Kasmera
versión impresa ISSN 0075-5222
Kasmera v.33 n.2 Maracaibo jul. 2005
Identification of Cryptococcus neoformans isolates using Staib agar without creatinine
Identificación de Aislados de Cryptococcus neoformans Usando Agar Staib sin Creatinina
Nardelli, Vanessa1; Pérez, Celina1; Mata-Essayag, Sofía1; Colella, María Teresa1; Roselló, Arantza1; Hartung de Capriles, Claudia1; Landaeta, María Eugenia2; Olaizola, Carolina1 y Magaldi, Sylvia1
1 Sección de Micología Médica, Instituto de Medicina Tropical, UCV, Caracas. 2Cátedra de Microbiología, Escuela de Medicina Luis Razetti, Universidad Central de Venezuela. Caracas, Venezuela. E-mail: somae50@hotmail.com
Resumen
En micología, el cultivo es la mejor manera de realizar el diagnóstico definitivo de una micosis. Muchos tipos de medios han sido desarrollados y modificados para tal fin, utilizando las propiedades bioquímicas de los hongos para reconocerlos. Los medios más comunes son Sabouraud, agar Malta, agar maíz, agar Papa-dextrosa, y agar Staib. Este último ha sido ampliamente usado para la identificación de levaduras del género Cryptococcus y en otros hongos. El objetivo de este estudio fue demostrar la utilidad del agar Staib y de un medio de Staib modificado, sin creatinina, en el aislamiento e identificación de hongos patógenos. Cuarenta y seis aislados de Cryptococcus neoformans más un control fueron identificados por criterios morfológicos y bioquímicos, usando la prueba de ureasa (agar urea de Christensen) y cultivo en medio Sablac. Las cepas fueron transferidas a placas de agar Staib estandar y de agar Staib sin creatinina. Las placas fueron incubadas a temperatura ambiente (26-28°C) durante una semana y luego fueron evaluadas las características morfológicas de las colonias y producción de fenoloxidasa. En 43 (91.5%) de las cepas estudiadas, se observó producción de fenoloxidasa (presencia de colonias marrones) tanto en el agar estandar como en el agar sin creatinina. Conclusión: el agar Staib sin creatinina es un medio excelente para la identificación de C. neoformans, con las ventajas de un bajo costo y fácil preparación.
Palabras clave: Agar Staib, Guizotia abyssinica, Cryptococcus neoformans, creatinina.
Abstract
In mycology, culture is the best way to demonstrate and identify pathogenic fungi. Many kinds of media have been developed and modified, using fungal biochemical properties to recognize them. The most common media are Sabouraud, Malt Agar, Cornmeal agar, Potato-dextrose agar, and Staib agar. Staib agar has been widely used for identification of yeasts from the genus Cryptococcus and other fungi. The aim of this study was to demonstrate the usefulness of Staib agar, and of a modified Staib medium without creatinine, in the isolation and identification of fungi. Forty-six Cryptococcus neoformans strains plus one control strain were identified by morphological and biochemical criteria, using urease test (Christensen urea agar) and culture in Sablac medium. The strains were then transferred into standard Staib and Staib without creatinine agar plates. Morphological features and phenoloxidase production were evaluated after one week incubation at room temperature (26-28°C). Culture in standard and modified Staib agar yielded brown color colonies (phenoloxidase production) in both media in 43 (91.5%) cases. Staib agar without creatinine is an excellent medium for identification of C. neoformans, with the advantage of a low cost and easy preparation.
Key words: Staib agar, Guizotia abyssinica, Cryptococcus neoformans, creatinine.
Recibido: 15-07-05 / Aceptado: 27-09-05
Introduction
Staib agar medium has been widely used for isolation and identification of yeasts from the genus Cryptococcus. It was developed in 1962, by the German mycologist Friedrich Staib, who thought of a culture medium made of Guizotia abyssinica, an oval black seed used for bird feeding. It is also used in India and Ethiopia for oil production (1, 2).
In his first studies, Staib employed powdered canary droppings, which is used by Cryptococcus neoformans as a nitrogen source. Thus, Staib observed a color change in the colonies, which grew in this medium, turning into a brown color after 4 days of incubation (3).
Later, Staib found that the color variation was not only dependent on the canary droppings, but on the food these birds consumed, principally made of Guizotia abyssinica seeds (3).
These seeds contain cafeinic acid, among other phenolic compounds, in which O-diphenol undergoes an oxidation process by action of the phenoloxidase enzyme, produced by C. neoformans, originating a melanin pigment which covers the yeast wall, thus turning the colonies growing in culture to a dark brown color. This feature differentiates C. neoformans from other yeasts. This reaction was named Brown color effect (BCE) by this author (2, 4). Then, BCE depends on the enzyme diphenoloxidase, an antioxidant, which helps the yeast to survive inside the host by the production of melanin (5).
This medium is prepared with glucose, creatinine and potassium phosphate (6). Creatinine is assimilated by C. neoformans; this is possible only in the presence of thiamine or other compounds like thiazol and pirimidine, which are in the seeds. Creatinine is used by the yeast as nitrogen source, its assimilation is especially important in serotypes B and C, but not A and D. This assimilation has been demonstrated by the presence of a green diffusible pigment (1, 2, 3, 7).
Staib medium is used routinely since the beginning of the AIDS epidemic, which increased the frequency of opportunistic infections, including fungal. Culture of samples (especially sputum) from AIDS patients directly onto this medium shows brown colonies of C. neoformans (5, 7-13).
For these reasons, we started to use this medium in our medical mycology laboratory, for the isolation and identification of C. neoformans in clinical samples from AIDS patients.
Initially, Staib agar was being prepared with the traditional method, but further on in the study there was a failure in the supply of some of the compounds, especially creatinine. For this reason the medium was modified, and prepared without creatinine. Surprisingly, C. neoformans colonies showed a deeper brown color than in the original Staib agar. We decided to continue using this medium with excellent results.
The aim of this study was to assess the usefulness of Staib agar without creatinine for the identification of C. neoformans, by the observation of the presence of pigment, macro and microscopic morphological development and time of growth, using strains from the Medical Mycology Department Culture Collection.
Materials and Methods
After certifying their pureness and viability, forty-six C. neoformans strains from the Medical Mycology Department Culture Collection were identified by morphological and biochemical criteria, using urease test (Christensen urea agar) (14) and culture in Sablac medium, which allows better capsule development. They were also identified with the Microscan Dade Behring Automated System.
C. neoformans (WC 1400 IMT) and Candida albicans (ATCC 90028) were used as urease positive and negative control isolates, respectively.
Additionally 5 isolates of Candida dubliniensis (S2-5, S2-6, S2-14, S636 and S645) from the Health Science Center, University of Texas (supplied by Dr. Anette Fothergill) were used to assess their development in Staib agar with and without creatinine.
All strains were then transferred into standard Staib and Staib without creatinine agar plates (two isolates per plate), they were incubated at room temperature (26-28°C) for one week, and then the morphological features were evaluated.
Canavanine-glycine-bromotimol blue (CGB) medium (15) was used to identify Cryptococcus isolates. All isolates were inoculated in this medium and incubated at room temperature for 5 days. Interpretation of results: yellow color: C. neoformans var neoformans, dark blue color: C. neoformans var gattii.
Descriptive statistics were used to analyze data obtained, using frequency tables, and percentage and sensitivity determination.
Results
During the course of this study we were forced to use Staib medium without one of its main components, creatinine. The experiments were continued using the modified medium. Later in the study, we were able to find the creatinine and nevertheless decided to use both kinds of Staib medium, the original and the modified.
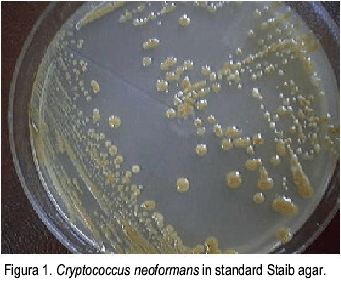
Forty-seven C. neoformans strains used for the study were tested for urease production, being all positive. The same strains were tested for phenoloxidase production, culturing in standard and modified Staib agar. They yielded brown color colonies in both media in 43 (91.5%) cases (Figura 1, 2). In 4 (8.5%) of the studied strains, the colonies remained cream color, in spite of being urease positive and identified by automated Microscan method (Table 1).
Table 1. Phenotypic differences of Cryptococcus neoformans in Staib agar with and without creatinine.
| Media | Diffusible Pigment | Colony | Strains | Microscopic Features | Growth Time (Hours) | Brown color colonies (Days) | Growth Temperature (°C) |
|
| No | Light brown | 33 | Encapsulated yeasts | 48-72 | 5-8 | 26-28 |
| Standard |
| Cream | – | – | – | – | – |
| Staib |
| Light brown | 10 | Encapsulated yeasts | 24-48 | 5-8 | 26-28 |
| Green | Cream | 4 | Encapsulated yeasts | 24-48 | – | 26-28 | |
| Staib without | No | Dark brown | 43 | Encapsulated yeasts | 48-72 | 5-8 | 26-28 |
| Creatinine |
| Cream | 4 | Encapsulated yeasts | 48-72 | – | 26-28 |
From the 47 isolates inoculated in CGB medium, only one showed dark blue color, which suggested C. neoformans var. gattii. This isolate came from a HIV seronegative patient. In standard Staib agar, colonies were light brown color, without green diffusible pigment; in Staib without creatinine, colonies were dark brown.
Five C. dubliniensis strains were inoculated on standard Staib agar and Staib agar without creatinine. In standard Staib agar, all 5 isolates showed the same characteristics: rough colonies, and formation of chlamydoconidea. On the other hand, on Staib agar without creatinine, neither of the isolates showed these features. Instead, colonies were smooth, and no chlamydoconidea were formed.
Discussion
In mycology, culture is the best method used to demonstrate the presence of pathogenic fungi. This is obtained by culturing a clinical sample in a nutrient rich medium, which feeds the fungus, in favorable temperature and time conditions, until it can be seen and identified.
During the history of mycology dozens of culture media have been developed, which have been made to highlight the morphological properties of different pathogenic fungi. The most common are Sabouraud agar, Malt agar, Cornmeal agar, Potato-dextrose agar, and Staib agar (6, 16, 17).
Staib in 1962, developed Staib agar, using Guizotia abyssinica seeds (1). This medium has been very useful in the identification of Cryptococcus strains, and moreover since the beginning of the AIDS epidemic, which has provoked a rise in fungal opportunistic infections.
Forty-three (91.5%) out of 47 C. neoformans strains, showed light brown colonies on the standard Staib medium and dark brown colonies on the modified Staib medium (without creatinine). There is no explanation for the different brown colors observed.
The other 4 (8.5%) strains showed cream color colonies on both media. Being these isolates from a culture collection, there is no information about their original color. Since these strains were 20 years old, and conserved in Castellani medium (distilled water) 18, maybe they could have lost the capacity of production of phenoloxidase in time, which is necessary to produce melanin (2, 5, 19-22). Melanin production is considered one of the main pathogenic factors of C. neoformans (22).
On the other hand, Staib, in 1962 determined that serotypes B and C, but not A and D assimilate creatinine from the medium, evidenced by the presence of a green diffusible pigment (1). In our study we found 14 strains that produced green diffusible pigment. We initially presumed that these were serotype B and C strains. However, when the isolates were inoculated in CGB medium, only one showed the characteristically dark blue color of C. neoformans var gattii. CGB medium is very specific for identification of this variety (15), and it is also useful for epidemiological studies of Cryptococcal disease.
Bennett et al. (1978) also found that certain C. neoformans var gattii (serotypes B and C) produced a green diffusible pigment in Staib medium. This phenomenon did not occur with C. neoformans var neoformans (serotypes A and D), which have a very slow assimilation of this compound. They explained that when serotypes B and C assimilate creatinine, it is metabolized producing ammonium, turning the medium to an alkaline pH and green color (7, 23, 24). For this reason the term diffusible green pigment used in published papers, actually refers to a color change in the medium provoked by the pH variation, and not to production of pigment. Since we didnt find similar results, we concluded that Staib medium is not a sensitive or specific way of identifying B and C serotypes.
Bennett et al in 1978, reported that not all C. neoformans strains assimilate creatinine in the same way. C. neoformans Serotype A grew more slowly (48-72 hours) in this medium (7), which also made creatinine assimilation slower. In our study, strains that assimilated creatinine between 48 and 72 hours of observation could correspond to serotype A (Table 1). This will be verified in future studies of strain serotyping.
In our revision of international literature, we found that in 1978, Paliwal and Randhawa (21) prepared a simplified Guizotia abyssinica seed medium, eliminating glucose, creatinine, and phosphate. This was evaluated for the isolation and presumptive identification of C. neoformans. The results of this study demonstrated that the simplified or modified G. abyssinica seed agar was superior to the complete medium for the rapid development of the brown pigment by C. neoformans. This finding is very similar to our results.
In the case of C. dubliniensis, Staib agar without creatinine was not useful in identifying these strains, as distinctive features were not present in this medium (rough colonies, presence of chlamydoconidea). For this reason, standard Staib agar continues to be the best medium to identify C. dubliniensis, as well as differentiating it from C. albicans (28).
Therefore Staib agar without creatinine can be used as a cheaper option, for the identification of C. neoformans, especially in laboratories with restricted economic resources.
It can be used for culture of clinical samples from patients with or without immune suppression, or AIDS patients, when cryptococcal disease is suspected, as recommended by other authors in the literature (5, 8, 10-12, 25, 26, 27, 29-31).
This medium was not useful in the morphological identification of C. dubliniensis or recognition of C. neoformans serotypes.
References
(1) Staib, F. Cryptococcus neoformans und Guizotia abyssinica (syn. G. Oleifera D. C.). Z Hyg. Infekt.-Kr. 1962; 148: 466-475. [ Links ]
(2) Nardelli, V.; Pérez, C.; Colella M.T.; Mata S.; Hartung C.; Roselló A.; Olaizola C., Landaeta ME. Identificación de diferentes hongos patógenos al hombre mediante el uso del medio Agar Staib. Antib. Infecc. 2004; 12 (1-2) (In press) [ Links ]
(3) Staib, F. New concepts in the occurrence and identification of Cryptococcus neoformans. Mycopathol Mycol Appl.1963;19:143-145. [ Links ]
(4) Staib, F. The green colour effect (GCE) of the killer strain Cryptococcus laurentii CBS 139 on Staib agar. Mycoses 1999; 42: 103-6. [ Links ]
(5) Golubev, W.I. and Staib, F. Green and brown colour effects in tremellaceous yeast fungi on Staib agar. Mycoses 2000; 43:1-5. [ Links ]
(6) Shields A.B. and Ajello L., Medium for selective isolation of Cryptococcus neoformans. Science 1966;151:208-209. [ Links ]
(7) Bennett J.E.; Kwon-Chung K.J. and Theodore T.S. Biochemical differences between serotypes of Cryptococcus neoformans. Sabouraudia. 1978; 16:167-174. [ Links ]
(8) Bava, A.J., Robles, A.M., Negroni, R., Arechavala, A., Bíarichi, M. Estudio de algunos aspectos epidemiológicos de 253 casos de criptococosis. Rev Iberoam Micol 1997; 14:111-114. [ Links ]
(9) Fernández, O.; Costa, T.; Costa, M.; Soares, A. et al. Cryptococcus neoformans isolados de pacientes com AIDS. Rev Soc Bras Med Trop. 2000; 33:75-78 [ Links ]
(10) Helou, S.; Robles, A.M.;, Arechavala, A.; Bianchi, M.; Negroni R. Criptococosis respiratoria en pacientes VIH positivos. Rev Iberoam Micol. 1999; 16: 126-129. [ Links ]
(11) Vidotto, V.; Defina, N.; Pugliese, A.; Aoki, S.; Nakamura, K.; Takeo K. Effect of different K+ concentrations on cryptococcus neoformans phenoloxidase activity Mycopathologia 2002;156: 171–176. [ Links ]
(12) Mitchel, T.; Perfect, J. Cryptococosis in the era of AIDS -100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995; 8: 515-548. [ Links ]
(13) Staib, F.; Seibold, M.; Antweiler, E.; Frohlich, B.; Weber, S.; Blisse A. The brown color effect (BCE) of Cryptococcus neoformans in the diagnosis, control and epidemiology of C. neoformans infections in AIDS patients. Zhl. Bakt. Hyg. I. Abt. Orig. A. 1987; 266: 167-77. [ Links ]
(14) Staib, F.; Blisse, A.; Randhawa H.S. Creatine and creatinine assimilation by Sporothrix schenckii. Zhl. Bakt. Hyg. I. Abt. Orig. A., 1972; 221: 94-99. [ Links ]
(15) Kwon-Chung, K.J.; Polacheck, I. Bennett JE. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) Cryptococcus neoformans var. gatii (serotypes B and C). J. Clin. Microbiol. 1982; 15(3):535-537 [ Links ]
(16) Borelli, D. Medios caseros para micología. Arch Venez Med Trop Parasitol Med 1962; 4: 301-309. [ Links ]
(17) Lacaz, CD.; Porto, E.; Costa, J. Micologia medica. Chapter 36. 8th ed. Sarvier. Sao Paulo 1991: 557-575 [ Links ]
(18) Hartung, C.; Mata, S. et al. Preservation of fungi in water: 20 years. Mycopathologia 106: 73-79, 1989 [ Links ]
(19) Hartung, C.; Navas, T.; Dolande, M.; Guaimare, G.; Reviakina V. Patogenicidad del Cryptococcus neoformans conservado en agua, según Castellani. Rev Inst Nac Hig Rafael Rangel. 1991; 22:19-22. [ Links ]
(20) Hopfer, R.; Groschel, D. Six-hour pigmentation test for the identification of Cryptococcus neoformans. J Clin Microbiol. 1975; 2: 96-98. [ Links ]
(21) Paliwal, D.K.; Randhawa, HS. Evaluation of a simplified Guizotia abyssinica seed medium for differentiation of Cryptococcus neoformans. J Clin Microbiol 1978; 7: 346-8. [ Links ]
(22) Polak, A. Melanin as a virulence factor in pathogenic fungi. Mycoses 1989; 33: 215-224. [ Links ]
(23) Bennett, J.E.; Kwon-Chung K.J.; Howard DH. Epidemiologic differences among serotypes of Cryptococcus neoformans. Am. J. Epidemiol. 1977;105: 582-586 [ Links ]
(24) Kwon-Chung, K.J.; Polacheck, I. and Bennett, JE. Improved diagnostic medium for separation of Cryptococcus neoformans var neoformans (Serotypes A and D) and Cryptococcus neoformans var gattii (Serotypes B and C). J. Clin. Microbiol. 1982; 15: 535-537. [ Links ]
(25) Bava AJ, Negroni R, Arechavala A, Robles AM, Bianchi M. Cryptococcal associated wíth AIDS in me Muñiz Hospital of Buenos Aires. Mycopathologia. 1997; 140:13-17. [ Links ]
(26) Bava AJ, Negroni R. Características epidemiológicas de 105 casos de criptococosis diagnosticados en la República Argentina entre 1981-1990. Rev. Inst. Med. Trop. Sao Paulo 1992; 34: 335-340. [ Links ]
(27) Tortorano AM, Vivianí MA, Rigoni AL, Cogfiati M, Roverselh A, Pagano A. Prevalence of serotype D in Cryptococcus neoformans isolates from HIV positive and HIV negative patients in ltaly. Mycoses 1997; 40: 397-302. [ Links ]
(28) Mata-Essayag S, Hartung de Capriles C, Sánchez L, Gallardo S, Colella MT, Magaldi S, Reyes H, Ontiveros J, Olaizola C, Suárez R. Aislamiento de Candida dubliniensis en Venezuela. Antib e Infecc. 2002; 10(4): 165-170 [ Links ]
(29) Denning DW, Stevens DA, Hamilton JR. Comparison of Guizotia abyssinica seed extract (birdseed) agar with conventional media for selective identification of Cryptococcus neoformans in patients with acquired immunodeficiency syndrome. J Clin Microbiol. 1990; 28: 2565-7. [ Links ]
(30) Rozembaum R, Rios AJ, Wanke B, Vieira W. Cryptococcus neoformans var gattii in a Brazilian AIDS patient. Mycopathologia. 1990; 112: 33-34. [ Links ]
(31) Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis 1995; 21: 28-34. [ Links ]












 uBio
uBio