Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos Latinoamericanos de Nutrición
versión impresa ISSN 0004-0622
ALAN vol.66 no.3 Caracas set. 2016
Antioxidant, cytotoxic and alpha-glucosidase inhibition activities from the Mexican berry Anacahuita (Cordia boissieri)
Ezequiel Viveros-Valdez, Carlos Jaramillo-Mora, Azucena Oranday-Cárdenas, Javier Morán-Martínez, Pilar Carranza-Rosales.
Universidad Autónoma de Nuevo León, Facultad de Ciencias Biológicas, San Nicolás de los Garza, Nuevo León. México. Instituto Mexicano del Seguro Social, Centro de Investigación Biomédica del Noreste, Monterrey, Nuevo León. México.
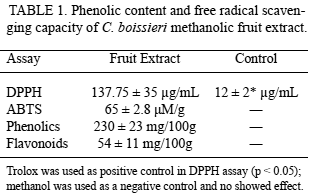
SUMMARY: This study describes the total phenolic and flavonoid content as well as cytotoxic, alpha-glucosidase inhibition and antiradical/antioxidant potential of extracts obtained from the edible fruits of Cordia boissieri, which is widely distributed throughout northeastern Mexico. Phenolic and flavonoid content were evaluated by means of the Folin-Ciocalteu method and aluminum chloride colorimetric assay respectively. The antiradical/antioxidant activity was determined by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging and Trolox Equivalent Antioxidant Capacity (TEAC) assays. Cytotoxic activity was assessed by means of human cancer cell lines (MCF-7 and HeLa), alpha-glucosidase inhibition was determined by colorimetric assay using p-Nitrophenyl α-D-glucopyranoside (PNPG) as a substrate. Results indicate that extract of C. boissieri fruit has a good antioxidant potential to show a EC50: 137.76 ± 35 μg/mL and 65 ± 2 μM/g in the DPPH and TEAC assays respectively, inhibitor of the enzyme alpha-glucosidase involved in sugar uptake (IC50: 215.20 ± 35 μg/mL), cytotoxic activities against MCF-7 (IC50: 310 ± 42 μg/mL) and HeLa (IC50: 450.4 ± 21 μg/mL) cancer cell lines as well as an important phenolic content with 230 ± 23 mg/100g and 54± 11 mg/100g of phenols and flavonoids totals respectively. These results point towards an interesting potential for the fruits of C. boissieri as chemopreventive properties and expand the possibilities for agro-industrial uses.
Key words: Fruits, antioxidants, alpha-glucosidase, chemopreventive.
Actividad antioxidante, citotóxica e inhibidora de la alpha glucosidasa por la baya mexicana Anacahuita (Cordia boissieri).
RESUMEN: Este estudio describe el contenido de fenoles y flavonoides totales, el efecto citotóxico, la inhibición de la enzima alfaglucosidasa y el potencial antirradical/ antioxidante del extracto obtenidos a partir de los frutos de Cordia boissieri, especie distribuida por todo el noreste de México. El contenido de fenoles y flavonoides totales se determinó por medio de los métodos de Folin-Ciocalteu y cloruro férrico respectivamente. La actividad antirradical / antioxidante se determinó mediante el secuestro del radical libre 2,2-difenil-1-picrilhidrazil (DPPH) y el ensayo de Capacidad Antioxidante Equivalente al Trolox (CAET). La actividad citotóxica se evaluó sobre las líneas celulares de cáncer humano (MCF-7 y HeLa), para determinar la inhibición de la enzima alfa-glucosidasa se utilizó el ensayo colorimétrico utilizando como sustrato p-Nitrofenil-α-D-Glucopiranósido (PNPG). Los resultados indican que el extracto del fruto de C. boissieri tiene un buen contenido de antioxidantes al mostrar una CE50 de 137.76 ± 35 μg/mL y de 65 ± 2 μM/g en los ensayos de DPPH y CAET respectivamente, un efecto inhibitorio interesante sobre la enzima alfa-glucosidasa, implicadas en la absorción de azúcar (CI50: 215.20 ± 35 μg/mL), efecto citotóxico contra las células cancerosas MCF-7 (CI50: 310 ± 42 μg/mL) y HeLa (450.4 ± 21 μg/mL), así como un importante contenido compuestos fenólicos con 230 ± 23 mg / 100g y 54 ± 11 mg / 100 g de fenoles y flavonoides totales, respectivamente. Estos resultados sugieren el potencial del fruto de C. boissieri como una fuente importante de compuestos quimiopreventivos y amplían las posibilidades para su aprovechamiento agroindustrial.
Palabras clave: Frutos, antioxidantes, alpha-glucosidasa, quimopreventivo.
Recibido: 15-04-2016
Aceptado: 27-06-2016
INTRODUCTION
In recent years there has been a growing trend of studies related to the bioactive properties and compounds present in plant foods; this is mainly due to scarce reports about deleterious side effects associated with their consumption (1,2). In fact, different investigations have shown that increased consumption of fresh fruits is related with a lower incidence of disease, particularly of degenerative ailments associated to the ageing process, including cancer prevention (3, 4).
Consumption of berries has increased over the last years, and different kinds of berries are being widely consumed across many countries, given their high contents of bioactive compounds and their health-beneficial effects (5). Their protective mechanisms are attributed to the presence of natural antioxidants, such as flavonoids, which help to scavenge free radicals and reactive oxidants in the body (6). In this context, the genus Cordia has been screened for biological activities (7, 8), however, the fruits of Cordia boissieri (Mexican anacahuita) remains unstudied, and in spite of they are used in traditional medicine to treat diseases such as coughs and colds (9). This is of particular interest, given that the species is common in the northeast regions of Mexico and it is not exploited. Cordia boissieri is a native North American evergreen tree which reaches 6 meters in height with a 3 to 4.5- meters spread, has silvery green leaves with a velvety texture, and white flowers appear year- round, these are followed by yellow-green fruits with a sweet flesh relished by birds and other wildlife; one of the major forms of human consumption is like jellies (10). The main objectives of this study were to evaluate the total phenolic and flavonoid content, antioxidant and α-Glucosidase inhibitory potential, as well as cytotoxic activity of the fruits from C. boissieri.
MATERIALS AND METHODS
Preparation of the fresh fruit extract
The fruits of Cordia boissieri were obtained from trees located in the municipality of San Nicolás de los Garza, Nuevo León (México) in a random sampling between the months of October and November of 2014. Taxonomic identification was provided by Dr. Marco Antonio Guzmán Lucio and a voucher specimen (Accession Number: 26307) was deposited in the FCBUANL herbarium.
The fresh fruits of C. boissieri were ground to a pulp and mixed with water 10% w/v. The juice (300 mL) was passed through an Amberlite XAD-7 column, then the column was washed with distilled water and eluted with 900 Ml methanol, the organic extract was concentrated in vacuo to dryness; the Amberlite-retained namely C. boissieri methanolic fruit extract (1.7 g) was stored at 4° C until use (11).
Chemicals and reagents
All reagents were of analytical grade and obtained from Sigma Aldrich Chemical Co. (Saint Louis, MO, USA), except the minimal essential medium (MEM) and fetal bovine serum, both were obtained from Invitrogen (Grand Island, NY, USA).
Determination of total phenolics
Total phenolic content was determined using the Folin-Ciocalteu reagent as described by Singleton and Rossi (12) with some modifications. One hundred μL of each sample, 250 μL of Folin-Ciocalteus reagent (1 N), 1250 μL of sodium carbonate (20%) and 400 μL of distilled water were placed into test tubes. The contents of each tube were homogenized and incubated for 2 h. The absorbance of each mixture was measured at 760 nm, with gallic acid as a standard. Total phenolic content was expressed as mg of gallic acid eq./100g of fresh fruit.
Determination of the flavonoid content
Flavonoid content was determined by use of the aluminum chloride colorimetric assay (+). The fruit extract (250 μL) or standard solution of catechin (50–500 mg/L) was mixed with 1,250 μL deionized water and 150 μl NaNO2. After standing at room temperature for 5 min, 150 μl of water solution of AlCl3 (2%) was added to the solution, followed by 500 μL of 1 M of NaOH after another 5 min. The absorbance in the reaction mixture was measured at 415 nm. Results were expressed as mg (+) Catechin eq. /100g of fresh fruit (13).
Free radical scavenging activity
The Trolox equivalent antioxidant capacity (TEAC) of the extracts was determined by the ABTS [2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)] cation radical discoloration assay (14) and the values are reported as μM Trolox/g of extract. The method is based on the consumption of the preformed ABTS+ in the presence of potassium persulfate followed at the maximum absorption of 734 nm. Addition of antioxidants to ABTS+ reduces it to ABTS. The assay was performed on 96 wells microplates, the absorbance of ABTS+ was adjusted to 0.70 ± 0.02. The decrease of the absorption was measured after 6 min. To determine the antiradical/antioxidant activity, 100 μl of DPPH (2 mg/L) were mixed with 100 μl of serial dilutions of the test solution in 96-well microplates; MeOH and Trolox® were used as a negative and positive controls, respectively. The decrease in absorbance at 517 nm was measured (13).
Cytotoxic Assay
The cancer cell lines MCF-7 and HeLa (American Type Culture Collection No. HTB-22 and CCL-2 respectively) were grown in minimal essential medium (MEM) with 10% fetal bovine, penicillin [100 U/mL], streptomycin [100 μg/mL] and incubated in an atmosphere of 5% CO2 at 37°C. Cytotoxic assays were performed in 96-well microplates containing 5 × 103 cells/well. Cell cultures were exposed to different concentrations of the fruit methanolic extract for 48 h. Cell viability was measured by WST-1 assay after treatment, to do this, 10 μL of WST-1 were added to each well and after 90 min of incubation, and the absorbance was measured at 450 nm. Taxol was used as positive control, and cell culture medium, and 1% DMSO were used as negative controls.
Alpha-Glucosidase Inhibition Assay
A modified version of the protocol described by Yang et al. (15) was employed in this study, wherein Saccharomyces cerevisiae α-Glucosidase (E.C.3.2.1.20) was suspended in 0.25 M phosphate buffer (pH 6.5). A separate 5 mM p-Nitrophenyl α-D-glucopyranoside (pNPG) solution was prepared using the same buffer. A sample of fruit methanolic extract was added to the enzymatic solution and allowed to incubate for 10 minutes at 37°C. Afterwards, the pNPG solution was added to the enzymatic-plant extract solution and allowed to incubate for additional 45 minutes at 37°C. Once the incubation period was over, the reaction was stopped by the addition of a 1 M solution of Na2CO3. Acarbose was used as positive control. The test solution was then analyzed using a spectrophotometer adjusted at 405 nm in order to evaluate enzymatic activity.
Statistical analysis
All data are given as the mean ± SD of three measurements. The concentration of the samples that inhibited 50% of cell growth (IC50), enzymatic reaction or half maximal effective concentrations (EC50) in DPPH radical scavenging activity was calculated from the log-dose inhibition growth curve obtained by a nonlinear regression algorithm, all values were compared by paired t tests using SPSS (Version 10 for Windows, SPSS Inc., Chicago, IL), p<0.05 was considered significant.
RESULTS
Phenolic compounds are extremely diverse in the plants. In the fruits, the phenolic compounds are related with sensory quality and in recent years they have gained interest for its beneficial effect protector to prevent damage by free radicals (16). The structures of this secondary metabolites contain a polyphenol structure with numerous hydroxyl groups that can donate electrons and thus stabilize free radicals. Exist numerous rapid techniques quantification of phenolic compounds by colorimetric assays, in this work it was used an extrapolation of our results obtained with phosphomolybdic-phosphotungstic acid reagent for total phenolics, the calibration equation was y = -0.0023+0.0057x (n = 3, r2 = 0.996) for gallic acid and a optimize spectrophotometric method based on flavonoid-aluminum chloride (AlCl3) complexation to determine the total flavonoid content, the calibration equation was y= 0.1187x-0.2218 (n = 3, r2 = 0.843) for catechin. Table 1 shows the phenolic content and free radical scavenging capacity of C. boissieri fruit extract, based in DPPH assay although there is a significant difference between the antioxidant activities of the fruit methanolic extract compared with trolox (water-soluble vitamin E analogue), it is important to mention that the trolox is a pure compound, while the extract is a mixture of compounds not determined. While for the cytotoxicity assay, the viability of each cell type was examined by the WST-1 reduction assay method. We have previously examined the relationship between culture cell density and its ability to form formazan (data not shown). The inhibition rate in Figure 1 displays the cytotoxicity of C. boissieri fruit extract at various concentrations after 48 h, which was estimated from the cell population of the control and cell populations after treatment. The cytotoxicity show a marking effect against on MCF-7 compared with HeLa cells, the taxol (paclitaxel), a natural antitumor compound, was more cytotoxic against cancer cell lines. The inhibition enzymatic assay showed that the fruits extract of C. boissieri against alpha glucosidase enzyme (Figure 2) depended on their concentration, the acarbose an anti-diabetic drug used to treat type 2 diabetes mellitus have more significant inhibition activity against alpha glucosidase than the fruit extract, but again, it is important note that this is a pure substance.

DISCUSSION
Many methods are used to evaluate antioxidant activities from natural compounds in foods. Two free radicals which are commonly used to assess antioxidant activity in vitro are 2, 2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 2, 2-diphenyl-1-picrylhydrazyl (DPPH), both methods employ the principle of a synthetic colored free radical, and the ability of a sample to scavenge the radical monitored by spectrophotometer. The ABTS assay is interesting in organic extracts because the wavelength absorption at 734 nm eliminates color interference, this assay is based on the decreasing absorbance changes of a blue/green to a colorless solution; whereas the DPPH assay is based on the reduction of the purple to yellow (17). According to our results, the Mexican anacahuita has good antioxidant potential with EC50 = 137.75 ± 35 μg/mL when compared with other wild un-common fruits like: Litchi chinensis (102 μg/mL), Lonicera caerulea (76.14 ± 4.04 to 134.92 ± 4.62 μg/mL), Physalis alkekengi (248 μg/mL) and vegetables like: Moringa oleifera (376 μg/mL), Chenopodium álbum (454.7 μg/mL) Caralluma tuberculata (695.7 μg/mL) (18- 21). ABTS results, on the other hand, were low (6.5 ± 0.28 μM/g) when compared to the Brazilian berries (6.3 ± 0.2 to 125 ± 9.7 μM/g). The same standing applies when phenolic content is compared to those same berries, rating a medium content according to the standards they employed. Total phenolic content is similar or superior to that exhibited by wild berries and cultivars (22, 23). On the other side, alpha-glucosidase is one of the enzymes responsible for breaking down carbohydrates to smaller sugar particles, like glucose, to facilitate their absorption. Alphaglucosidase inhibitors block by competitive and reversible interaction these intestinal enzymes; they slow the digestion of carbohydrates and delay glucose absorption. This results in a smaller and slower rise of blood glucose levels following meals, and throughout the day, for this reason it is important to study alpha glucosidase inhibitors as possible nutraceuticals (24); in this regard, the anacahuita showed moderate inhibitory activity (Figure.1) when compared to the blueberries used in the previously mentioned report, however inhibition was on par or superior when compared to that displayed by root extracts of four species of Flemingia (25), as well as when compared to controls. The cytotoxic effects of C. boissieri fruits against HeLa cells it was good (Figure.2) if is compared with previous reports of non-traditional fruits and analyzed under similar conditions, like: Pyracantha coccinea (IC 500 μg/mL), Zosima absinthifolia (IC 1 mg/mL), Cipadessa baccifera (133 μg/mL), Solanum erianthum (142 μg/mL) (26-29) on the other side, the methanolic fruit extract of C. boissieri was poorly active against MCF-7 cell line in comparative with fruit extracts of B. racemosa (57 μg/mL) and H. sabdariffa (112 μg/mL), but good when compared to malay apple (Syzygium malaccense with IC50=632.3 μg/ml) (30,31). Taking into account the above the cytotoxicity of methanolic extracts from C. boissieri (Mexican anacahuita) is moderate compared to other uncommon fruits, however, it could be a good source of compounds with antioxidant activity and potential alpha-glucosidase inhibitory effect
CONCLUSION
Perspectives of utilization of Latin American native fruits are relatively big. The research accomplished indicated the fruits of C. boissieri (Mexican anacahuita) as potential source of bioactive substances that could be considered like a possible nutraceutical product.
AKNOWLEDGEMENTS
The autors are grateful to PROMEP, for funding the project 103.5/13/6044.
REFERENCES
1. Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, Li HB. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20(12): 21138-21156. [ Links ]
2. Ozturk A, Demirsoy L, Demirsoy H, Ozturk S. Quality characteristics and phenolic compounds of european pear cultivars. Afr J Tradit Complement Altern Med 2015, 12 (5): 63-69. [ Links ]
3. Da Silva Pereira AC, Wurlitzer NJ, Dionísio AP, Lacerda Soares MV, Rocha Bastos MS, Alves RE, Montenegro Brasil I. Synergistic, additive and antagonistic effects of fruit mixtures on total antioxidant capacities and bioactive compounds in tropical fruit juices. Arch Latinoam Nutr 2015, 65(2): 119-127. [ Links ]
4. Kanera IM, Bolman CA, Mesters I, Willems RA, Beaulen AA, Lechner L. Prevalence and correlates of healthy lifestyle behaviors among early cancer survivors. BMC Cancer 2016, 16(1):4. [ Links ]
5. Abrantes Sarmento JD, Dantas de Morais PL, Irael de Souza F, Ramos da Costa L, de Assis Melo NJ. Bioactive compounds and antioxidant activity of Ximenia americana coming from different collection sites. Arch Latinoam Nutr 2015, 65(4): 263-270. [ Links ]
6. Sakulnarmrat K, Konzcak I. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chem 2012, 134(2012): 1011-1019. [ Links ]
7. Jamkhande PG, Barde SR, Patwekar SL, Tidke PS. Plant profile, phytochemistry and pharmacology of Cordia dichotoma (Indian cherry): A review. Asian Pac J Trop Biomed 2013, 3(12): 1009-1012. [ Links ]
8. Hernandez T, Canales M, Teran B, Avila O, Duran A, Garcia AM, Hernandez H, Angeles-Lopez O, Fernandez-Araiza M, Avila G. Antimicrobial activity of the essential oil and extracts of Cordia curassavica (Boraginaceae). J Ethnopharmacol 2007, 111(1): 137–141. [ Links ]
9. Martínez M. Las plantas medicinales de México. 6a ed, México: Ediciones Botas; 1967. [ Links ]
10. Alvarado Vázquez M, Rahim Foroughbakhch P, Jurado Y Enrique, Rocha E Alejandra. Caracterización morfológica y nutricional del fruto de anacahuita (Cordia boissieri A. DC.) en dos localidades del Noreste de México. Pitón 2014, 85-90. [ Links ]
11. Quispe C., Viveros-Valdez E, Yarleque JA, Arones MR, Paniagua JC, Schmeda-Hirschmann G. High speed centrifugal countercurrent chromatography (HSCCC) isolation and identification by LC-MSn analysis of the polar phenolics from Vasconcellea quercifolia. J Chil Chem Soc 2013, 58 (3):1830-1835. [ Links ]
12. Singleton VL, Rossi Jr, JA. Colorimetric of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am J Enol Viticult 1965, 16(3): 144–158. [ Links ]
13. Viveros-Valdez E, Rivas-Morales C, Carranza-Rosales P, Mendoza S, Schmeda-Hirschmann G. Free radical scavengers from the Mexican herbal tea poleo (Hedeoma drummondii). Z Naturforsch c 2008, 63(5-6): 341-346. [ Links ]
14. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio Med 1999, 26 (9-10): 1231-1237. [ Links ]
15. Yang Z, Wang Y, Wang Y, Zhang Y. Bioassayguided screening and isolation of α-Glucosidase and tyrosinase inhibitors from leaves of Morus alba. Food Chem 2011, 131(2011): 617-625. [ Links ]
16. Oliveira de Lacerda de L, Carvalho Veras de M, Melo L. Health promoting and sensory properties of phenolic compounds in food. Revista Ceres 2014, 61: 764-779. [ Links ]
17. Ademola Ayeleso O, Oluwafemi Oguntibeju O, Nicole Brooks L. In Vitro Study on the Antioxidant Potentials of the Leaves and Fruits of Nauclea latifolia. Scientific World Journal 2014, 2014: 437081. [ Links ]
18. Prakash D, Upadhyay G, Pushpangadan P, Gupta C. Antioxidant and free radical scavenging activities of some fruits. J Complement Integr Med 2011, 8(1): Article 23. [ Links ]
19. Laczkó-Zöld E, Zupkó I, Réthy B, Csedo K, Hohmann J. Antioxidant activity of the fruits and hydrophilic compounds of Physalis alkekengi. Acta Pharm Hung 2009, 79(4):169-73. [ Links ]
20. Rop O., Řezníček V, Mlček J, Juríková T, Balík J, Sochor J, Kramářová D. Antioxidant and radical oxygen species scavenging activities of 12 cultivars of blue honeysuckle fruit. Hort Sci 2011, 38(2): 63-70. [ Links ]
21. Khattak KF. Nutrient composition, phenolic content and free radical scavenging activity of some uncommon vegetables of Pakistan. Pak J Pharm Sci 2011,24(3):277-83. [ Links ]
22. Rufino MSM, Alves RE, de Brito ES, Pérez-Jiménez J, Saura-Calixto F, Mancini-Filho J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem 2010, 121(2010): 996–1002. [ Links ]
23. Wang SY, Camp MJ, Ehlenfeldt MK. Antioxidant capacity and a-glucosidase inhibitory activity in peel and flesh of blueberry (Vaccinium spp.) cultivars. Food Chem 2012, 132(2012): 1759–1768. [ Links ]
24. Podsędek A, Majewska I, Redzynia M, Sosnowska D, Koziołkiewicz M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J Agric Food Chem 2014, 62(20): 4610-4617. [ Links ]
25. Hsieh P-C, Huang G-J, Ho Y-L, Lin Y-H, Huang S-H, Chiang Y-C, Tseng M-C, Chang Y-S. Activities of antioxidants, α-Glucosidase inhibitors and aldose reductase inhibitors of the aqueous extracts of four Flemingia species in Taiwan. Bot Stud 2010, 51: 293-302. [ Links ]
26. Vahabi L, Monajemi R, Hosseini S.A. The Cytotoxic Effect of Methanolic Extract of Pyracantha coccinea M. Roemer Fruit on Hela cell line, Antioxidant Capacities and Total Phenol Contents of Methanolic and Aquatic Extract of this fruit. Biomed Pharmacol J 2015, 8. [ Links ]
27. Razavi SM, Ghasemiyan A, Salehi S, Zahri F. Screening of biological activity of Zosima absinthifolia fruits extracts. EurAsia J BioSci 2009, 4:25-28. [ Links ]
28. Kavitha KR, Bopaiah AK, Ramakrishnaiah H, Naveen Kumar N. Cytotoxicity of Cipadessa baccifera (Roth.) Miq., on HeLa, Jurkat, MCF-7, and KB cell lines. Int J Pharm Bio Sci 2015, 6(3): 230 – 237. [ Links ]
29. Radhika Mahadev H, Ramakrishnaiah H, Krishna V, Deepalakshmi AP, Naveen Kumar N. Cytotoxic Activity Of Methanolic Extracts of Solanum erianthum D. Don. Int J Pharm Pharm Sci 2014, 7(2):106-108. [ Links ]
30. Norliyana Amran, Anis Najwa Abdul Rani, Roziahanim Mahmud, Khoo Boon Yin. Antioxidant and Cytotoxic Effect of Barringtonia racemosa and Hibiscus sabdariffa Fruit Extracts in MCF-7 Human Breast Cancer Cell Line. Pharmacognosy Res 2016, 8(1): 66–70. [ Links ]
31. Rabeta MS, Chan S, Neda GD, Lam KL, Ong MT. Anticancer effect of underutilized fruits. Int Food Res J 2013, 20(2): 551-556. [ Links ]