Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Latinoamericana de Metalurgia y Materiales
versión impresa ISSN 0255-6952
Rev. LatinAm. Metal. Mater. v.29 n.1 Caracas ene. 2009
An attempt to synthesize mnnb2s4 and the structural characterization of mnnb3s6.
Jines Contreras 1, Belkis Ramírez 1, Luis Nieves 2, Héctor Romero 2, José M. Delgado 1*
1 Lab. de Cristalografía, LNDR-X, Facultad de Ciencias, Universidad de Los Andes, Mérida 5101, Venezuela.
2 Lab. de Magnetismo en Sólidos, Facultad de Ciencias, Universidad de Los Andes, Mérida 5101, Venezuela. * e-mail: migueld@ula.ve
Disponible en: www.polimeros.labb.usb.ve/RLMM/home.html
Abstract
In spite of several attempts at synthesizing MnNb2S4, a II- -III2-VI4 semiconducting material with potentially interesting magnetic properties, MnNb3S6, a closely related compound was always prepared as the majority phase. The material was characterized using X-ray Powder Diffraction data. The refinement of the structure of this material carried out by the Rietveld Method produced results consistent with a previously reported study. The unsuccessful attempts of producing MnNb2S4 raise doubts about the existence of this compound.
Keywords: Semiconductors, X-ray diffraction, Crystal structure
Resumen
A pesar de numerosos intentos realizados en la preparación del MnNb2S4, un material semiconductor del tipo II- -III2-VI4 con propiedades magnéticas potencialmente interesantes, siempre se obtuvo como producto mayoritario MnNb3S6, un compuesto estequiométricamente relacionado. El material obtenido se caracterizó mediante técnicas de difracción de Rayos-X. El refinamiento de la estructura, realizado por el Método de Rietveld, produjo resultados consistentes con un estudio previamente reportado. La imposibilidad de obtener MnNb2S4, luego de numerosos intentos, genera dudas acerca de la existencia de este compuesto.
Palabras Claves: Semiconductores, Difracción de Rayos-X, Estructura Cristalina
Recibido: 11-Jul-2008; Revisado: 05-May-2009; Aceptado: 05-May-2009 Publicado On-Line: 29-Jun-2009
1. INTRODUCTION
Semiconducting compounds of the II- -III2-VI4 family of materials have been extensively studied because of the diverse structural characteristics and the great variety of physical properties of technological interest they display. Particularly, interesting magnetic behaviors such as low dimensionality, spin glass, among other phenomena, have been reported for some of the compounds containing a transition 3d element [1]. These materials are members of one of four possible families of four-fold defect derivatives of the II-VI binaries in which the II cation has been substituted by two or three types of cations and an array of vacancies is introduced (the other families are II2- -IV-VI4, I-III-IV- -VI4 and I-II-V- -VI4) [2].
Structural characterization studies of several members of the II- -III2-VI4 family of semiconductors, based on single crystal and powder diffraction techniques, indicate that they crystallize predominantly in three main structure-types, all with a closed-packed array of anions:
-
A spinel-type structure (a cubic structure with the cations partially occupying the available octahedral and tetrahedral sites in a 2:1 ratio),
-
Two sphalerite-related structures: stannite- and kesterite-derivative structures (tetragonal structures with the cations solely occupying some of the tetrahedral sites).
-
A set of layered structures of the ZnIn2S4-type (different polytypes with the cations partially occupying, in different ratio, the octahedral and tetrahedral sites available).
It has been our interest to expand the knowledge about the structural characteristics of tetrahedral and other related semiconducting compounds. In this work, the results of the attempts to prepare a II-III2- -VI4 compound in the Mn-Nb-S system are presented. Since the compound synthesized in these attempts was in fact MnNb3S6, its structure was characterized by X-ray Powder Diffraction Techniques.
2. EXPERIMENTAL Part
2.1 Synthesis
Several experiments were set up intending to prepare MnNb2S4 using conventional solid state reaction of the constituent elements in stoichiometric proportions. In a typical run, the reaction was carried out in a quartz ampoule evacuated to approximately 10-5 torr, sealed, and gradually heated from room temperature at the following rate: the reacting mixture was heated slowly to 220°C in 10 hours, to 440°C in 15 hours, where it stays during 24 hours. Then, the temperature was raised to 750°C in 15 hours, staying there 5 hours. Finally, it reached 1100°C in 16 hours. The sample was maintained at this temperature for 8 hours. Then, the ampoule was slowly cooled down to 850°C where it stayed for 10 days. It was finally brought to room temperature at a rate of 2°C per minute. A few different heating regimes were also tried. Several attempts performed to grow single crystals of MnNb2S4 using vapor transport techniques were unsuccessful.
2.2 X-Ray Diffraction
X-ray powder diffraction patterns of the most homogeneous products were collected at room temperature with a Siemens D5005 diffractometer, using CuKa radiation and equipped with an incident beam Ge(111)-Johannson-type monochromator (l=1.5406 Å). This instrument was working in a q/2q configuration, at 40 kV and 30 mA. The specimen was scanned in the 2q range of 5°-100°, the scan step was 0.02°, and the time of counting in every step was 40 s.
3. RESULTS AND DISCUSSION
The patterns recorded were processed and analyzed with the package Jade 5.0 (MDI) [3]. A qualitative phase analysis of the diffraction patterns of the products (Figure 1) led to the identification of MnNb3S6, as the major phase after search/match procedures carried out with the JADE program. A large number of peaks of the diffraction patterns recorded are consistent with the peaks contained in entry PDF-4+ No. 00-022-0723 [4], corresponding to the above mentioned phase. A small number of additional weak diffraction maxima, present in most of the patterns registered, suggests the presence of MnS [PDF-4+, No. 00-006-0518] and a NbS2-based misfit layer structure similar to (NdS)1.18NbS2 [PDF-4+, No. 00-081-1272] as minor phases. The number of peaks belonging to MnS in the different patterns was always greater than the number of peaks assigned to the NbS2-based composite.
In spite of several attempts to prepare MnNb2S4 by solid state reaction, this compound was not obtained. In the product of the reactions, the primary phase was always MnNb3S6. It is noteworthy that similar results have been reported by van Laar et al. [5] after trying to prepare compounds of compositions M0.50NbS2 for M = V, Mn, Fe, Co and Ni. These authors reported that in all their attempts with Mn the resulting phase was Mn0.33NbS2; that is, MnNb3S6. It must be noted that Eibschütz et al. [6] reported the preparation and X-ray powder diffraction pattern of several sulfides with composition MNb2S4 (M = Mn, Fe, Co, Ni and Cu). The X-ray patterns were indexed on the basis of an orthorhombic unit cell having about the same unit cell constants as berthierite (FeSb2S4). Later, van Laar and Ijdo [7] indicated that the powder patterns of these compounds could be indexed on the basis of a hexagonal cell. The results of the powder diffraction studies presented in the last two publications have been incorporated in the last release of the Powder Diffraction File, PDF-4+ [4], as entries 00-021-0552 and 00-026-1243, respectively. The Quality Mark assigned by the Editors of the PDF-4+ database to these patterns are: B (Blank) for the first one and O (Low precision) for the second one. Furthermore, in the comment section of entry 00-026-1243, it is stated that the O mark was assigned because of the very bad fit of the data.
On the other hand, the powder diffraction pattern calculated, assuming that MnNb2S4 exhibits the berthierite-structure type, as stated by Eibschütz et al. [6], is notably different to the experimental pattern registered, presented in Figure 1. This fact clearly indicates that it is not true that the structure of berthierite (FeSb2S4) is the base of the structure of the material synthesized by these authors.
The indexing of the most intense diffraction maxima of the patterns performed with the computer program Dicvol04 [8] consistently produced the hexagonal unit cell reported for MnNb3S6 (Table 1). The de Wolf (M20) [9] and Smith-Snyder (F33) [10] figure-of-merits are included.
Table 1. Indexing and evaluation of the pattern recorded for MnNb3S6.
|
| DICVOL06 |
|
| NBS*AIDS83 |
| a | 5.7787(4) Å |
| a | 5.7768 (4) Å |
| c | 12.626(2) Å |
| c | 12.623 (2) Å |
| V | 365.13 Å3 |
| V | 364.82 Å3 |
| M20 | 39.7 |
| M20 | 44.3 |
| F33 | 19.0 (0.0156, 43) |
| F30 | 20.7 (0.017, 85) |
Table 2 contains the results of the evaluation of the entire diffraction pattern and the final unit cell parameters obtained by least-squares refinement using the program NBS*AIDS83 [11].
Table 2. Powder diffraction data obtained after the evaluation with NBS*AIDS83 of the pattern recorded
| 2qobs (°) | dobs (Å) | Int (%) | hkl | 2qcalc (°) | dcalc (Å) | D2q (°) |
| 14.037 | 6.3023 | 100 | 0 0 2 | 14.016 | 6.3117 | -0.021 |
| 17.743 | 4.9934 | 3.80 | 1 0 0 | 17.709 | 5.0029 | -0.034 |
| 19.086 | 4.6450 | 5.9 | 1 0 1 | 19.061 | 4.6509 | -0.025 |
| 27.697 | 3.2173 | 5.4 | 1 0 3 | 27.671 | 3.2202 | -0.026 |
| 28.261 | 3.1544 | 10.1 | 0 0 4 | 28.248 | 3.1558 | -0.013 |
| 30.926 | 2.8883 | 18.8 | 1 1 0 | 30.925 | 2.8884 | -0.001 |
| 31.742 | 2.8159 | 12.6 | 1 1 1 | 31.745 | 2.8156 | 0.003 |
| 34.117 | 2.6251 | 29.5 | 1 1 2 | 34.099 | 2.6265 | -0.018 |
| 36.594 | 2.4529 | 4.1 | 2 0 1 | 36.582 | 2.4537 | -0.012 |
| 37.773 | 2.3790 | 9.5 | 1 1 3 | 37.735 | 2.3813 | -0.038 |
| 39.968 | 2.2533 | 4.2 | 1 0 5 | 39.956 | 2.2539 | -0.012 |
| 42.374 | 2.1307 | 66.7 | 1 1 4 | 42.375 | 2.1307 | 0.001 |
| 42.968 | 2.1027 | 9.7 | 0 0 6 | 42.942 | 2.1039 | -0.026 |
| 47.801 | 1.9007 | 7.4 | 1 1 5 | 47.797 | 1.9009 | -0.004 |
| 53.845 | 1.7008 | 13.6 | 1 1 6 | 53.851 | 1.7006 | 0.006 |
| 54.984 | 1.6682 | 16.1 | 3 0 0 | 55.004 | 1.6676 | 0.020 |
| 57.042 | 1.6128 | 8.4 | 3 0 2 | 57.061 | 1.6123 | 0.019 |
| 58.413 | 1.5782 | 15.4 | 0 0 8 | 58.423 | 1.5779 | 0.010 |
| 60.444 | 1.5299 | 5.2 | 1 1 7 | 60.454 | 1.5297 | 0.010 |
| 62.987 | 1.4741 | 5.0 | 3 0 4 | 62.972 | 1.4744 | -0.015 |
| 64.440 | 1.4443 | 5.3 | 2 2 0 | 64.446 | 1.4442 | 0.006 |
| 64.900 | 1.4352 | 4.4 | 2 2 1 | 64.918 | 1.4348 | 0.018 |
| 66.299 | 1.4083 | 6.3 | 2 2 2 | 66.323 | 1.4078 | 0.024 |
| 67.583 | 1.3846 | 9.0 | 1 1 8 | 67.575 | 1.3848 | -0.008 |
| 71.848 | 1.3125 | 10.3 | 2 2 4 | 71.804 | 1.3132 | -0.044 |
| 75.176 | 1.2625 | 5.5 | 0010 | 75.186 | 1.2623 | 0.010 |
| 80.569 | 1.1910 | 5.2 | 226 | 80.596 | 1.1907 | 0.027 |
| 83.448 | 1.1571 | 6.3 | 1 1 10 | 83.481 | 1.1567 | 0.033 |
| 84.303 | 1.1475 | 11.9 | 3 2 0 | 84.281 | 1.1477 | -0.022 |
| 89.716 | 1.0918 | 4.4 | 4 1 0 | 89.721 | 1.0917 | 0.005 |
| 91.406 | 1.0759 | 5.3 | 4 1 2 | 91.427 | 1.0757 | 0.021 |
| 96.559 | 1.0317 | 8.4 | 4 1 4 | 96.559 | 1.0317 | 0.000 |
| 99.706 | 1.0074 | 5.2 | 3 2 6 | 99.689 | 1.0076 | -0.017 |
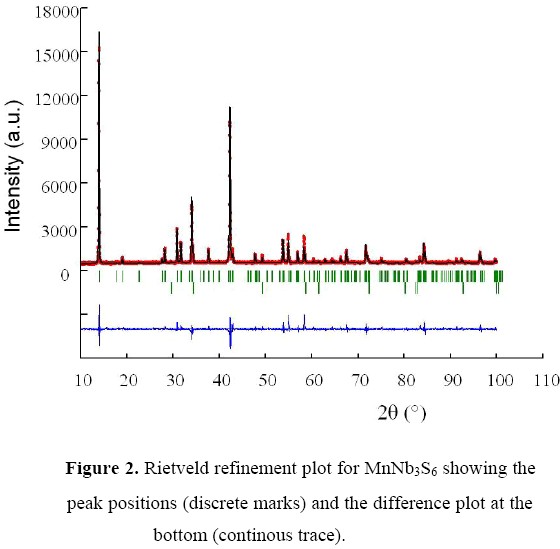
The structure reported by Anzenhofer et al. [12] was used as the starting model in the refinement performed with the program FullProf, as implemented in computer package Winplotr [13]. The background (a fifth order polynomial in 2q) was refined simultaneously with other profile and structural parameters. The peak shapes were modeled with a pseudo-Voight function. MnS was incorporated in the refinement as a second phase. Its crystal structure data were taken from the PDF-4+ database [4] (space group: Fm-3m (N° 225), a = 5.2238(4) Å; Mn and S atoms at 4a and 4b sites, respectively). The data were corrected for preferred orientation (002) due to the presence of platy crystallites, following the Dollase formulism as implemented in Winplotr [13]. The refined pattern is shown in Figure 2. The crystal data obtained are contained in Table 3. The most relevant bond distances and angles are given in Table 4.
Table 3. Crystal data obtained for MnNb3S6 after the Rietveld refinement. Space group: P6322 [No. 182] a = 5.7808(4) Å, c = 12.629(1) Å, V = 365,48(6) Å3
| Atom | Wyc Pos. | x | y | z | Biso(Å2) |
| Mn(1) | 2(c) | 1/3 | 2/3 | 1/4 | 1.0 |
| Nb(1) | 2(a) | 0 | 0 | 0 | 1.0 |
| Nb(2) | 4(f) | 1/3 | 2/3 | 0.9999(4) | 1.0 |
| S(1) | 12(i) | 0.337(3) | 0.009(2) | 0.3718(5) | 1.5 |
Rp = 8.9; Rwp = 12.4; Rb = 14.0; Rexp = 13.1
Table 4. Bond distances and angles of the structure of MnNb3S6.
| Characteristics | Values |
| Bond: Distance (Å) |
|
| Nb1-S1 x 6 | 2.515(2) |
| Nb2-S1 x 3 | 2.490(1) |
| Nb2-S1 x 3 | 2.552(1) |
| Mn1-S1 x 6 | 2.501(1) |
|
|
|
| Atoms: Angle (°) |
|
| S1-Nb1-S1 x 3 | 80.23(3) |
| S1-Nb1-S1 x 3 | 133.12(3) |
| S1-Nb1-S1 x 3 | 136.95(3) |
| S1-Nb1-S1 x 5 | 82.98(3) |
|
|
|
| S1-Nb2-S1 x 3 | 135.92(3) |
| S1-Nb2-S1 x 3 | 80.03(3) |
| S1-Nb2-S1 x 3 | 133.99(3) |
| S1-Nb2-S1 x 3 | 83.91(3) |
| S1-Nb2-S x 3 | 82.31(2) |
|
|
|
| S1-Mn1-S1 x 6 | 86.03(3) |
| S1-Mn1-S1 x 3 | 93.39(3) |
| S1-Mn1-S1 x 3 | 94.55(3) |
| S1-Mn1-S1 x 3 | 179.15(3) |
The atomic disposition in MnNb3S6 can be described based on the structure of NbS2. The relationship between the two cells in the basal plane is shown in Figure 3a. The Mn atoms (Mn0.33) orderly occupy one of the three different octahedral sites available in the NbS2 structure. These octahedral sites correspond to the 2c Wyckoff position of space group P6322 (No. 182): 1/3, 2/3, 1/4. They are depicted in Figure 3b.
4. CONCLUSIONs
In spite of numerous attempts at synthesizing MnNb2S4, a II- -III2-VI4 semiconducting material with potentially interesting magnetic properties, MnNb3S6, a closely related compound was always prepared as the majority phase. These unsuccessful tries cast doubts about the existence of MnNb2S4. The structure of MnNb3S6, the resulting compound, was refined using the Rietveld Method.
5. ACKNOWLEDGEMENTS
This work was supported by CDCHT-ULA (Proyecto C-961-99-05-A) and FONACIT (Proyecto LAB-97000821).
6. REFERENCES
1. Nikirofov KG. Prog. Cryst. Growth Ch. 1999; 39 (1): 1-104. [ Links ]
2. Delgado JM, Crystal Chemistry of Diamond-like and other Derivative Semiconducting Compounds. In: Tomlinson RD, Hill AE, Pilkington RD (eds.), Ternary and Multinary Compounds: Proceedings of the 11th International Conference, Institute of Physics Conference Series, Number 152. Bristol (UK): Institute of Physics Publishing, 1998, 45-50. [ Links ]
3. Jade 6.5, Materials Data Inc., 1224 Concannon Blvd. Livermore, CA 94550, 2003. [ Links ]
4. PDF-4+, International Centre for Diffraction Data, 12 Campus Boulevard, Newtown Square, PA 19073-3272, 2007. [ Links ]
5. Van Laar B, Rietveld HM, Ijdo DJM. J. Solid State Chem 1971; 3 (2): 154-160. [ Links ]
6. Eibschütz M, Hermon E, Shtrikman S. Acta Cryst. 1967; 22 (6): 944-945. [ Links ]
7. Van Laar B. Ijdo DJW, Acta Cryst. 1969; B25 (5): 993-994. [ Links ]
8. Boultif A, Louër D. J. Appl. Cryst 2004; 37 (5): 724-731. [ Links ]
9. de Wolf PM. J. Appl. Cryst 1968; 1 (2): 108-113. [ Links ]
10. Smith GS, Snyder RL. J. Appl. Cryst 1979; 12 (1): 60-65. [ Links ]
11. Mighell AD, Hubbard CR, Stalick JK, NBS*AIDS80: A Fortran Program for Crystallographic Data Evaluation, Tech. Note 1141. Washington DC (USA): National Bureau of Standards, 1981. [ Links ]
12. Anzenhofer K, van Den Berg JM, Cossee P, Helle JN. J. Phys. Chem. Solids 1970; 31 (5): 1057-1067. [ Links ]
13. Roisnel T, Rodríguez-Carvajal J, WinPLOTR: a Windows Tool for Powder Diffraction Patterns Analysis. In: Delhez R, Mittenmeijer EJ (eds.), Materials Science Forum: Proceedings of the 7th European Powder Diffraction Conference (EPDIC 7), volumes 378-381. Barcelona (Spain): Trans Tech Publications Inc., 2000, p.118-123. [ Links ]