Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Latinoamericana de Metalurgia y Materiales
versión impresa ISSN 0255-6952
Rev. LatinAm. Met. Mat. v.22 n.1 Caracas ene. 2002
R. F. E. Guerrero1, R. R. Talavera2, V. M. C. Meneses2.
1.Universidad Iberoamericana, Prolongación Paseo de la Reforma 880, Lomas de Santa Fe, México D.F. 01210, México. rodolfo.estrada@uia.mx
2.Instituto de física UNAM, Departamento de FATA. A.P. 1-1010 Querétaro Qro. 76001, México. rogelior@servidor.unam.mx
ABSTRACT.
In this work a description about the behavior of the porosity in membranes made from cellulose acetate and polyacrylic acid were carried out. In this description a mathematical model which describes the variation of the diameter of the pores was obtained. The results shows that the size of the pores vary as a function of the time employed during a thermal treatment. To obtain the mathematical model experimental data were analyzed by the use of the software Graphical Analysis.
Key words: Pore size, Cellulose acatate, Poly acrilic acid, Membranes.
RESUMEN
En este trabajo se presenta una descripción acerca del comportamiento de la porosidad en membranas hechas a partir de acetato de celulosa y poli (ácidoi acrílico). En esta descripción se obtuvo un modelo matemático que describe la variación del diámetro de los poros. Los resultados mostraron que el tamaño de los poros varía como una función del tiempo de tratamiento térmico. Para obtener el modelo matemático los datos experimentales fueron analizados con el uso del programa Graphical Analysis.
Palabras clave: Tamaño de poro, Acetato de celulosa, poli (ácido acrílico), Membranas.
1. Introduction
The use of polymeric membranes for filtration and separation processes is a field that has attracted the attention from the scientist dedicated to the research in this area of knowledge [1]. This work is the next step from a previous work [2] in which a novel kind of membranes where the porous size can be adjusted at will in the very same membrane was reported. Some investigations has been focused to obtain new materials with adequate properties to be applied in separation of gases, and some experimental and theoretical work has been developed [3]. The use of cellulose acetate to produce membranes with adequate porosity has attracted the attention from the scientific community due, among other reasons, to the important applications as a biological material [4]. Nevertheless, just a few effort has been dedicated to the investigation about the way in which the porosity of a membrane can be controlled [5]. In this work, the results obtained with membranes made from the cross linking of cellulose acetate and polyacrylic acid, shows that the porosity can be controlled varying some parameters as the time of thermal treatment once that the membrane has been formed.
2. Experimental
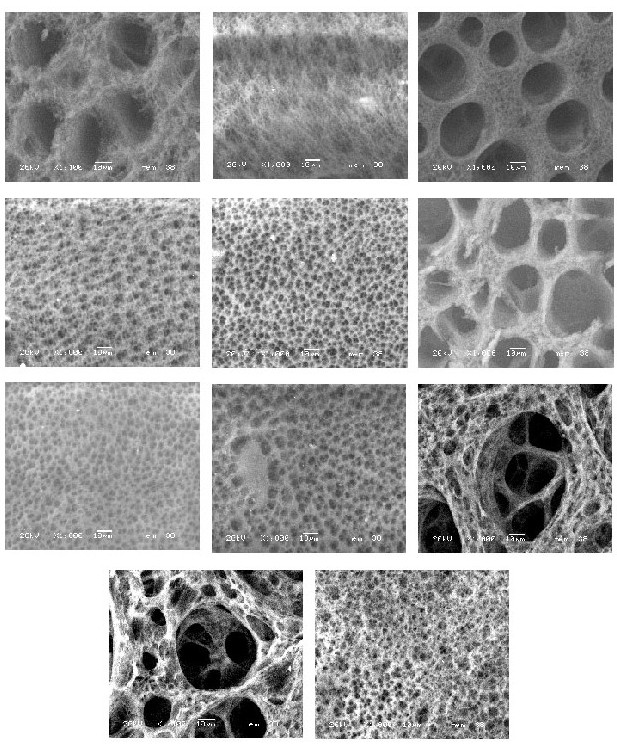
The membranes were obtained from a solution prepared dissolving 8g of cellulose acetate (Fluka Chemika) in 100 ml of glacial acetic acid (Aldrich) at room temperature, once the cellulose acetate has dissolved 10 ml of polyacrylic acid with molecular weight of 30000 g/mol (Aldrich) is added and the solution is heated at a temperature of 60 °C during 30 minutes. Then the solution is allowed to cool down to room temperature under room conditions and is stored and allowed to set for 3 days. To obtain the membranes the solution is cast into a flat glass mold, 10 cm diameter, flouting onto an ice-water mixture at 4 °C for 30 seconds. The whole mold with the solution is immersed into the same water, once that the membrane is formed, is collected from the mold into the cool water, then the membrane is washed in a bath of water at 60 °C varying the time from 0.5 to 5 minutes in regular intervals of 0.5 minutes for each sample, with this procedure 11 membranes was obtained. To determine the behavior of the pore size, the membranes were characterized by using a low vacuum scanning electron microscope (JEOL LV 5900) operated at an acceleration voltage of 20 kV and a pressure of 20 Pa. To investigate the behavior of the porosity, a plot of the diameter of the pores against the time of thermal treatment was made by using the software Graphical analysis.
Fig. 1. Sequence of micrographs obtained by LV SEM.
3. Results
In figures 1A to 1K the images obtained in the microscope are shown, as can be appreciated the diameter of the pores vary as the time of thermal treatment was increased. All the micrographs were taken at 20 Pa of pressure and an acceleration voltage of 20 Kv. Figure A corresponds to the membrane used as reference, the rest of the membranes corresponds to the time intervals from 0.5 minutes To 5 minutes.This variation is presented in table 1.
Table 1. Variation of pore diameter and time
As can bee appreciated from table 1, the variation in pore diameter presents a periodic behavior as the time of thermal treatment is increased. The temperature of thermal treatment and the thicknes of all the membranes was the same.
In figure 2 a plot of the pore diameter against the time of thermal treatment is shown.
Fig. 2. Plot of the pore diameter Vs time of thermal treatment.
In the plot of figure 2 can be better appreciated the periodic variation of the pore size as the time is increased.
The experimental data of the plot in figure 2 can be well fitted to the equation
![]()
Were P is the pore diameter at a particular immersion time t, t0 is the time in which the pore P0 reaches the maximum size.
The values of the parameters in the equation are:
P0 = 120 µm, t0 = 4.4 minutes, a = 14 and b = 2.
In figure 3 a plot of the experimental data with the fit curve is presented.
Fig. 3. Plot of the pore diameter as a function of the immersion time.
From the fitting one can see thet the maximum pore diameter is 60 microns and occur at a time t0 = 2.4 min. From these values the period can be calculated and is T = 1.8 min, the frequency of oscilation is
f = 0.55 rpm.
4. Conclusions
The pores size of a membrane made with the cross linking of cellulose acetate and polyacrylic acid can be controlled varying the time of thermal treatment after the membrane has been formed. This adjustable pore membranes is part of a series of intelligent membranes which are in development.
ACKNOWLEDGEMENTS
The authors would like to thank to materials management of the Instituto Nacional de Investigaciones Nucleares México for their technical support. CONACyT México for the project “Desarrollo de materiales cerámicos para aplicaciones biomédicas” 32605-U.
5. References
1 Yoshihito Osada and Tsutomu Nakagawa, Membrane Science and Technology, Marcel Deeker, USA, (1992), 239-358[ [ Links ]STANDARDIZEDENDPARAG]
2 R.F Estrada,. R. Rodríguez and Castaño V.M, Int, Jour. of Polimeric Materials, in press (2001) [ Links ]
3 F.A. Ruiz-Treviño and D.R. Paul, J. Applied Polym Science Part B., 36, (1998), 1037. [ Links ]
4 S. Park and J. Crank, Difusion in Polymers, Academic Press, N.Y (1968), 277-319[ [ Links ]STANDARDIZEDENDPARAG]
5 Ulrich Merten, Desalination by Reverse Osmosis, MIT, USA, (1966), 55-89. [ Links ]
6 George Odian, Principles of Polymerization, John Wiley, USA, (1981). [ Links ]