the cases, and 86% of them were anemic. The highest incidence of anemia was found in children, with a prevalence of 100% in infants of both sexes. The high prevalence of anemia, iron and folic acid deficiencies found, indicates an important health and nutrition problem that should be immediately and properly addressed.
The number of cases of anemia due to iron deficiency could be underestimated, since ferritin concentration increased as a acute phase protein, although prevalence data was also analyzed with a cutoff point of 30 μg/L for ferritin concentration.
Key words: Anemia, iron deficiency, folic acid, malaria, Indians.
Prevalencia de anemia y deficiencias de hierro, ácido fólico y vitamina B12 en una comunidad indígena del Amazonas Venezolano con alta incidencia de malaria
RESUMEN.
El objetivo de este trabajo fue estudiar la prevalencia de anemia y de las deficiencias de hierro, ácido fólico y vitamina B12 en Betania del Topocho, población indígena de la etnia Piaroa, del Estado Amazonas, Venezuela, una zona con alta incidencia de malaria. Se estudiaron 184 sujetos de todas las edades que asistieron al Centro de salud para despistaje de malaria. Se realizó hematología completa por Coulter counter, la cuantificación de ferritina por ELISA con anticuerpos monoclonales, y la determinación de ácido fólico y vitamina B12 séricas por un ensayo inmunoradiométrico. La prevalencia de anemia fue de 89.6% y las deficiencias de hierro, ácido fólico y vitamina B12 afectaron 37.1, 70.3 y 12.4% de la población estudiada, respectivamente. La infección con Plasmodium fue detectada por diagnóstico molecular en el 53.2% de los casos, y 86% de ellos eran anémicos. La mayor incidencia de anemia fue encontrada en niños, con una prevalencia del 100% en lactantes de ambos sexos. La alta prevalencia de anemia y deficiencias de hierro y ácido fólico indican un problema de salud y de nutrición importante en esta comunidad que debe ser tratada de forma adecuada y urgente. Los casos de anemia debidos a deficiencia de hierro, podrían estar siendo subestimados, ya que la ferritina sérica es también una proteína de fase aguda que aumenta en caso de infección, aunque los datos de prevalencia fueron también analizados con un punto de corte de 30 μg/L para la concentración de ferritina.
Palabras clave: Anemia, deficiencia de hierro, ácido fólico, malaria, indígenas.
Recibido: 01-11-2007 Aceptado: 17-01-2008
INTRODUCTION
Anemia and iron deficiency constitute the most frequent nutritional problems worldwide. Some age groups are more vulnerable to suffer this deficiency, especially as a consequence of increased requirements and/or losses of the mineral. For this reason infants, preschoolers and pregnant and childbearing age women are more susceptible to iron deficiency (1,2). Folic acid is required for some vital functions such as the synthesis of nucleic acids, blood cells and neural tissue. Also, it has an important role in the synthesis of methionine and as a precursor of SAM (S-adenosyl methionine), universal donor of methyl groups for more than 100 organic reactions (3,4). Folic acid deficiency is very prevalent in the world and some consequences of this deficiency include: macrocitic or megaloblastic anemia, increased incidence or severity of some types of cancer (5-7), neural tube defects (8,9), increased serum homocysteine levels and cardiovascular alterations (10-12). has an important role in the synthesis of methionine and as a precursor of SAM (S-adenosyl methionine), universal donor of methyl groups for more than 100 organic reactions (3,4). Folic acid deficiency is very prevalent in the world and some consequences of this deficiency include: macrocitic or megaloblastic anemia, increased incidence or severity of some types of cancer (5-7), neural tube defects (8,9), increased serum homocysteine levels and cardiovascular alterations (10-12).
On the other hand, Vitamin B12 deficiency is less common and usually affects elderly people and strict vegetarians (3,13). Deficiency of this vitamin is recognized for its effect on hematopoyetic and neural systems, causing an important impairment of DNA replication, producing irreversible neuropathy and discontinuous, diffuse and progressive nerve demielinization (14,15).
Nutritional information, and especially on prevalence of anemia and deficiencies of iron, folic acid and vitamin B12, is scarce in Indigenous populations living far from big cities. In Venezuela, this is valid even in some Indigenous communities partially or totally incorporated to the same level of development achieved in the rest of the country. That is the case of the Piaroa community studied in this work, which is located in close proximity to a populated center and has adapted to some urban costumes, while preserving some ancestral traditions and habits. The socioeconomic situation and the difficulties to access services associated with urban centers (i.e. year round supply of varied food sources), as well as religious or cultural practices, may act in some cases as factors that increase the probability of anemia, iron deficiency and in general, malnutrition.
The objective of this work was to determine the prevalence of anemia and deficiencies of iron, folic acid and vitamin B12 in Betania del Topocho, a rural Piaroa community, located in close proximity to Puerto Ayacucho capital city of the Amazonas State in Venezuela. This is a region with a high prevalence of malaria.
MATERIALS AND METHODS
According to the information provided by the Venezuelan National Institute of Statistics and based on projections from 2001 Venezuelan population census, the Amazonas State population during 2005 was 134,594 (16). The community studied named Betania del Topocho, belongs to the Atures Municipality and is located 50 kilometers north of Puerto Ayacucho, the capital city of the state. At the time of the study, the community consisted of 500 Piaroa individuals living in individual houses (one per family), which differ from the traditional communal house typical of the Piaroa people. The community has access to electricity and water services, markets, a rural medical facility attended by a nurse and one elementary school (17). The Piaroa ethnia represents approximately 3% of all indigenous population in Venezuela.
Sample collection was performed in the community medical facility and our research team was accompanied by a representative of the Ministry of Health who was collecting samples for malaria diagnosis. The sampling process was carried out during 3 consecutive days, and each individual, including parents or guardians for children and adolescents under 18 years of age, was informed, using interpreters, about the objectives of the study. Each individual was informed that participation consisted in donating a blood sample to determine hemoglobin, ferritin, folic acid and vitamin B12 concentrations and that the results would be informed to each participant. They were informed that data on gender, age, height and weight, was also required.
In the community studied most individuals speak only Piaroa and we contacted 2 members of the community who spoke Spanish and acted as interpreters. Information about the study was exchanged with participants, fluently and without problems.
The sample consisted in 208 individuals of both sexes and ages between 1 and 94 years, which represents 41.6% of the total population of the community, based on data from 2001 National Census. Sample was taken from all individuals that attended the medical facility because they knew about our presence in the community and consented to participate in the study. Since it was a self-selected sample, the age and gender distribution of the sample was compared against the indigenous communities of Amazonas State.
Blood samples were taken from the antecubital vein of the arm, after proper antisepsis, with alcohol and sterile cotton swabs. The content of the syringe was distributed in 2 tubes: with or without ethylene diamino tetra acetic acid (EDTA) as anticoagulant. Determinations of hemoglobin concentration were performed in a Coulter Counter in the Laboratory of the hospital Jose Gregorio Hernández at Puerto Ayacucho, the capital city of the State. Samples in tubes without anticoagulant were centrifuged 2 to 4 hours after collection, the serum was separated, aliquoted, and frozen at –20ºC until use for serum iron (18), unsaturated iron binding capacity (UIBC) (19), total iron binding capacity (TIBC) (19), ferritin, folic acid and vitamin B12 determinations. Serum ferritin concentration was determined by an ELISA (Enzyme linked immunosorbent assay) (20), developed in our laboratory using monoclonal antibodies raised against human ferritin. Intra and inter-assay variation is 5 and 7%, respectively and the assay has been validated against International standards. Also, internal controls of known concentration (low, medium and high) are run in each plate.
Serum folic acid and vitamin B12 determinations were performed in a sub sample by a radio immunoassay from DPC (Diagnostic Product Corporation, Los Angeles, California) (21), which contains a high affinity folate-binding protein from milk to measure folic acid, and intrinsic factor purified from hog to measure vitamin B12 by competitive binding. It is a radioactive method non susceptible to antibiotics and metothrexate that allows the simultaneous determination of folic acid and vitamin B12 concentrations in serum, plasma or blood, which can be calculated from standard curves. Anemia was diagnosed when hemoglobin concentration was < 11 g/L in children less than 4 years, <11.5 g/L in children 4 to 10.9 years, <12 g/L for all non-pregnant women older than 11 years of age, <11 g/L for pregnant women, <12.5 for boys 11 to 12.9 years and < 13 for men older than 13 years. The cutoff points used for iron deficiency were serum ferritin concentrations <10 μg/L for individuals less than 14 years old and <12 μg/L for the rest of the age groups. Since
there was an important prevalence of malaria and infections in general, ferritin data was also analyzed using a cutoff point of 30 μg/L, as suggested by WHO (2). The cutoff points used for serum folic acid were <3 ng/mL for severe deficiency, and 3 to 6 ng/mL for moderate deficiency.
Vitamin B12, deficiency was defined as serum concentration below 200 pg/mL, normality range varied from 200 to 900 pg/ ml and excess >900 pg/mL (3, 21). Unless otherwise indicated, prevalence of folic acid deficiency refers to cases with severe and moderate deficiency, with a cutoff point <6 ng/ml. Analyses of results were performed calculating the prevalence of anemia and deficiencies of iron, folic acid and vitamin B12 separately and then correlating these deficiencies as causes of anemia, according to age and gender. ANOVA with Bonferroni as a post-test was used to compare differences in biochemical variables by age and gender.
RESULTS
The population studied included subjects of all ages and gender that assisted to the community medical facility for diagnosis of malaria and consented to participate in the study. Since the sample was self-selected and we did not have access to the community age and gender distribution, the comparison was performed against the indigenous communities of Amazonas State. Regarding age, distribution was similar in both locations: 10.4 and 10.5% for 1-3 years old children, 29.7 and 24.1% for 4-10 years, 17.6 and 22.3 for 11-20 years, 28.6 and 28.0 for 21-40 years and 13.7 and 15.1% for 41 and more years, in the community and the State respectively. Gender distribution in the community presented a predominance in females and was different from the State: 58.8% females and 41.2% males for the community and 48.2% females and 51.8% males for the Amazonas State.
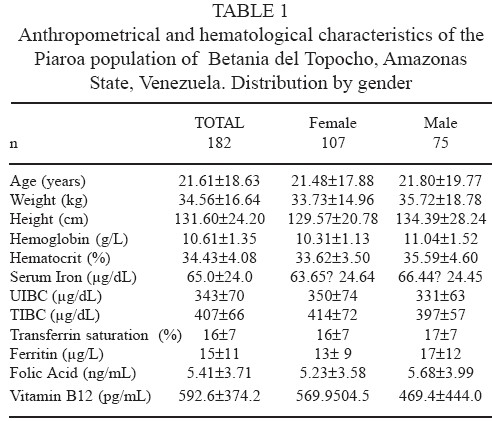
As shown in Table 1 the hematological evaluation of the whole group and classified by gender, revealed an anemic population with a mean hemoglobin concentration of 10.61 g/L. Biochemical parameters for measuring iron metabolism of the individuals, showed low levels for serum iron and transferrin saturation, as well as for UIBC and TIBC. Iron stores, measured by serum ferritin concentration, are slightly above the cutoff point for iron deficiency of 12 μg/L, being classified as a moderately iron deficient population. Also, serum folic acid levels revealed a moderately deficient population.
The only nutritional parameter evaluated that showed adequate serum levels at population level, was vitamin B12, with a mean concentration of 592.6 pg/mL, considerably above the cutoff point for deficiency of 200 pg/mL.
Population studied was classified by age and gender, showing that all groups presented borderline or deficiency values for hemoglobin, ferritin and folic acid. Children in general, especially infants less than 3 years old, presented the lowest levels for hemoglobin and ferritin regardless of gender (Table 2). In the case of serum folic acid, deficiency is moderate in all groups older than 10 years of age of both sexes. In children, values registered were above the cutoff point of 6 ng/mL, except for girls between 1 and 3 years old, with a mean concentration of 4.51 ng/mL. The differences in all biochemical parameters studied, were not different statistically, between age and gender groups.
The prevalence of anemia and deficiencies of iron, folic acid and vitamin B12 by age and gender is shown in Table 3. The highest prevalence of anemia was found in infants of both sexes, reaching 100%. Anemia affected the whole group studied, but was especially high in females of all ages, including women older than 40 years, with a prevalence of 80%. The prevalence of iron deficiency was also higher in females, affecting all ages studied. In males, iron deficiency was less prevalent than in women, and was higher in children below 3 years of age. Regarding folic acid and vitamin B12 deficiencies, the highest prevalence was found in males for both nutrients. The prevalence of folic acid deficiency was high and affected all age groups, while vitamin B12 deficiency was lower and less homogeneous in distribution, showing no cases of deficiency in women older than 40 years of age, probably due to a small sample size (n=52).
tabla 3. Prevalence of anemia and definciencies of irons, folic acid and vitam B12 and prevalence of combinaned anemia + iron defency or anemia + iron deficiency + filic acid deficiency+ in the Piaro community of Betania del Topocho, Amazon State, Venezuela. Distribution by age and gender.
| Gender and age (years) | Anemia | Iron Deficiency | Folic Acid Deficiency | Vit B12 Deficiency | Anemia + Iron Deficiency | Anemia + Folic Acid Deficiency | Anamia + Iron Deficiency +Acid Folic Deficiency |
| Prevalence(%) | |||||||
| FEMALE | |||||||
| 1-3 | 100 | 56 | 75 | 0 | 56 | 75 | 60 |
| 4-10 | 100 | 31 | 50 | 10 | 29 | 60 | 22 |
| 11-20 | 90 | 55 | 90 | 20 | 50 | 60 | 50 |
| 21-40 | 94 | 41 | 80 | 5 | 41 | 75 | 46 |
| 41-+ | 80 | 33 | 38 | 0 | 33 | 38 | 20 |
| TOTAL | 93.45 | 43.2 | 66.6 | 7.0 | 41.8 | 61.6 | 39.60 |
| MALE | |||||||
| 1-3 | 100 | 50 | 75 | 25 | 50 | 25 | 0 |
| 4-10 | 91 | 36 | 50 | 0 | 35 | 50 | 13 |
| 11-20 | 83 | 25 | 88 | 22 | 17 | 75 | 50 |
| 21-40 | 65 | 26 | 100 | 9 | 25 | 73 | 60 |
| 41-+ | 95 | 18 | 57 | 33 | 20 | 43 | 50 |
| TOTAL | 85.80 | 31.08 | 74.0 | 17.8 | 29.4 | 53.2 | 36.80 |
| TOTAL | 89.63 | 37.10 | 70.3 | 12.4 | 35.6 | 57.4 | 38.2 |
The prevalence of iron deficiency increases to 92% when the cutoff point for iron deficiency is set in 30 μg/L. In this scenario, the prevalence of combined anemia and iron deficiency reached 93.3 % indicating that all anemia cases presented iron deficiency. For the combination of anemia, iron and folic acid deficiencies, the prevalence was 58.1%.
When the sample was analyzed as a whole, (Figure 1), there was high prevalence of anemia and folic acid deficiency (89.6 and 70.3%, respectively) with a moderate and atypical prevalence of iron deficiency (37.1%), and a low prevalence of vitamin B12 deficiency (12.4%). When a cutoff point of 30 was used for ferritin concentration, iron deficiency reached 91.6% of prevalence. It was also found that 86% of the individuals with molecular diagnosis of malaria, presented anemia.
DISCUSSION
The study performed in this community showed a high prevalence of anemia and folic acid deficiency, that is evident not only by individual data but also by the mean levels obtained for the entire population. These low general mean values, indicate that the majority of the population studied is affected by these deficiencies, which requires an immediate and massive nutritional intervention. The sample studied included individuals from all age groups, and although the individuals voluntarily included themselves in the study, they represented almost 50% of the community and had the same age distribution found for the indigenous community of the whole Amazonas State. Gender distribution however, showed and increase percentage of females compared to the state population, probably due to the interest of mothers to obtain malaria diagnoses for their children.
Prevalence of anemia reached values close to 90% for the total population and 100% for boys and girls with less than 3 years of age. For the last few years, data obtained in Venezuela and in other underdeveloped countries, seem to indicate that other factors, besides iron deficiency, could have a role in the reported prevalence of anemia (22). Results from this work show that anemia due to iron deficiency account only for 35.6% of the cases, with a total prevalence of anemia of 89.6%, These results were obtained with the cutoff points for normal populations, and it can be noticed that all iron deficient cases studied were anemic, although it is clear from the high prevalence of anemia registered, that there were other causes of anemia.
The cases of anemia that could be due to folic acid deficiency reach 26.7%, using the cutoff of <3ng/ml for severe folic acid levels, or 57.4% with the cutoff of 6 ng/mL, for severe and moderate folic acid deficiency. With the first cutoff point, there is a 27.3% of anemia cases (to complete the total prevalence of 89.6%) due to other causes, different for iron and folic acid deficiencies, and could have an non-nutritional origin, while the more broad cutoff point, could explain the rest of the cases not produced by iron deficiency, although an important number of cases (38.2% of the anemic population) presented combined iron and folic acid deficiencies. One problem to clarify the origin of the anemia is that ferritin is an acute phase protein, that is elevated in case of infections, and in this population the proportion of anemia
produced by iron deficiency could be underestimated, since the diagnosis of iron deficiency was based only on ferritin concentration. The fluctuating and relatively moderate prevalence of iron deficiency by age and gender, was probably due to the fact that ferritin was not only reflecting iron stores, but also the presence of infections in those individuals. It is possible that the difference found in this study between prevalence of anemia and iron deficiency was due to incidence of infections, especially malaria, with a direct effect not only on erythrocytes, but on serum ferritin concentration. As mentioned before, 53% of the population studied presented malaria infection and from this group, almost 90% was anemic. As a matter of fact, when the cutoff point for ferritin concentration was adjusted 30 μg/L, the prevalence of iron deficiency increased to 92% of the cases studied, and could explain all the cases of anemia reported. Also, the prevalence of combined anemia, iron and folic acid deficiencies increased to 58% indicating that combined or multiple deficiency of nutrients is the main problem in this population, along with infections.
It is interesting to highlight that the treatment for these populations is multifactorial: a treatment for the infectious process in a first phase and then begin the adequate feeding or the introduction of specific nutrients that are in deficit. The proper improvement in quality of life, education and access to services are also necessary.
The scarce reports on nutritional status in Indigenous populations in Venezuela, indicate that there in an important nutritional problem. A report on 97 preschool and scholar Piaroa children living in 9 Communities of Amazonas State, showed a prevalence of anemia of 80% and 48% in boys and girls, respectively (23). The study of a Yucpa Indigenous community in a western region of Venezuela (24), reported that the prevalence of anemia was 71% and in half of the cases, there was no iron, folic acid or vitamin B12 deficiencies. The preva-prevalence found in the present study, and folate deficiency was 12.9%. The authors explain the presence of anemia without iron deficiency, to the incidence of infectious diseases such as hepatitis, parasitic, skin and gastrointestinal tract infections. In another study from the same authors (25) in 2 communities of Bari indians, they report a high prevalence of anemia not due to iron deficiency, attributing the results to nutritional deficiencies and high incidence of infections.
The high prevalence of malaria, anemia, iron and folic acid deficiency indicate a health and nutrition problem in this population, that should be properly and urgently addressed. Anemia incidence could be due to nutritional deficiencies of iron and folic acid, as well as to malaria. Anemia cases due to iron deficiency could be underestimated, since the biochemical indicator used to measure iron reserves (serum ferritin) is also an acute phase reactant that increases in cases of infection, although, as shown in this work, changing the cutoff point for this protein concentration, could help in obtaining a general idea of the magnitude of the problem.
ACKNOWLEDGMENT
Authors are grateful to the Community of Betania del Topocho from Amazonas State, Venezuela for their participation in the study, to Licentiate Irma Rodríguez and to the Centro Amazónico de Investigación y Control de Enfermedades Tropicales (CAICET) from Amazonas State, for their collaboration in sample collection and processing. We also thank Venezuelan Air Force for transportation during the study.
REFERENCES
1. International Anemia Consultative Group (INACG). Guidelines for the eradication of iron deficiency anaemia. A report of the International Nutritional Anaemia Consultative Group. Washington, DC: The Nutrition Foundation. 1977. [ Links ]
2. WHO-UNICEF. Indicators and strategies for iron deficiency and anaemia programs. World Health Organization Technical Report. September 1993. [ Links ]
3. ILSI-OPS. International Life Science Institute (ILSI), Pan American Health Organization (OPS). Ácido fólico y Vitamina B12. In: Conocimientos actuales sobre nutrición. Seventh Edition. Ziegler y Filer Eds. Washington DC, USA. p 235-263. 1997. [ Links ]
4. Machlin L, Hüni J. Vitamin A. Folic Acid. Vitamins, basics. Hoffmann-La Roche LTD. Basel Switzerland. pp 37-40, 49- 51. 1994. [ Links ]
5. Giovanucci E, Stampfer M. Folate, methionine and alcohol intake and the risk of colorectal adenoma. J Nat Cancer Inst. 1993; 87: 895-904. [ Links ]
6. Woon Choi S, Friso S, Dolnikowski G, Bagley P, Edmonson, A, Smith D, Mason J. Biochemical and molecular aberrations in the rat colon due to folate depletion are age-specific. J Nutr. 2003; 133: 1206-1212. [ Links ]
7. Woon Choi S, Mason J. Folate and carcinogenesis: an integrated scheme. J Nutr. 2000; 130: 129-132. [ Links ]
8. Czeizel A. Folic acid in the prevention of neural tube defects. J Ped Gastroent. 1995; 2: 4-16. [ Links ]
9. Medical Research Council. Prevention of neural tube defects: results of the Medial Research Council Vitamin Study. Lancet 1991; 338: 131-137. [ Links ]
10. Brattström L, Wilcken D. Homocysteine and cardiovascular disease: cause or effect? Am J Clin Nutr. 2000; 72: 315- 323. [ Links ]
11. McKinley M, McNulty H, McPartlin J, Strain J, Pentieva K, Ward M, Weir D, Scott J. Low-dose vitamin B6 effectively lowers fasting plasma homocysteine in healthy elderly persons who are folate and riboflavin replete. Am J Clin Nutr. 2001; 73: 759-764. [ Links ]
12. Stipanuk M. Folic acid, vitamin B12 and vitamin B6. In: Biochemical and physiological aspects of human nutrition. W.B Saunders Company. Philadelphia pp 483-518. 2000. [ Links ]
13. Hebert V. Staging vitamin B12 status in vegetarians. Am J Clin Nutr. 1994; 59(Suppl): 1213S- 1222S. [ Links ]
14. Allen L. Vitamin B12 metabolism and status during pregnancy, lactation and infancy. Adv Exp Med Biol. 1994; 352: 173- 186. [ Links ]
15. Rodríguez G. Ácido fólico y vitamina B12 en la nutrición humana. Rev Cub Alim Nutr. 1998; 12(2):107-119. [ Links ]
16. Instituto Nacional de Estadística. Proyecciones del Censo 2001. http://www.ine.gov.ve. Information obtained in November 2006, and January 2008. [ Links ]
17. World Press. Informe de una dinámica de grupo con la cooperativa Ärümechä en la comunidad piaroa de Betania del Topocho, Estado Amazonas. 2005. http://babilonia. thinkertothinker.com/. Consulted on December 2006 and January 2008. [ Links ]
18. International Committee for Standardization in Hematology. Recommendation for measurement of serum iron in human blood. Brit J Haematol. 1978; 38:291-294. [ Links ]
19. International Committee for Standardization in Hematology. The measurement of total and saturated iron-binding capacity in serum. Brit J Haematol. 1978; 38: 281-290. [ Links ]
20. Flowers C, Kuizon M, Beard S, Skikne B; Covell A, Cook J. A serum ferritin assay for prevalence studies of iron deficiency. Am J Hematol. 1986; 23: 141-151. [ Links ]
21. DPC Diagnostic Product Corporation. Dualcount Solid Phase No Boil Assay for Vitamin B12 and Folic Acid. Brochure included with the kit. California USA. 1999. [ Links ]
22. Suárez T, Torrealba M, Villegas N, Osorio C, García-Casal MN. Deficiencias de hierro, ácido fólico y vitamina B12 en relación a anemia, en adolescentes de una zona con alta incidencia de malformaciones congénitas en Venezuela. Arch Latinoam Nutr. 2005; 55 (2): 118-123. [ Links ]
23. Hidalgo G. Vitamina A, anemia y antropometría nutricional en pre-escolares y escolares Piaroa, Estado Amazonas. (Vitamin A, anemia and nutritional anthropometrics in Piaroa preschoolers and scholars, Amazonas State). Thesis for Master in Nutrition from Universidad Simón Bolívar. Caracas, Venezuela pp 61-66. 2002. [ Links ]
24. Diez-Ewald M, Torres-Guerra E, Leets I, Layrisse M, Vizcaíno G, Arteaga M. Anemia en poblaciones indígenas del Occidente Venezolano. Invest Clín. 1999; 40 (3): 191-202. [ Links ]
25. Diez-Ewald M, Torres-Guerra E, Leets I, Layrisse M, Vizcaíno G, Arteaga M. Prevalencia de anemia y deficiencias de hierro, ácido fólico y vitamina B12 en 2 comunidades Bari al oeste de Venezuela. Invest Clín. 1997; 38 (4): 191-201. [ Links ]