Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Ciencias Veterinarias
versión impresa ISSN 0258-6576
Rev. Fac. Cienc. Vet. vol.55 no.2 Maracay dic. 2014
The Kidney and Adrenal Gland of the African Palm Squirrel Epixerus ebii: A Microanatomical Observation
El Riñón y la Glándula Adrenal de la Ardilla de la Palma Africana Epixerus ebii: Una Observación Microanatómica
Ikpegbu Ekele*,1, Nlebedum Uchenna*, and Ibe C.S.*
*Department of Veterinary Anatomy, Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria
Correo-E: fikpegbu@yahoo.com
1 A quien debe dirigirse la correspondencia (To whom correspondence should be addressed)
Abstract
The kidneys and adrenal glands of the African palm Squirrel (Epixerus ebii), were subjected to histological and mucin histochemical studies. The kidney was covered by a capsule of periodic acid of shiff (PAS) positive connective tissue fibres. The kidney parenchyma was composed of large peripheral cortex and smaller centrally placed medulla. The cortex contained renal corpuscle, proximal convoluted tubules, distal convoluted tubules and juxtaglomerullar complex. At the hilus, the renal artery and vein, ureters, and nerve fibres were surrounded by adipose tissue. The renal medullar was composed of Henles loop and collecting ducts. The adrenal gland cortex contained zona glomerulosa, zona fasciculate and zona reticulata. The zona fasciculata was the largest of the zones containing polyhedrally shaped cells with less basophilic nuclei and very foamy cytoplasm. Some cells of zona fasciculate were binucleated. The adrenal medulla contained mostly glandular cells, few ganglion cells and capillaries. This study establishes that the organs investigated are typical of mammals. It will fill the knowledge gap, help wildlife clinicians in disease diagnosis of these organs in this species.
(Key words: Diagnosis; adrenal glands; kidneys; squirrels; animal histology; animal disease; wildlife; Nigeria)
Resumen
Los riñones y las glándulas adrenales de la ardilla de la Palma Africana (Epixerus ebii) fueron objeto de estudios histológicos y de estudios histoquímicos con mucina. El riñón estaba cubierto por una cápsula de fibras de tejido conectivo que resultaron positivas a la coloración con ácido peryódico de Schiff (PAS). El parénquima renal estaba constituido por una gran corteza periférica y una médula más pequeña, ubicada centralmente. La corteza contenía el corpúsculo renal, los túbulos contorneados proximales, los túbulos contorneados distales y un complejo yuxtaglomerular. A nivel del hilio del órgano, la arteria y vena renales, los uréteres y las fibras nerviosas estaban rodeados de tejido adiposo. La médula renal estaba compuesta por el asa de Henle y los túbulos colectores. La corteza de la glándula adrenal contenía las zonas glomerular, fasciculada y reticular. La zona fasciculada era la mayor de las tres y contenía células con forma poliédrica con núcleos menos basofilícos y citoplasma muy esponjoso. Algunas células de la zona fasciculada eran binucleadas. La médula adrenal contenía principalmente células glandulares y pocas células ganglionares y capilares. Los resultados de este estudio establecieron que los órganos investigados son típicos de los mamíferos. Esta investigación llenará la brecha de conocimiento existente y ayudará a los clínicos de fauna silvestre en el diagnostico de enfermedades de estos órganos en esta especies.
(Palabras clave: Diagnóstico; glándulas suprarrenales; riñones; ardilla; histología animal; enfermedades de los animales; fauna; Nigeria)
Recibido: 05/09/14 - Aprobado: 20/11/14
Introduction
Rodent biology has become an area of increasing research interest. This may be attributed to their use as a ready source of animal protein, experimental animals, and zoonotic diseases [1-3]. The African Palm Squirrel (Epixerus ebii), is a popular dark brown coloured rodent native of Eastern Nigeria. Its activities include pest on fruits and crops, exotic in game reserve; and a source of meat to locals As part of our continued investigation on its biology, a research was performed on the micro-morphology of the kidneys and adrenal glands to establish their normal histology, as there is dearth of information in available literature. These organs have elicited a lot of research interest in other animals like the effect of medroxy progesterone acetate on adrenal glands of Indian Palm Squirrel (Funambulus pennati Wroughton) [4]; volume estimation of the bovine renal parenchyma [5]; morphological features of fetal and adult adrenal glands in kano brown goats (Capra hircus) [6].
This study is very significant because the kidneys are involved in regulating body homeostasis [7-11]; while the adrenal glands play important role in controlling the hydro-electrolytic balance, and in pH regulation; and also the flight and fight response of the body [12]. In this paper, we present our findings on the histology and mucin histochemistry of the kidneys and adrenal glands of the African palm squirrel. The result will be of interest to zoologists, histologists, physiologists, and pathologists, especially those involved in wild animal practice.
Materials and Methods
Five adult African palm squirrels (Epixerus ebii) of both sexes were captured in the wild from Olokoro Umuahia in the State of Abia, Nigeria, from March to November 2012, using metal cage traps for the study. Olokoro Umuahia is located in the rainforest vegetation of southern Nigeria, which is characterized by heavy rains and thick well grown mangrove forest trees. The animals were immediately transferred to the veterinary anatomy laboratory of Michael Okpara University of Agriculture, Umudike, for acclimatization. During this period, the animals were fed with grasses, oil palm fruit and water ad libitum. The squirrels on the day of sacrifice, were euthanized with deep chloroform sedation . The animals averaged a weight of 163.5g after weighing with a Mettler balance (Model Ohaus scout PRO-200) with a sensitivity of 0.1g. The animals were placed on dorsal recumbency and cut open through mid ventral incision from the inguinal region to the mandibular symphysis. The kidneys and adrenal glands were dissected out and slices fixed in 10% neutral buffered formalin. The tissues were passed through graded ethanol, cleared in xylene, impregnated and embedded in paraffin wax. Sections of 5µm thick were obtained with a Leitz microtome model 1512. They were stained with haematoxylin and eosin [13] for light microscopy examination and periodic acid of Schiff (PAS) procedure with and without prior digestion with diastase [14, 15]. The slides were examined and photomicrographs taken with a Motican 2001 camera (Motican U.K.) attached to an Olympus microscope.
Results
Kidneys: The kidneys were coated by two sheaths. An outer single layer of simple squamous cells and inner layer of thin regular collagen fibres of about four cells layer thick (Figure 1). The cortex contained prominent renal corpuscles while the medulla was composed mainly of medullary rays of collecting ducts at low magnification (Figures 2, 3). At the hilus, the renal vein, renal artery, ureters, and nerve fibres were surrounded by a dense layer of unilocular adipose tissue and scanty loose irregular connective tissue fibres (Figure 2).
In the cortex, the renal corpuscle contained a tuft of glomerular vessels inside the Bowmans cup. The urinary space was prominent and clear (Figure 3). The proximal convoluted tubules (PCT) were lined by dark staining simple cuboidal cells with prominent brush border that reduced the luminal diameter or even partial occlusion in some PCT (Figure 3). The distal convoluted tubule (DCT) was lined by light staining simple cuboidal cells with no brush border (Figure 1). The DCT simple cuboidal cells, which were very close to the renal corpuscle, changed to simple columnar cells, thus forming the macula densa of the juxtaglomerular complex or apparatus (JGC) (Figure 3). In most microscopic view in the cortex, more PCTs were observed than renal corpuscles and DCTs.
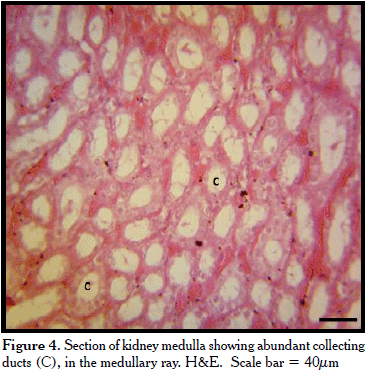
The medulla contained mostly collecting ducts lined by simple cuboidal epithelium and few loops of Henle lined by simple squamous cells (Figure 4).
Mucin histochemistry reaction revealed that the epithelial basement membrane of the kidney tubules, ureters, and blood vessels was PAS positive (Figure 5). The tunica media of the renal artery was PAS positive (Figure 6), while the poorly developed tunica media; and well developed tunica adventitia were PAS negative.
Adrenal Glands: At low magnification, the adrenal glands were coated by a thin capsule. The gland parenchyma contained a large cortex and a relatively small medulla (Figure 7). The cortex contained three clearly demarcated zones- an outer subcapsular basophilic area of about 1/3 of the cortex; a middle lighter staining area with abundant vacuoles about ½ of the cortex; and an inner basophilic peri-medulla zone about 1/6 of the cortex (Figure 7). The medulla was light stained with blood vessels (Figure 7).
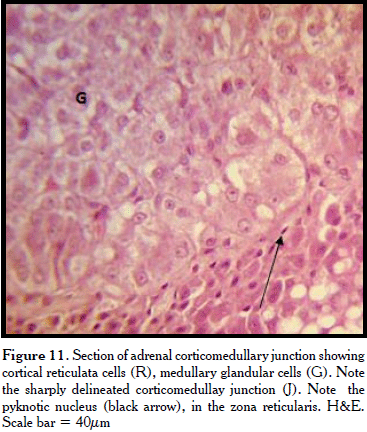
At higher magnification, the capsule contained thin layer of PAS positive connective tissue fibres whose trabeculae penetrated the cortical parenchyma (Figs. 7, 8, 9). The cortical subcapsular zone (zona glomerulosa) contained cords of cells. These polyhedral cells arranged in acini had centrally placed basophilic nuclei and evenly acidophilic cytoplasm (Figure 8). Few vacuolated structures and blood vessels were also observed. In the middle cortical zone (the zona fasciculata), the cords contained polyhedral- shaped cells with less basophilic vesicular nuclei with foamy cytoplasm (Figure 10). Some cells were binucleated. The blood vessels were more abundant while the vacuoles were larger and more numerous. The inner third zone (the zona reticulata) contained freely anastomosing cells. The cell nucleus was very basophilic to pyknotic while the cytoplasm was less foamy than the fasciculata cells (Figure 10). Some reticulata cells contained brown pigments. These adrenal cortical zones were not clearly demarcated. Abundant blood vessels were observed, especially near the corticomedullary junction which was well delineated (Figure 11).
The medulla contained glandular cells, ganglion cells, and capillaries, including some sinusoids (Figure 11). The basement membrane of the capillary endothelium was PAS positive. The glandular cells were large polyhedral to columnar cells with vesicular nuclei and slightly basophilic granular cytoplasm.
Discussion
The renal capsule of regular collagen tissue maintains kidney shape and protects the underlying tissue. A thin fibro-muscular capsule has been seen in the Camelus dromedaries [11]; but a capsule containing collagen and reticular fibres has been reported in the rabbit [16]. The differences in capsular composition may be related to functional adaptation or species variation. The simple squamous cells of the mesothelium are part of peritoneal coat on the kidney. The adipose tissue observed is part of the peri-renal fat that helps hold the kidneys in place in the abdomen [17]. This well developed unilocular renal adipose tissue suggests that the animal is actively feeding and not hibernating, as adipose tissue physiology of hibernating mammals has been described in literature [18]. The absence of brown fat also supports the fact that tree squirrels like the species under study do not hibernate. The presence of adipose tissue around the kidney hilus, surrounding its borders has been reported in the Camelus dromedaries [11]; and albino rats [19]. The presence of the renal artery, renal vein, ureters and nerve fibres at the hilus is typical of mammalian kidneys [20].
The PCT epithelium of simple cuboidal cells agrees with the findings recorded in albino rats [19]. The more abundant PCT than DCT in this species has been reported in the hedgehog [21]. This relative difference can be related to the increased need for selective re-absorption of essential substances like water, glucose, amino acids and various ions, which the animal has to adequately conserve in the wild. The same reason can be adduced for the well developed collecting ducts that will enable the Palm Squirrel conserve water in the wild since access to water bodies is limited, hence the most probable source of water will be re-absorbed from ingested food through the large intestine. Species differences in rodent renal histology have revealed that structure is related to function especially with variation in habitat and the need for resources conservation [22]. The presence of PAS positive basement membrane and renal artery tunica media fibres indicates glycogen storage and this has been reported [21]. The absence of PAS positive entities especially in the luminal surface of the tubules has been reported in hedgehog [21], while the positive PAS reaction of kidney tubules in birds have been associated with aiding the elimination of uric acid from urine [23]. The presence of macula densa is very significant as its complex with JG cells, the juxtaglomerular complex produces renin, which regulates the secretion of aldosterone by cells the zona glomerulosa of the adrenal glands. The presence of macula densa and renin in JG cells has been reported in the hedgehog like other mammals [21].
The relatively larger adrenal cortex and smaller medulla is typical of mammalian adrenal cortex [24]. The observation of the zona fasiculata as the most abundant portion of the cortex has been reported in the porcupine Hystrix cristata [24]; kano brown goats Capra hircus [6]. The presence of vesicular zona fasciulata cells nuclei indicates an active cell involved in protein synthesis most probably the glucocorticoids cortisone and cortisol. The mostly foamy cytoplasm of the zona fasciculata cells and vacuoles is due to the presence of fat droplets [24]. Also the pyknotic nuclei seen in the reticularis cells have been reported in goats and rabbits, where they are related to apoptosis, as cells originating from outermost layers of zona glomerulosa migrate to the reticulate for their final death [26, 27]. This migration theory can also explain the presence of brown pigments of the zona reticulata, which are intracellular deposits and debris due to age long cell activity. The abundant vascularization reflects the need to effectively transport hormones produced by the adrenal glands to the target organ, being a ductless gland.
The absence of clear demarcation of the adrenal cortical zones has been reported in the goat, where the zona fasciculata and zona reticularis lacked clear distinction [28], this contrasts with the histology in the rabbit [27] and the buffalo [29], where the zones were clearly delineated.
The clearly delineated corticomedullary junction as observed in this study has also been seen in rabbits [27] and day old foal [30]. But an intermingled cortical and medullary junction has been observed in birds [31]; a festooned corticomedullary junction has been reported in the whale and it was related to its pseudolobulated appearance [32]. The presence of well developed abundant medullary glandular cells can be related to the need to produce adequate epinephrine and norepinephrine for flight and fight response. This is important for the survival of the Palm Squirrel in the wild, especially escape from predators. The neurotransmitter norepinephrine, released at the sympathetic end of adrenergic fibres is of immense physiologic importance [33].
In conclusion, the results of the findings of the micro-morphology study of the kidneys and adrenal glands of the Palm Squirrel, will help physiologists and zoologists in understanding its functional adaptation in the wild. It will also facilitate clinicians in disease diagnosis in the species, especially the wild life Veterinarians.
Reference
1. Asibey EOA, Addo PG.. The grasscutter, a promising animal for meat production. In: Turnham D (ed). African perspective practices and policies supporting sustainable development (Scandinavian seminar college Denmark, in association with Weaver Press, Harare, Zimbabwe). Available in: http://www.cdr.dk/sscafrica/as & adgh.htm. [access date: 19 abril 2013]. 2000. [ Links ]
2. Ogunsanmi AO, Ozegbe PC, Ogunjobi O, Taiwo VO, Adu JO. Haematological plasma biochemistry and whole blood minerals of the captive adult African grasscutter (Thryonomis swinderianus Temminck). Trop Vet. 2002; 20: 27. [ Links ]
3. Opara MN, Ike KA, Okoli IC. Haematology and plasma biochemistry of the wild adult African grasscutter (Thrynomys swinderianus). J Amer Sci. 2006; 2: 17-22. [ Links ]
4. Pradhan VP. The effect of medroxy progesterone acetate on adrenal gland of Indian Palm Squirrel Funambulus pennati (Wroughton). Internat J of Life Sci. 2013; 1(3):198-201. [ Links ]
5. Lenger OF, Akosman MS. An urological model: volume estimation of the bovine renal parenchyma. Bulgarian J Agric Sci. 2013; 19(4): 848-853. [ Links ]
6. Nwaogu IC, Francis B. 2009. Morphological features of fetal and adult adrenal glands in kano brown goats (Capra hircus). Anim Res Internat. 2009; 6(1):953-957. [ Links ]
7. Moffat DB. The Mammalian Kidney. Cambridge University Press, London, 1975; p. 1-211. [ Links ]
8. Holz PH, Raidal SR. Comparative renal anatomy of exotic species. Vet. Clin. North Am Exot Anim Pract. 2006; 9:1-11. [ Links ]
9. Oyeanusi BI, Adeniyi AA, Ayo JO, Ibe CS, Onyeanusi CG. A comparative study of the urinary system of the African giant rat (Cricetomys gambianus Waterhouse) and the Wistar rat. Pak J Nutri. 2009; 8: 1043-1047. [ Links ]
10. Olukole SG. Morphometric analysis of the kidneys of the adult domesticated African great cane rat (Thryonomys swinderianus). Eur J Anat. 2009; 13(3):117-120. [ Links ]
11. Salehi E, Sharifabad MM. 2012. Kidney morphogenesis during development in Camelus dromedaries embr yos. J Anim Vet Adv. 2012; 11(6):822-825. [ Links ]
12. Malatesta M, Fakan S, Zancanaro C. Cell and tissue structural modifications in hibernating dormice. Hystrix It. J. Mamm. 2005; 16(1):41-52. [ Links ]
13. Bancroft J.D and Stevens A. 1990. Theory and practice of histological techniques. Third Edition. Churchill Livingstone, London. p. 88-89. [ Links ]
14. Lillie RD, Greco J. Mact diastase ptyalin in place of saliva in the identification of glycogen. Stain Tech. 1947; 22:67-70. [ Links ]
15. Ikpegbu E, Nlebedum UC, Nnadozie O, Agbakwuru I. Fast Green FCF or Ehrlichs hematoxylin as counterstain to periodic acid Schiff reaction: A comparative study. Histologic. 2011; 54:29-30. [ Links ]
16. Al-Jeboril JGA, Al-Badri AMS, Jassim BA. 2014. Study of the anatomical and histomorphological description of the kidney in adult white rabbits female New Zealand strain. Wld J Pharm Pharmaceut Sci. 2014; 3(6):40-51. [ Links ]
17. Singh J. Textbook of Human Histology. Jaypee Brothers, India. 2006; p. 70-72. [ Links ]
18. Himms-Hagen J. Brown adipose tissue and cold acclimatation. In: Trayhurn, P., and Nicholls, D.G. (eds). Brown Adipose Tissue. Edward Arnold, London: 1986; 214-268. [ Links ]
19. Al-Samawy ERM. Morphological and histological study of the kidneys on the Albino rats. Al-Anbar J Vet Sci. 2012; 5(1):115-119. [ Links ]
20. Sandeep GJ, Deepak AY, Gorahnath RT. Case report: Varicosity of the communicating vein between the left renal vein and the left ascending lumbar vein mimicking a renal artery aneurysm: Report of an unusual site of varicose veins and a novel hypothesis to explain its association with abdominal pain. Indian J Radiol Imaging. 2011; 21(1): 24-27. [ Links ]
21. Nabipour A, Dehghani H. Light and electron microscopic features of the kidney in hedgehog (Hemiechinus auritus). J Vet Anat. 2012; 5(1): 91-106. [ Links ]
22. Samuelson, D. A 2007: Textbook of Veterinary Histology. Saunders, St. Louis. USA. 2007; p. 372-390. [ Links ]
23. Casotti G. Effects of season on kidney morphology in house sparrows. J. Experimental Biol. 2001; 204: 1201-1206. [ Links ]
24. Yilmazl S, Girgin A. 2005. Light and electron microscopic observations on the structure of the porcupine (Hystrix cristata) adrenal gland. Vet Arhiv. 2005; 75 (3): 265-272. [ Links ]
25. Peng KM, Song H, Liu HZ, Zhang JB, Lu ZQ, Liu ZW, Liu YX. Histological study of the adrenal gland of african white rhinoceros. Pak Vet J. 2012; 32(3): 394-397. [ Links ]
26. Roy MK, Sinha RD, Prasad J. 1987. Certain micrometric observation on the adrenal cortex of normal and vasectomized goats. Ind J Anim Sci. 1987; 57(11): 1163-1165. [ Links ]
27. Farhan OR. 2013. Morphological study of adrenal gland in prenatal and postnatal periods of domestic Rabbit. Bas J Vet Res. 2013; 12(1): 253-264. [ Links ]
28. Eurell JA. Veterinary Histology. Tenton new Media. Library of congress. 2004; 34-36. [ Links ]
29. Mohammed NI. Anatomical and Histological study of Adrenal Gland in Iraqi Buffalo Bubalus bubalis with reference to the Seasonal Changes. MSc.thesis, Veterinary Medicine College, Baghdad University. 2007; p.190. [ Links ]
30. Sharma A. Histomorphological observation on the adrenal gland of day old foal. Ind J Vet Sci. 2013; 1(2): 31-38. [ Links ]
31. Basha SH, Kannan TA, Geetha RC. Age related of the adrenal gland in Japans quail (Coturnix coturnix japonica). Tamilnadu J Vet Anim Sci. 2009; 5(5): 198-202. [ Links ]
32. Carballeria A, Brown JW., Fishman LM, Trujillo D, Odell DK. The adrenal gland of stranded Whales (Kogia breviceps and Mesoplodon europaeus): Morphology, hormonal contents and biosynthsis of corticosteroids. Gen Comp Endocrinol. 1987; 68(2): 293-303. [ Links ]
33. Engelhardt WV, Breves G. Nebennierenhormone. In Physiologie der Haustiere, Stuttgart: Enke im Hippokrates Verlag. 2000; p. 345-354. [ Links ]