Interciencia
versión impresa ISSN 0378-1844
INCI v.26 n.12 Caracas dic. 2001
PROTEINS, AMINO ACIDS AND FATTY ACIDS COMPOSITION OF NUTS FROM THE MEXICAN ENDEMIC RARITY, Pinus maximartinezii, AND ITS CONSERVATION IMPLICATIONS
Lauro López-Mata
Lauro López-Mata. Biologist, Universidad Nacional Autónoma de México (UNAM). M.Sc. in Botany, Colegio de Postgraduados. Ph.D. in Biology, University of North Caroline, Chapel Hill, USA. Researcher and Professor in Botany, Instituto de Recursos Naturales, Colegio de Postgraduados, UNAM. Address: Colegio de Postgraduados, 56230 Montecillo, Texcoco, Estado de México, México. e-mail: lauro@colpos.colpos.mx
Summary
Seeds of blue pine, Pinus maximartinezii, have long been used for human consumption. However, no chemical analysis of its nutrient components has been carried out. In this paper, the content of kernel proteins, amino acids and fatty acids is reported and compared with some pinyon pines and other nut species. Proximate analysis showed that blue pine contains about 31% crude protein, 66% defatted protein, 42% crude fat, 2% carbohydrates, 9% crude fibre, 4% ash, and 4% moisture. It contains 18 different amino acids, including all the essential ones, and six different fatty acids; of these about 84% are unsaturated. Nuts of blue pine are of outstanding dietary value and appear to be a promising resource, but the impact of seed harvesting size on the population stability needs to be thoroughly evaluated in order to insure its sustainable use. A conservation strategy for P. maximartinezii, should urgently focus on protecting the specific habitat where this endemic rarity occurs.
Resumen
Las semillas del pino azul, Pinus maximartinezii, han sido usadas desde hace mucho tiempo para consumo humano. Sin embargo, ningún análisis químico de sus componentes nutritivos ha sido llevado a cabo. En este artículo se reporta el contenido de proteína, amino ácidos y ácidos grasos de su nuez y se le compara con algunas especies de pinos piñoneros y otras nueces. El análisis proximal mostró que el pino azul contiene 31% de proteína cruda, 66% de proteína desgrasada, 42% de grasa cruda, 2% de carbohidratos, 9% de fibra cruda, 4% de cenizas y 4% de humedad. Contiene 18 amino ácidos distintos, incluyendo a todos los esenciales, y seis ácidos grasos diferentes, 84% de ellos insaturados. La nuez del pino azul es de valor dietético sobresaliente y parece ser un recurso promisorio. Sin embargo, el impacto de la magnitud de la cosecha de semillas sobre la estabilidad poblacional necesita ser cuidadosamente evaluada para asegurar su uso sostenido. Una estrategia de conservación para P. maximartinezii debe concentrarse en la protección del hábitat donde ésta rareza endémica ocurre.
Resumo
As sementes do pinho azul, Pinus maximartinezii, têm sido usadas desde muito tempo para consumo humano. Porém, não foi feita nenhuma análise química de seus componentes nutritivos. Neste artigo se reporta o conteúdo de proteína, amido ácidos e ácidos grassos de sua castanha e é comparado com algumas espécies de pinhos, pinheiros e outras castanhas. A análise proximal mostrou que o pinho azul contém 31% de proteína crua, 66% de proteína sem gordura, 42% de gordura crua, 2% de carboidratos, 9% de fibra crua, 4% de cinzas e 4% de umidade. Contém 18 amido ácidos distintos, incluindo a todos os essenciais, e seis ácidos grassos diferentes, 84% deles insaturados. A castanha do pinho azul é de valor dietético sobressalente e parece ser um recurso promissório. Porém, o impacto do tamanho da colheita de sementes sobre a estabilidade populacional necessita ser cuidadosamente avaliada para assegurar seu uso sustentado. Uma estratégia de conservação para P. maximartinezii deve concentrar-se na proteção do habitat onde esta rareza endêmica ocorre.
KEY WORDS / Pinus maximartinezii / Rare and Endemic Pine / Conservation / Dietary Value / Chemical Composition /
Recibido: 12/06/2001. Aceptado: 22/10/2001
Introduction
One of the greatest number of pine species throughout the world is found in México (Farjon and Styles, 1997; Martínez, 1948; Perry, 1991; Styles, 1993). About 50% of the approximately 100 pine species currently recognized are native to Mexico (Farjon and Styles, 1997; Little and Critchfield, 1969; Perry, 1991). Of these, nearly a dozen produce edible seeds with highly nutritious nuts and palatable flavor that makes them worth gathering (Crawford, 1995; Duke, 1989). It has been well documented that pinyon pine nuts rank high in food value among wild and commercial nuts (Crawford, 1995; Botkin and Shires, 1948; Duke, 1989; Duke and Atchley, 1986; Han and Hwang, 1990; Lanner, 1981; Sagrero-Nieves, 1992; Yoon et al., 1989).
Pinus maximartinezii (Rzedowski), subsection Cembroides sensu Little and Critchfield (1969) bears one of the largest seeds among the edible pine nuts world-wide (Rzedowski, 1964). Nevertheless, although seeds of P. maximartinezii have been used for a long time as a source of food for the local population, their chemical analysis has been neglected so far. This paper reports the chemical composition of P. maximartinezii nuts including a proximate analysis, proteins, amino acids, and fatty acids contents, and intends to attract attention on the importance of ecological knowledge within the context of its conservation and sustainable management.
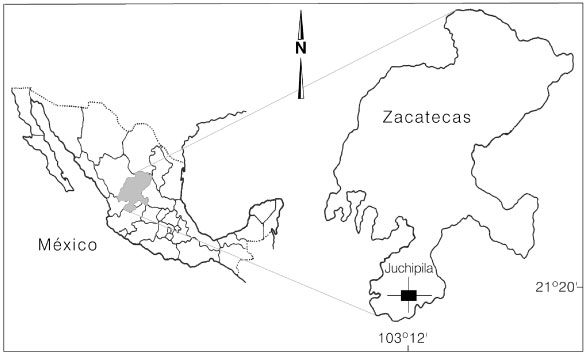
P. maximartinezii, commonly known as Martinez pine, Zacatecas pine or blue pine, is a rare, endemic and threatened species known only from a single, small and localized population in the southernmost state of Zacatecas, Mexico (Rzedowski, 1964). Blue pine is distributed on the Sierra de Morones, approximately at 21º20-21º22 N and 103º12-103º15 W and from 1650 to 2500m elevation on a mountain locally known as Cerro de Piñones, Juchipila county (Figure 1). The species is restricted to sedimentary calcareous substrates all over its range, however, it is relatively common on rock outcrops and limestone soils on strong slopes (30-55°) with rolling rocks. Donahue and Mar-López (1995) described superficial soils as sandy loams with a pH of 7.2-7.5 on West slopes and as clay loams with pH of 6.8-7.0 on Southwest slopes. These authors estimated the total number of mature trees in 2000-2500 individuals. However, their observations must be taken with caution because they did not conducted a systematic sampling to estimate densities on any stands.
Figure 1. Geographic distribution of the endemic rarity, Pinus maximartinezii Rzedowski at Juchipila county, Zacatecas State, México.
P. maximartinezii grows sparsely on flat terrain and more densely on low to deep slopes. Trees are usually 5-15m in height and 10-30cm in diameter at breast height (DBH). In humid protected ravines they may reach 20-22m and, exceptionally, 110cm DBH. The crown is rather open and rounded, with large somewhat horizontal and pendent branches. Cones are borne singly or in clusters of 2-3 on the tip of the shoots; they are oblong and extremely large, 18-25cm by 10-15cm at the widest portion, green (brown when ripe) and of up to 2kg fresh weight. Cone scales open widely when ripe, releasing seeds soon after maturation. They usually open in September-October, after the rainy season and before the first fall frost. After seed dispersion cones remains attached to the shoots or limbs for two years. On mast years, seeds may fall over a period of 6-8 weeks. They are shelled, stoned, and susceptible to predation by a local gray squirrel and blue jay birds, both of which are also dispersal agents. P. maximartinezii, as all pinyon pines in the Haploxylon group, has wingless seeds that are exceptionally large, ranging 17.5-27.4mm long, 9-13.1mm wide, 7-10.2mm thick and 4.8-7.3 mg fresh weight.
Local weather is semiarid, with temperatures from 18 to 22°C. Approximately 75% of the annual rainfall, estimated in about 700-850mm, occurs during June-September. The frost-free period is approximately 275 days, from mid February to November. Frost periods occur mostly in December or January, but may occasionally be present by mid November. Three major causes threat the persistence of P. maximartinezii: (1) Local habitat fragmentation directly related to recent land-use changes, resulting in a great surface area reduction of its natural range as well as in the species habitat deterioration and soil erosion; (2) Overgrazing and induced forest fires, which directly prevent seedlings recruitment; and (3) The current seed over-harvesting and illegal national and international trade. By virtue of these facts blue pine is an endangered species after the Mexican norm, NOM-059-ECOL-1994 (Anonymous, 1994). In addition, more recently Ledig et al. (1999) based on allozyme frequencies, alleles per locus, and gametic disequilibrium measures for nine major polymorphic loci, have shown that P. maximartinezii may have survived an extreme bottleneck.
Materials and Methods
Cones of P. maximartinezii were harvested directly from more than 50 randomly selected mature trees. Cones were dried in open sunlight for two to three weeks. Then, 250 cones were randomly selected, broken down and cleaned up to collect complete seeds. In the laboratory, seeds were cleaned, mixed and stored in airtight plastic bags at room temperature. Nuts were carefully fractionated in small pieces, stored in glass containers at 4°C, and samples of 250g were taken for analyses.
Proximate analysis
Three replicates were examined for oil, protein, ash, and moisture contents. Ash was determined by incineration in a muffle furnace at 525ºC (AOAC, 1984). Nitrogen content was estimated by the Kjeldahl method, and crude protein content was calculated using the conversion factor Nx6.25. Crude fiber, fat, and moisture were determined in accordance with the standard methods of AOAC (1984).
Fatty acid analysis
Fatty acid methyl esters were analyzed by gas-liquid chromatography (GLC). Samples were injected into a Hewlett-Packard HP 5890 gas chromatograph (GC), with a flame ionization detector (FID) coupled to an electronic integrator. The GC was equipped with a 60-m SP-1000 capillary column (0.25mm ID, µm film thickness). The oven was programmed as follows: 60ºC to 220ºC at 20ºC/min. The final oven temperature was maintained for 10 min. The injector and detector temperatures were both 220ºC. Fatty acid methyl esters were identified by comparing their retention times to those of pure standard fatty acid methyl esters. Fatty acid methyl esters were quantified according to their percentage area obtained by integration of peak areas.
Amino acid analysis
Amino acid composition was analyzed by ion exchange chromatography following the method of Lucas and Sotelo (1982). Defatted samples were hydrolyzed with HCl (6.0 mol/l) at 145ºC for 4h. At the end of hydrolysis, the hydrolysate was adjusted to pH 6.8. According to Lucas and Sotelo (1982), 1ml hydrolysate was diluted with a 1ml buffer pH 1.3, and from this dilution a 100µl aliquot was injected on a Technicon amino acid analyzer model NC2P (Technicon International, Geneva). Tryptophan was isolated by enzymatic hydrolysis with pepsin and panchreatine reaction for 24h. A hydrolysate aliquot was made to react with p-dimethylaminobenzaldehyde and the product was quantified with a spectrophotometer at 590 nm and standard Tryptophan curve (Lucas and Sotelo, 1980; Ras et al., 1974).
Results and Discussion
Proximate analysis
Table I shows the proximate analysis of P. maximartinezii nuts along with other nine edible pine species. Nut composition varies largely among different pine species. P. maximartinezii contains one of the highest protein values among all other pine nut species, either crude protein (31.3%) or defatted protein (65.6%). It is lowest in carbohydrates, relatively high in fiber and ash, and average in moisture (Table I). Other edible nut pine species such as P. monophylla and P. quadrifolia are among the lowest in crude protein and the highest in carbohydrates. On the other hand, P. cembra, P. cembroides, P. edulis, P. koraiensis, and P. sibirica contain intermediate quantities of both crude protein and carbohydrates. P. maximartinezii compares favorably with the Italian stone pine P. pinea, the Korean pine P. koraiensis, the Siberian pine P. sibirica, and the digger pine P. sabiniana. Blue pine is similar to Italian stone pine in protein content, as well as in carbohydrates and fats, but significantly higher in fiber (Table I). P. maximartinezii contains more crude protein than both, P. koraiensis and P. sibirica and is similar to that of P. sabiniana. It should be noticed that for pines, defatted protein is not directly comparable to the usual nut analysis; therefore, it is not possible to compare defatted protein contents among pine species because data has not been reported.
The dietary value of P. maximartinezii results favorable when compared with some commercial nuts. For instance, pecan (Carya illinoensis) contains 9-10% crude protein, 71-73% fat, and 11-15% carbohydrate; peanut (Arachis hypogaea) contains 26% crude protein, 39% fat, and 24% carbohydrate; cashew (Anacardium occidentale) contains 17.2% crude protein, 45.7% fat, and 29.3% carbohydrate; while English walnut (Juglans regia) contains 15% crude protein, 68% fat, and 12% carbohydrate (Botkin and Shires, 1948; Duke, 1989; Lanner, 1981). Therefore, the dietary and biological value of blue pine proteins exceeds that of commercial nuts.
Amino acid analysis
Just as valuable as the protein content is the protein quality of P. maximartinezii, largely determined by the amino acid composition and digestibility of the protein. The importance of essential amino acids in the diet is emphasized in several studies, further supported by the International Committee of FAO/WHO (1990). Eighteen of the twenty amino acids are found in nut protein of blue pine (Table II), as in Korean pine (Han and Hwang, 1990), while all twenty are present in P. edulis and P. monophylla (Lanner, 1981). The nutritional value of blue pine nuts lies partly in the fact that all essential amino acids such as Arginine, Methionine, Tryptophan, Lysine, Leucine, Isoleucine, Phenylalanine, Valine, Threonine, and Histidine are present in significant concentrations, above 1% (Table II). In contrast, in Korean pine only Arginine, Aspartic acid, Glutamic acid, and Methionine content surpass 1% (Han and Hwang, 1990).
All ten essential amino acids but Tryptophan are found in nuts of blue pine (Table II) in relatively high percentages as compared with that of P. koraiensis and contains high percentages of Aspartic and Glutamic acids, as well as Glycine, Alanine, Serine, and Proline, all of which surpass those of Korean pine.
Studies by Massieu et al. (1950) have indicated that Lysine, Tryptophan, and Methionine are limited in the Mexican diet, specially in the poorest people living in the rural country. Besides its role as one of the limiting essential amino acids in protein metabolism, Tryptophan is a precursor for the synthesis of the neurotransmitter serotonin and Tryptamine, as well as for the synthesis of the antipellagra vitamin nicotinic acid and the epiphyseal hormone melatonin (Heine et al.,1995). Tryptophan and its metabolites regulate neurobehavioral effects such as appetite, sleeping-walking-rhythm and pain perception. Although these three essential amino acids are found in blue pine nuts, they occur in relatively low percentages. Nevertheless, blue pine nut is exceptionally rich in protein and contains a high proportion of essential amino acids, including Arginine, Leucine, Isoleucine, and in a lesser extent Valine, all quite important in the Mexican diet which is based mainly on corn. In this respect, the essential amino acids of blue pine nuts compare relatively well with the levels suggested by the FAO/WHO (1990, Table II).
A single-source protein seldom serves independently as a source of dietary amino acids. It works in concordance with other equally important proteins. Hence, the most important characteristic of each protein in the diet is its ability to fill the gaps between the quantities of essential amino acids needed and those supplied by other foods. When examined from this standpoint, blue pine nut proteins appear to be more valuable as a source of supplementary proteins. Blue pine nut is deficient in Methionine, Lysine, Tryptophan, and possibly Threonine. Such an essential amino acid limitation must be considered when blue pine nut proteins are added for nutritional purposes. It is therefore necessary to supplement blue pine proteins with these amino acids when they are the sole source of protein. Alternatively, blue pine proteins can be blended with proteins from other oilseed proteins, legumes, and cereals to provide a good amino acid balance.
Fatty acid analysis
P. maximartinezii nuts contain palmitic, stearic, oleic, linoleic, arachidic, and behenic acids, as well as acids with Carbon chains larger than C20 (Table III). The most abundant fatty acids in blue pine are the unsaturated linoleic and oleic acids, which comprise about 84% of the total fatty acids. Studies conducted by Yoon et al. (1989), and Han and Hwang (1990) on P. koraiensis, revealed relatively high levels of the unsaturated oleic and linoleic acids, which comprise about 73% of the total. Sagrero-Nieves (1992) working on three different seed coat phenotypes of P. cembroides from Mexico also quantified high levels of both oleic and linoleic acids, which accounted for about 82% of total fatty acids. Likewise, Lanner (1981) reported that unsaturated fatty acids from P. edulis and P. monophylla are the oleic, linoleic, and linolenic acids, which altogether account for about 85% of the total.
The essential unsaturated linoleic acid content in P. maximartinezii is high as compared with that of P. cembroides and P. koraiensis. Linoleic acid is of biological importance for the biosynthesis of arachidonic acid (not present in plants), and the absence of these two fatty acids in the diet may result in certain pathological conditions, and eventually death, in experimental animals (Lenninger, 1975). The total fatty acids found in nuts of P. maximartinezii also suggest that they are of high food quality, similar to that reported for P. cembroides, P. edulis, P. koraiensis, and P. monophylla. However, based on unsaturated fatty acids content alone, nuts from P. maximartinezii are of similar quality than those of P. edulis and P. monophylla, and higher than P. koraiensis and P. pinea. Blue pine unsaturated fatty acids, moreover, are higher than some commercial nuts like pecans (73%), English walnuts (68%), and peanuts (29%) (Botkin and Shires, 1948; Lanner, 1981).
From an ecological standpoint, P. maximartinezii energy, primarily stored in form of proteins, lipids, or carbohydrates (either starches or sugars) may be crucial very early in its life-history. For instance, nut energy allocation to roots, stem, and cotiledonary leaves, may be quite critical, during the seedling establishment phase, in determining seedling growth rates just before becoming self-sustaining, as it is for most long-lived plants (Fenner, 1987; Harper, 1977). Although seed energy of P. maximartinezii is relatively high as compared with other pine species, the ecological importance of the amount of energy stored in the nut will depend, however, on the form in which it is packed and mobilized. It is known that lipids, for instance, contain more energy per unit weight than carbohydrates (starches) but they are more slowly mobilized. Likewise, sugars are more readily available than starches. On the other hand, predator-disperser animals like squirrels and birds, tend to prefer large seeds, but quantifying the size effect is complicated due to variation in seed abundance (specially for mast-seeding trees) besides nut nutritional quality and the identity of the predator-disperser vector (Thompson, 1987).
Before any management plans are implemented for this species, it is necessary to take into account the potential impact of seed harvesting on the current population growth. P. maximartinezii is an endemic and threatened species because of its small population size, extremely restricted distribution and its habitat conversion, which is directly associated to recent land-use changes resulting in a reduction of its natural range, soil erosion and habitat fragmentation. Other factors, such as the overgrazing and induced forest fires, which result in prevention of recruitment of seedlings and saplings and the current seed over harvesting and illegal trade also threat the long-term persistence of blue pine. Levels of seed harvesting must be investigated because they may prevent natural regeneration and, in the long-term, forest growth. Research in that direction is being conducted to evaluate the maximum level of seed harvesting that warrants population stability. Blue pine represents, therefore, a good opportunity to conciliate conservation values with management and economic development.
Conclusions
The large edible nuts of P. maximartinezii are of outstanding dietary value as compared with other pinyon pine species and commercial nuts. They are a remarkable source of protein, essential amino acids and unsaturated fatty acids for human nutrition. However, the utilization of seeds needs to be thoroughly evaluated since species management may be hindered by the lack of basic ecological research. Sound management proposals for P. maximartinezii require a comprehensive perspective on conservation and development, and an integrated approach to decision-making. Such proposals should take into account an integrated research of the social, economical, and political aspects locally involved, all of them focusing on protecting the specific habitat where this endemic rarity occurs.
ACKNOWLEDGEMENTS
The author thanks Teresa Terrazas and Edmundo García-Moya for their useful comments to an earlier manuscript. Financial support was provided by the National Commission for the Knowledge and Use of Biodiversity (CONABIO) contract FB305/H140. Chemical analyses were carried out in the Laboratorio de Química Analítica at the Facultad de Química, UNAM.
REFERENCES
1. Anonymous (1994) Norma Oficial Mexicana NOM-059-ECOL-1994. Diario Oficial de la Federación. pp 35-45 [ Links ]
2. AOAC (1984) Official Methods of Analysis. 14th edition. Association of Official Analytical Chemists. Washington, DC. 501 pp. [ Links ]
3. Botkin CW, Shires LB (1948) The composition and value of piñon nuts. New Mexico Agric Exp Stn Bull 344. pp. 3-14. [ Links ]
4. Crawford M (1995) Nut pines. Yearbook Western Australian Nut and Tree Crops Association 19: 56-66. [ Links ]
5. Donahue JK, Mar-López C (1995) Observations on Pinus maximartinezii Rzed. Madroño 42: 19-25. [ Links ]
6. Duke JA (1989) CRC Handbook of Nuts. CRC Press. Boca Raton, Florida. 368 pp. [ Links ]
7. Duke JA, Atchley AA (1986) CRC Handbook of Proximate Analysis Tables of Higher Plants. CRC Press. Boca Raton, Florida. pp. 130,332. [ Links ]
8. FAO/WHO (1990) Protein quality evaluation. In Report of a Joint FAO/WHO Expert Consultation. Food and Agriculture Organization of the United Nations, Rome, Italy, p 23. [ Links ]
9. Farjon A, Styles BT (1997) Pinus (Pinaceae). Flora Neotropica Vol. 75. The New York Botanical Garden, New York. pp. 221-224. [ Links ]
10. Fenner M (1987) Seedlings. New Phytol. 106: 35-47. [ Links ]
11. Han SS, Hwang BH (1990) Analysis of amino acid, fatty acid, and vitamin in Korean pine (Pinus koraiensis) seeds. J. Korean For. Soc. 79: 345-351. [ Links ]
12. Harper JL (1977) Population Biology of Plants. Academic Press, Orlando, Florida. 892 pp. [ Links ]
13. Heine W, Radke M, Wutzke KD (1995). The significance of tryptophan in human nutrition. Amino acids 9: 191-205. [ Links ]
14. Lanner RM (1981) The Piñon Pine. A Natural and Cultural History. University of Nevada Press, Reno. Nevada. 101 pp. [ Links ]
15. Ledig TF, Thompson M, Bermejo-Velázquez B, Eguiluz-Piedra T, Hodgskiss PD, Jonson DR, Dvorak WS (1999). Evidence for an extreme bottleneck in a rare mexican pinyon: genetic diversity, disequilibrium, and the mating system in Pinus maximartinezii. Evolution 53: 91-99. [ Links ]
16. Lenninger AL (1975) Biochemistry. 2nd edition. Worth Publishers Inc. New York. 1104 pp. [ Links ]
17. Little EL, Critchfield WB (1969) Subdivisions of the genus Pinus (Pines). Miscellaneous Publication 1144. Forest Service, USDA, Washington DC, USA. 51 pp. [ Links ]
18. Lucas B, Sotelo A (1982) Amino acid determination in pure proteins, foods and feeds using two different hydrolysis methods. Anal. Biochem 123: 349-356. [ Links ]
19. Lucas B, Sotelo A (1980) Effect of different alkalies, temperatures and hydrolysis times on tryptophan determination in pure proteins and foods. Anal. Biochem 109: 192-197. [ Links ]
20. Martínez M (1948) Los Pinos Mexicanos (2nd ed). Ediciones Botas, México City, D.F. 361 pp. [ Links ]
21. Massieu GH, Guzmán J, Cravioto RO, Calvo de la Torre J (1950) Contenido de aminoácidos indispensables en algunas semillas mexicanas. Ciencia 10: 142-144. [ Links ]
22. Perry JP (1991) The Pines of Mexico and Central America. Timber Press. Portland, Oregon. USA. 231 pp. [ Links ]
23. Ras RMV, Tara MR, Krishnan CK (1974) Colorimetric estimation of tryptophane content of pulses. J. Food Sci. Technol. 11: 213-216. [ Links ]
24. Rzedowski J (1964) Una nueva especie de pino piñonero del Estado de Zacatecas (México). Ciencia 23: 17-20. [ Links ]
25. Sagrero-Nieves L (1992) Fatty acid composition of Mexican pine nut (Pinus cembroides) oil from three seed coat phenotypes. J. Sci. Food Agric. 59: 413-414. [ Links ]
26. Styles BT (1993) Genus Pinus: a Mexican Purview. In Ramamoorthy TP, Bye R, Lot A, Fa J (Eds) Biological Diversity of Mexico: Origins and Distribution. Oxford University Press. New York. pp 397-420. [ Links ]
27. Thompson K (1987) Seeds and Seed Banks. New Phytol. 106: 23-34. [ Links ]
28. Yoon TH, Im KJ, Koh ET, Ju JS (1989) Fatty acids composition of Pinus koraiensis seeds. Nutr. Res. 9: 357-361. [ Links ]












 uBio
uBio