Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos Latinoamericanos de Nutrición
versión impresa ISSN 0004-0622
ALAN vol.65 no.3 Caracas set. 2015
Aflatoxin M1 in pasteurized, UHT milk and milk powder commercialized in Londrina, Brazil and estimation of exposure
Joice Sifuentes dos Santos*, Vanessa R França, Shiguedy Katto, Elsa H W Santana
Program on Milk Science and Technology, University North of Paraná (UNOPAR), Londrina, PR, Brazil.
SUMMARY.
Aflatoxin M1 (AFM1) is found in milk and other excretion products after aflatoxin B1 intake. AFM1 is carcinogenic to humans, and known levels of dairy product contamination is important to understand the risks to which the population is exposed. The occurrence of AFM1 was evaluated in 42 milk samples commercialized in Londrina, Parana State, Brazil and this rate of occurrence was used to estimate this exposure. AFM1 determina tion was ca rried out by ELISA, and was detected in 100 % samples at levels ranging from 0.01 to 0.81 µ
Key-words: Mycotoxins; aflatoxin M1 (AFM1); dairy products; ELISA; estimated daily intake
Aflatoxina M1 em leite pasteurizado e UHT e leite em po comercializados em Londrina, Brasil e exposicao estimada.
RESUMO.
Aflatoxina M1 (AFM1) e encontrada no leite e em outros produtos de excrecao apos o consumo de aflatoxina B1. AFM1 e carcinogenica para humanos, e avaliar os niveis de contaminacao em produtos lacteos e importante para conhecer os riscos aos quais a populacao esta exposta. A ocorrencia de AFM1 foi avaliada em 42 amostras de leite comercializadas em Londrina, Estado do Parana, Brasil, e sua ocorrencia foi utilizada para estimar sua exposicao. A determinacao de AFM1 foi avaliada por ELISA, e foi detectada em 100% das amostras, em niveis variando de 0,01 a 0,81 µ
Palavras-chave:Micotoxinas; aflatoxina M1 (AFM1); produtos lacteos; ELISA; exposicao diaria estimada
Recibido: 30-02-2015 Aceptado: 28-04-2015
INTRODUCTION
Aflatoxins are fungus secondary metabolites that contaminate cereals and other products of vegetable origin. Aflatoxin B1 (AFB1) is the most common and the most toxic aflatoxin. AFB1 is amongst the most potent genotoxic and carcinogenic substances known to date and is classified as Group 1 by the International Agency for Research on Cancer – carcinogenic to humans (1).
After ingesting AFB1 contaminated feeds, a part is degraded in the rumen, resulting in the formation of aflatoxicol. The remaining fraction is absorbed in the digestive tract by passive diffusion and is hydroxylated in the liver to aflatoxin M1 (AFM1) (2). Circulating AFM1 can be excreted in the urine or appear in milk (3). This excretion is also observed in human milk. AFM1 was originally classified as Group 2B – possibly carcinogenic to humans, in 1993, but subsequent evidence of its cytotoxic, genotoxic and carcinogenic effects have led to a new categorization of AFM1 as Group 1 (1).
In Brazil, the national milk production in the first trimester 2014 was estimated at 6.2 billion liters (4), and Paraná State is the third major producer in the country. Since the presence of AFM1 and other contaminants in milk is of public health concern, the Ministry of Agriculture, Livestock and Food Supply instituted the National Plan for Residue Control (PNCR) in 1999 (5). The PNCR has the basic function to regulate, control and survey the food chain. Their actions aim to know and prevent the violation of secure levels or the maximum permitted levels of authorized substances, as well as the occurrence of residues prohibited in the country. The Milk PNCR stipulates the analysis of AFM1 in 100 milk samples per year by immunoassay ELISA or high performance liquid chromatography. Samples are collected randomly on a weekly basis by the Federal Inspection Service and sent to official or accredited laboratories
The aim of the present study was to evaluate the natural occurrence of AFM1 in pasteurized, ultra heat treated milk and milk powder from Londrina, Paraná State, Brazil and estimate the daily AFM1 intake of consumers.
MATERIAL AND METHODS
Samples
A total of 42 samples of pasteurized, ultra heat treated (UHT) milk and milk powder were collected randomly in July 2014 from supermarkets in Londrina, Paraná State, Brazil. Sample information was taken from the labels. Samples were produced in six different States of Brazil. According to information from the packaging, the pasteurized milk samples were produced in July 2014. The UHT milk samples were produced in March, April, May and June 2014. The powder milk samples were produced in December 2013, February, April, May and June 2014. Before being analyzed, the pasteurized milk samples were stored at 4 ºC and the UHT milk and milk powder samples at room temperature. The analyses were made within the expiration period.
Aflatoxin M1 determination
Milk powder samples were weighed and homogenized with distilled water, warmed to 50 oC in a water bath and prepared as fluid milk samples. The samples were defatted by centrifugation at 3500 g at 15 oC for 10 min. The defatted supernatant was subjected to the ELISA test for AFM1 (Ridascreen, R-Biopharm AG, Germany). According to the manufacturer, the limit of detection is 5 ng/L for fluid milk (corresponding to 0.004 µ
Aflatoxin M1 dietary intake estimations
The intake estimates were based on the food consumption data collected by the National Household Food Budget Survey, POF 2008–2009, conducted by the Brazilian Institute for Geography and Statistics from May 2008 to May 2009. The data from the survey were presented as the average daily food intake (g/day) for three different age groups: adolescents (10 to 19 years old), adults (20 to 64 years old), and the elderly (over 65). The data referred to raw food consumption. The sampling survey consisted of 13,569 households and 34,003 inhabitants. The body weight (b.w.) was calculated as the arithmetic mean of the median from the age groups (6).
The estimates of dietary exposure to AFM1 were calculated from the amount of the compound found in dairy products (ìg/kg), the daily intake of dairy products by the age groups (g), and the mean body weight of the age groups (Kg).
Statistical analysis
AFM1 levels in pasteurized, UHT milk and milk powder that did not fit normal distribution, were analyzed using the Kruskal-Wallis non-parametric test. Differences were considered to be significant at p < 0.05.
RESULTS
AFM1 was detected in 100 % samples (Table 1), with levels ranging from 0.01 to 0.81 ìg/kg, and a mean of 0.13 µ
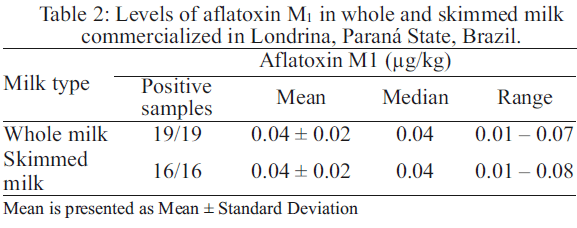
To calculate the estimated daily AFM1 intake through fluid milk and milk powder, the contamination of whole milk and skimmed milk was calculated (Table 2).
Determination of the exposure degree is one of the most important parameters to assess risk from chemical compounds. The Estimated Daily Intake (EDI) of AFM1 from fluid milk and milk powder (Table 3) was obtained using the amount of food consumed and the corresponding mean concentrations of AFM1 detected in each food group, taking into account the mean body weight of the age groups. Since all samples were positive for AFM1, the real concentration was used in the calculation (18). According to the IBGE (6), whole milk consumption was estimated at 38.6 g/day by adolescents, 31.5 g/day by adults and 45.6 g/day by the elderly. Skimmed milk consumption by adolescents, adults and the elderly was 2.5, 4.4 and 9.7 g/day, respectively. Milk powder consumption by adolescents, adults and the elderly was 0.3, 0.3 and 0.4 g/day, respectively. Considering the ingestion of these three dairy products, the AFM1 EDI ranged from 0.384 ng/kg b.w. per day for adults to 0.559 ng/kg b.w. per day for the elderly (Table 3).
DISCUSSION
AFM1 was detected in all samples investigated. It is well known that heat processing is not able to degrade mycotoxins (7, 8). However, in milk powder processing, the water elimination process produces the milk solids, and the heat-resistant contaminants such as mycotoxins concentrate.
Brazilian legislation sets a maximum permitted level of AFM1 for fluid milk of 0.5 µ/kg and for milk powder of 5 µ/kg (9). All the samples evaluated were within these values. The European Commission legislation (10) establishes a limit of 0.05 µ/kg of AFM1 in raw milk, heat-treated milk and milk for the manufacture of milk-based products. Two samples of pasteurized milk (28.6 %), five samples of UHT milk (71.4 %) and three samples of milk powder (42.8 %) exceeded this limit.
In Nigeria, AFM1 was detected in cow milk from 2.04 to 4.00 µ/L (11). In Jordan, Herzallah (12) observed very high levels of AFM1 in raw and pasteurized sheep, cow and goat milk, ranging from 0.16 to 5.23 µ/kg in samples collected in the winter. However, levels were lower than limit of detection of the method (0.05 µ/kg) in samples collected in the summer. The author attributed the difference to the low feed quality offered to the animals during the winter. In Morroco, AFM1 was detected in 13 samples (27%), ranging between 0.01 and 1 µ/kg (13). Four samples were above the European legislation limit.
Previous studies also detected AFM1 in samples from Brazil, but few studies were performed in Parana State. Singular result of AFM1 in pasteurized milk was observed by Santos et al. (14), who did not find this contaminant in any of the 82 samples analyzed in a region of Parana State. Oliveira et al. (15) analyzed 75 samples from Minas Gerais State and showed that 23 (30.7%) samples contained AFM1. Different from the present study, all of the contaminated samples were found to be higher than the maximum acceptable limits for fluid milk in Brazil. Samples of pasteurized and UHT milk from Sao Paulo (48 samples) were analyzed by Oliveira et al. (16). The authors observed a 77.1% frequency of positive samples, with levels of 0.06 µg/L for pasteurized milk and 0.07 µg/L for UHT milk. Also in Sao Paulo, Shundo et al. (17) investigated 125 samples, and a high AFM1 frequency was also observed (95.2%). Thirty three percent of the samples exceeded the limit established by the European Commission.
In the Lebanon, Hassan and Kassaify (19) reported a lower EDI of 0.14 ng/kg b.w. per day in local dairy products, such as fresh milk, yogurt and different types of cheese (akkawi, kashta, karishe, halloum and shanklish). Duarte et al. (20) observed an even lower AFM1 EDI through milk consumption of a Portuguese average adult citizen of 0.08 ng/kg b.w. per day. Similar values were reported by Cano-Sancho et al. (21) in Spain for the adult population of 0.305 ng/kg b.w. per day. Because aflatoxins are carcinogenic, international expert committees (22) did not specify a tolerable daily intake (TDI) for these substances and concluded that daily exposure as low as <1 ng/kg b.w. contributed to the risk of liver cancer. It was therefore recommended that levels should be reduced to as low as reasonably achievable. Kuiper-Goodman (23) has proposed a TDI for AFM1 determined by dividing the median toxic dose (TD50) by an uncertainty factor of 5000. The proposed value is 0.2 ng/kg of b.w., a value equivalent to a risk level of 1:100.000. Taking these values into account, the Londrina population is under toxicological risk to AFM1 exposure.
CONCLUSION
Levels of AFM1 in fluid milk and milk powder commercialized in Londrina, Paraná State, Brazil are below the maximum permitted levels established by Brazilian Legislation. However, considering the European Commission legislation, some samples exceeded the limit. The estimated daily intake of AFM1 through dairy products for adolescents, adults and the elderly in Londrina poses a toxicological risk for the population. The most effective method of controlling AFM1 in dairy products is to reduce the contamination of animal feed with AFB1 by applying preventive measures in terms of temperature and humidity control that reduce fungal growth.
ACKNOWLEDGMENTS
The author thanks the Coordination for the Improvement of Higher Education Personnel – CAPES, for the fellowship of Post-Doctoral National Program granted to Joice Sifuentes dos Santos.
REFERENCES
1. IARC. International Agency for Research on Cancer. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. In. Monographs on the evaluation of carcinogenic risks to humans, Vol. 82 (pp. 171-274). Lyon: IARC. 2002. [ Links ]
2. Kuilman MEM, Maas RFM and Fink-Gremmels J. Cytochrome P450-mediated metabolism and cytotoxicity of aflatoxin B1 in bovine hepatocytes. Toxicol In Vitro. 2000; 14:321-327.
3. Fink-Gremmels J. Mycotoxins in cattle feeds and carry-over to dairy milk: a review. Food Addit Contam. 2008; 25:172-180. [ Links ]
4. IBGE. Abate de animais, produção de leite, couro e ovos. http://www.ibge.gov.br/home/estatistica/indicadores/ agropecuaria/producaoagropecuaria/abate-leitecouro- ovos_201401_2.shtm. 2014. [ Links ]
5. Brasil. Ministério da Agricultura Pecuária e Abastecimento. Instrução Normativa n. 42, de 20 de dezembro de 1999. Plano Nacional de Controle e Resíduos em Produtos de Origem Animal. Brasília. 1999. [ Links ]
6. IBGE. Instituto Brasileiro de Geografia e Estatística. Pesquisa de orçamentos familiares 2008–2009. http://www.ibge.gov.br/home/estatistica/populacao/con dicaodevida/pof. 2011. [ Links ]
7. Picinin LCA, Cerqueira MMOP, Vargas EA, Lana AMq, Toaldo IM and Bordignon-Luiz MT. Influence of climate conditions on aflatoxin M1 contamination in raw milk from Minas Gerais State, Brazil. Food Control. 2013; 31:419-424.
8. Van Egmond HP, Paulsch WE, Veringa HA and Schuller PE. The effect of processing on the aflatoxin M1 content of milk and milk products. Arch Inst Pasteur Tunis. 1977; 54:381-390.
9. Brasil. Agência Nacional de Vigilância Sanitária. Resolução RDC nº 7, de 18 de fevereiro de 2011. Dispõe sobre limites máximos tolerados (LMT) para micotoxinas em alimentos. Diário Oficial da União, 22 de fevereiro de 2011. 2011. [ Links ]
10. Commission Regulation. EU n. 165/2010 of 26 February 2010. Amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. 2010.
11. Atanda O, Oguntubo A, Adejumo O, Ikeroah J and Akpan I. Aflatoxin M1 contamination of milk and ice cream in Abeokuta and Odeda local governments of Ogun State, Nigeria. Chemosphere. 2007; 68:1455– 1458.
12. Herzallah SM. Determination of aflatoxins in eggs, milk, meat and meat products using HPLC fluorescent and UV detectors. Food Chem. 2009; 114:1141-1146. [ Links ]
13. El Marnissi B, Belkhou R, Morgavi DP, Bennani L and Boudra H. Occurrence of aflatoxin M1 in raw milk collected from traditional dairies in Morocco. Food Chem Toxicol. 2012; 50:2819–21.
14. Santos AL, Bando E and Machinski Junior M. Ocorrência de aflatoxina M1 em leite bovino comercializado no estado do Paraná, Brasil. Semin-Cienc Agrar. 2014; 35:371-374.
15. Oliveira CP, Soares NFF, Oliveira TV, Baffa Junior JC and Silva WA. Aflatoxin M1 occurrence in ultra high temperature (UHT) treated fluid milk from Minas Gerais/ Brazil. Food Control. 2013; 30:90-92.
16. Oliveira CA, Rosmaninho J and Rosim R. Aflatoxin M1 and cyclopiazonic acid in fluid milk traded in São Paulo, Brazil. Food Addit Contam. 2006; 23:196-201.
17. Shundo L, Navas SA, Lamardo LCA, Ruvieri V and Sabino M. Estimate of aflatoxin M1 exposure in milk and occurrence in Brazil. Food Control. 2009; 20:655-657.
18. IPCS/GEMS. International Programme on Chemical Safety. Food Euro workshop on reliable evaluation of low level contamination of food, Appendix 5, Kulmbach, Germany, May 1995.
19. Hassan HF and Kassaify Z. The risks associated with aflatoxins M1 occurrence in Lebanese dairy products. Food Control. 2014; 37:68-72.
20. Duarte SC, Almeida AM, Teixeira AS, Pereira AL, Falcão AC, Pena A and Lino CM. Aflatoxin M1 in marketed milk in Portugal: Assessment of human and animal exposure. Food Control. 2013; 30:411-417. [ Links ]
21. Cano-Sancho G, Marin S, Ramos AJ, Peris-Vicente J and Sanchis V. Occurrence of aflatoxin M1 and exposure assessment in Catalonia (Spain). Rev Iberoam Micol. 2010; 27:130-135. [ Links ]
22. JECFA. JECFA - Joint FAO/WHO Expert Committee on Food Additives. Safety evaluation of certain mycotoxins in food. In Prepared by the 56th meeting of the food additives Series No. 47. http://www.inchem. org/documents/jecfa/jecmono/v47je01.htm. Retrieved in 10.09.14 2001.
23. Kuiper-Goodman T. Uncertainties in the risk assessment of three mycotoxins: aflatoxin, ochratoxin and zearalenone. Can J Physiol Pharm. 1990; 68:1017-1024. [ Links ]