Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Latinoamericana de Metalurgia y Materiales
versión impresa ISSN 0255-6952
Rev. LatinAm. Metal. Mater. vol.35 no.1 Caracas jun. 2015
X-ray powder diffraction data and rietveld refinement of the ternary semiconductor chalcogenides AgInSe2 and AgInTe2
Gerzon E. Delgado*1, Asiloé J. Mora1, Carlos Pineda2, Rosario Ávila-Godoy3, Solange Paredes-Dugarte4
1: Laboratorio de Cristalografía, Departamento de Química. Facultad de Ciencias, Universidad de Los Andes, Mérida, Venezuela,
2: Núcleo Universitario Alberto Adriani, Universidad de Los Andes, El Vigía, Venezuela.
3: Laboratorio de Análisis Químico y Estructural de Materiales, Departamento de Física, Facultad de Ciencias, Universidad de Los Andes, Mérida, Venezuela.
4: Laboratorio de Caracterización de Materiales, Instituto de Investigaciones en Biomedicina y Ciencias Aplicadas, Universidad de Oriente, Cumaná, Venezuela.
*e-mail: gerzon@ula.ve
ABSTRACT
The ternary chalcogenides AgInSe2 and AgInTe2 were studied by X-ray powder diffraction structure refinement using the Rietveld method. Both compounds crystallizes with a chalcopyrite structure in the space group I ![]() 2d (N° 122), Z = 4, and unit cell parameters a = 6.0988(2) Å, c = 11.7086(6) Å, V = 435.51(3) Å3 for AgInSe2 and a = 6.4431(4) Å, c = 12.6362(9) Å, V = 524.57(6) Å3 for AgInTe2. Improved X-ray powder diffraction data are reported with figures of merit M19 = 84.0, F19 = 40.7 (0.0071, 66) for AgInSe2, and M20 = 80.8, F23 = 39.0 (0.0075, 79) for AgInTe2.
2d (N° 122), Z = 4, and unit cell parameters a = 6.0988(2) Å, c = 11.7086(6) Å, V = 435.51(3) Å3 for AgInSe2 and a = 6.4431(4) Å, c = 12.6362(9) Å, V = 524.57(6) Å3 for AgInTe2. Improved X-ray powder diffraction data are reported with figures of merit M19 = 84.0, F19 = 40.7 (0.0071, 66) for AgInSe2, and M20 = 80.8, F23 = 39.0 (0.0075, 79) for AgInTe2.
Keywords: Semiconductors, Chalcogenides, Rietveld refinement, X-ray powder diffraction data.
Datos de difracción de rayos-X y refinamiento rietveld de los semiconductores calcogenuros ternarios AgInSe2 y AgInTe2
RESUMEN
Los calcogenuros ternarios AgInSe2 y AgInTe2 se estudiaron mediante refinamiento Rietveld utilizando datos de difracción de rayos-X en muestras policristalinas. Ambos compuestos cristalizan con una estructura tipo calcopirita en el grupo espacial I ![]() 2d, (N° 122), Z = 4, y parámetros de celda unidad a = 6.0988(2) Å, c = 11.7086(6) Å, V = 435.51(3) Å3 para AgInSe2 y a = 6.4431(4) Å, c = 12.6362(9) Å, V = 524.57(6) Å3 para AgInTe2. Se reportan mejores datos de difracción de polvo con figuras de mérito M19 = 84.0, F19 = 40.7 (0.0071, 66) para AgInSe2, y M20 = 80.8, F23 = 39.0 (0.0075, 79) para AgInTe2.
2d, (N° 122), Z = 4, y parámetros de celda unidad a = 6.0988(2) Å, c = 11.7086(6) Å, V = 435.51(3) Å3 para AgInSe2 y a = 6.4431(4) Å, c = 12.6362(9) Å, V = 524.57(6) Å3 para AgInTe2. Se reportan mejores datos de difracción de polvo con figuras de mérito M19 = 84.0, F19 = 40.7 (0.0071, 66) para AgInSe2, y M20 = 80.8, F23 = 39.0 (0.0075, 79) para AgInTe2.
Palabras Claves: Semiconductores, Calcogenuros, Refinamiento Rietveld, Datos de difracción de polvo.
Recibido: 20-01-2014; Revisado: 09-05-2014
Aceptado: 08-07-2014; Publicado: 20-07-2014
1. INTRODUCTION
Chalcogenide semiconductors of the type Ag-In-VI (VI = S, Se, Te) have been studied because of their possible technological applications as photo-voltaic detectors, solar cells, light emitting diodes, modulators, filters and their use in nonlinear optics [1-2]. Following the rules of formation of semiconductor compounds [3], in the system Ag-In- VI, stoichiometric compounds with three compositions can be form: AgInVI2, AgIn3VI5 and AgIn5VI8. These materials belong to the normal structure compounds (I-III-VI2) and the defect structure compounds (I-III3-□-VI5, I-III5-□2-VI8), respectively [4].
From the crystallographic point of view, in the Ag- In-S system, the AgInS2 ternary compound crystallizes in two polymorphs: a tetragonal chalcopyrite-type phase (space group I ![]() 2d) and an orthorhombic wurtzite-like phase (space group Pna21). These phases were simultaneously characterized from a unique X-ray powder diffraction pattern [5]. In the Ag-In-Se and Ag-In- Te systems, the ternaries AgInSe2 [6] and AgInTe2 [7] were reported, at room temperature, in undistorted chalcopyrite-type structures. Both materials have been studied under high pressure and temperature and have been reported to have a firstorder structural phase transition from chalcopyrite structure to a cation-disorder NaCl-like structure [8]. In particular, for these ternaries chalcopyrite phases appear reported only poor quality X-ray powder diffraction data in the Powder Diffraction File PDFICDD [9], as shown in Table 1.
2d) and an orthorhombic wurtzite-like phase (space group Pna21). These phases were simultaneously characterized from a unique X-ray powder diffraction pattern [5]. In the Ag-In-Se and Ag-In- Te systems, the ternaries AgInSe2 [6] and AgInTe2 [7] were reported, at room temperature, in undistorted chalcopyrite-type structures. Both materials have been studied under high pressure and temperature and have been reported to have a firstorder structural phase transition from chalcopyrite structure to a cation-disorder NaCl-like structure [8]. In particular, for these ternaries chalcopyrite phases appear reported only poor quality X-ray powder diffraction data in the Powder Diffraction File PDFICDD [9], as shown in Table 1.
Therefore, in this work, we present the detailed structural characterization of the AgInSe2 and AgInTe2 compounds using Rietveld refinement and reported better X-ray powder diffraction data.
2. EXPERIMENTAL PART
The samples were synthesized by the melt and annealing technique. Stoichiometric quantities of Ag, In and Se (Te) powders with a nominal purity of 99.99 wt% (Fisher Scientific), were evacuated in sealed quartz ampoules and deposited into an one zone furnace, and then submitted to direct fusion. The mixture, en each case, was slowly heated up to 250ºC at a rate of 5ºC/hour. The ampoules were kept at this temperature for a period of 6 hours. Then, the temperature was gradually raised at the same rate up to 590°C. It was kept at this condition for 24 hours. Subsequently, it was heated at 800ºC at 10°C/hour and remained at this temperature for 3 hours. Then, the reacted mixtures were heated up to 1050°C at the same rate. Finally, the furnace was turned off and the ingots were cooled to room temperature in about a day.
Chemical analysis of the resultant ingots were carried out with a Hitachi S-2500 SEM equipped with a Kevex EDX accessory. Three different regions of each ingot were scanned. The error standardless analysis was around 5%.
Small quantities of each sample were ground mechanically in an agate mortar and pestle. The resulting fine powders were mounted on a flat zerobackground holder (a plate of single crystalline silicon cut parallel to the 510 lattice planes) covered with a thin layer of petroleum jelly. The X-ray powder diffraction data were collected at room temperature, in θ/θ reflection mode using a Siemens D5005 diffractometer (Bragg-Brentano geometry) equipped with an X-ray tube (CuKα radiation: λ= 1.54059 Å; 40kV, 30mA) and a diffracted beam graphite monochromator. Fix 1 mm aperture slit, 1 mm divergence slit and a 0.1 mm monochromator slit and a 0.6 mm detector slit were used. The specimens were scanned in the 2θ range of 10-100°, the scan step was 0.02°, and the time of counting in every step was 35s. Quartz was used as an external standard.
3. RESULTS AND DISCUSSION
3.1 Chemical analysis
The following average atomic percentages were obtained from the EDX study: Ag (24.4%), In (26.7%), Se (48.9%) for AgInSe2, and Ag (23.5%), In (26.3%), Te (51.2%) for AgInTe2. These results are in good quality agreement with the ideal composition 1:1:2 for each compound.
3.2 X-ray powder diffraction
The 19 (AgInSe2) and 23 (AgInTe2) peak positions measured from the patterns were input to the autoindexing program Dicvol04 [10]. Unique solutions were readily obtained in a tetragonal system. A comparison of the powder patterns taking into account the chemical composition, crystal system and cell parameters, showed that these materials are isostructural with the AgInS2 chalcopyrite phase, which crystallize in the tetragonal space group I ![]() 2d (Nº 122) [5]. The complete powder diffraction dataset of both compounds were reviewed and the unit cell refined with the NBS*AIDS program [11] in the space group I
2d (Nº 122) [5]. The complete powder diffraction dataset of both compounds were reviewed and the unit cell refined with the NBS*AIDS program [11] in the space group I ![]() 2d. From these analyses, the refined unit cell parameters given in the Tables 2 and 3 were obtained. These tables contain the observed and calculated X-ray powder diffraction data for AgInSe2 and AgInTe2, with the MN [12] and FN [13] figures of merit.
2d. From these analyses, the refined unit cell parameters given in the Tables 2 and 3 were obtained. These tables contain the observed and calculated X-ray powder diffraction data for AgInSe2 and AgInTe2, with the MN [12] and FN [13] figures of merit.

These powder data are improved that the three pattern with low quality (PDF 35-1099, PDF 38- 0952, PDF 75-0118) reported for AgInSe2 and the two (PDF 49-1301, PDF 75-0119) reported for AgInTe2 in the ICDD Powder Diffraction Files [9].
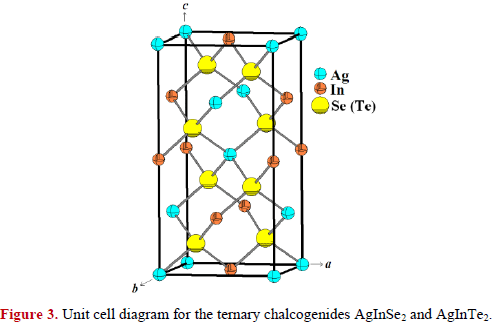
The crystal structure refinement was performed by means of Rietveld method [14], using the Fullprof program [15]. The starting parameters were taken from the AgInSe2 and AgInTe2 undistorted chalcopyrite-type structures [6,7] with the unit cell given in Tables 2 and 3. The structural refinement was carried out following the same procedure employed elsewhere [5, 16]. Details of the Rietveld refinement results for each compound are given in Tables 4 and 5. These tables contain the atomic coordinates, isotropic temperature factors and bond distances for each compound. Figures 1 and 2 shows the observed, calculated and difference profile for the final Rietveld refinements of AgInSe2 and AgInTe2. Figure 3 shows the unit cell diagram of the semiconducting compounds prepared with the program Diamond [17].


The ternary chalcogenides AgInSe2 and AgInTe2 are chalcopyrite-like compounds and can be described as derivatives of the sphalerite structure. In this type of materials, each cation is surrounded by four anions (Se or Te), and each anion is surrounded by two Ag and two In atoms. This array is expected for adamantane compounds [3]. The bond distances Ag-Se (Te) and In-Se (Te) are in good agreement with similar distances in other adamantane-type compounds found in the ICSD database [18], and agrees with the bond strength reported from ab initio calculations [19].
4. CONCLUSIONS
The crystal structure of the ternary semiconductor chalcogenides AgInSe2 and AgInTe2 were Rietveld refined using X-ray powder diffraction data. These compounds crystallize in the chalcopyrite-type structure and are reported improved X-ray powder diffraction data.
5. ACKNOWLEDGEMENTS
This work was supported by CDCHTA-ULA and FONACIT (Lab-97000821).
6. REFERENCIAS
1. El-Korashy A, Abdel-Rahima MA, El-Zahed H. Thin Solid Films 1999; 338, 207-212. [ Links ]
2. Orlova NS, Bodnar IV, Kudritskaya EA. Inst. Phys. Conf. Series 1998; 152, 147-150. [ Links ]
3. Parthé E, en: Westbrook JH, Fleischer RL (Eds.), Intermetallic Compounds, Principles and Applications, Vol. 1, John Wiley & Sons, Chichester, UK, 1995; 14, 343-362. [ Links ]
4. Delgado JM. Inst. Phys. Conf. Series 1998; 152, 45-50. [ Links ]
5. Delgado GE, Mora AJ, Pineda C, Tinoco T. Mater. Res. Bull. 2001; 36, 2507-2517. [ Links ]
6. Benoit P, Charpin P, Lesueur R, Djega-Mariadassou C. Jap. J. App. Phys. 1980; 9, 85-88. [ Links ]
7. Hahn H, Frank G, Klinger W, Meyer AD, Stöerger G. Z. Anorg. Allg. Chem. 1953; 271, 153-170. [ Links ]
8. Jayaraman A, Dernier PD, Kasper HM, Maines RG. High-Temp. High-Pressures. 1977; 9, 97-102. [ Links ]
9. PDF-ICDD; Powder Diffraction File (Set 1-63), International Centre for Diffraction Data, 12 Campus Boulevard, Newtown Square, PA, USA (2011). [ Links ]
10. Boultif A, Louër D. J. Appl. Cryst. 2004; 37, 724- 731. [ Links ]
11. Mighell AD, Hubbard CR, Stalick JK. National Bureau of Standards, USA, 1981, Tech. Note 1141. [ Links ]
12. de Wolff PM. J. Appl. Cryst. 1968; 1, 108-113. [ Links ]
13. Smith GS, Snyder RL. J. Appl. Cryst. 1979; 12, 60-65. [ Links ]
14. Rietveld HM. J. Appl. Cryst. 1968; 2, 65-71. [ Links ]
15. Rodriguez-Carvajal J. Fullprof (version April 2013), Laboratoire Léon Brillouin (CEA-CNRS), France. [ Links ]
16. Delgado GE, Mora AJ, Marcano G, Rincón C. Mater. Res. Bull. 2003; 38, 1949-1955. [ Links ]
17. Branderburg K Diamond; Crystal Impact GbR. version 2.0 (1998). [ Links ]
18. ICSD; Inorganic Crystal Structure Database (Release 2008-01) Karlsruhe, Germany (2008). [ Links ]
19. A.V. Kopytov, A.V. Kosobutsky, Phys. Solid State 2009; 51, 2115-2120. [ Links ]