Interciencia
versión impresa ISSN 0378-1844
INCI v.34 n.5 Caracas mayo 2009
b-n-acetylglucosaminidase production by Lecanicillium (Verticillium) lecanii ATCC 26854 by solid-state fermentation utilizing shrimp shell
Esteban Barranco Florido 1, Patricia Bustamante Camilo 2, Lino Mayorga-Reyes 3, Rina González Cervantes 4, Patricia Martínez Cruz 5 and Alejandro Azaola 6
1 Ph.D. in Biotechnology, Universidad Autónoma Metropolitana (UAM), Mexico. Lecturer-Researcher, UAM-Xochimilco, Mexico. Address: Departamento de Sistemas Biológicos, Unidad Xochimilco, UAM. Calz. Del Hueso 1100, Coyoacán C.P. 04960. México D.F. e-mail: barranco@correo.xoc.uam.mx
2 M.Sc. in Pharmaceutical Sciences, UAM, Mexico. Doctorate Student in Biological Sciences, UAM-Xochimilco, México. e-mail: pbcamilo@correo.xoc.uam.mx
3 Ph.D. in Biotechnology, Cinvestav-IPN, Mexico. Lecturer-Researcher, UAM-Xochimilco, Mexico. e-mail: lmayorga@correo.xoc.uam.mx
4 Ph.D. in Biological Sciences, UAM, Mexico. Lecturer-Researcher, UAM-Xochimilco, Mexico. e-mail: gcrm4280@correo.xoc.uam.mx
5 M.Sc.in Biotechnology, Universidad Nacional Autónoma de México. Lecturer-Researcher, UAM-Xochimilco, Mexico. e-mail: pmartine@correo.xoc.uam.mx
6 Ph.D. in Biological Sciences, UAM, México. Lecturer-Researcher, UAM-Xochimilco, Mexico, México. e-mail: azaola@correo.xoc.uam.mx
SUMMARY
Lecanicillium (Verticillium) lecanii produced chitinases using shrimp shell as inducer. Maximum production of b-N-acetylglucosaminidase was measured at 80h. Enzyme stability was obtained at temperatures ranging from 30 to 40°C and maximum activity at 50°C, pH 6.0. Enzyme activity increased with Ba2+, Co2+, Fe3+ and Zn2+. Bioassays against the phytopathogenic fungus Oidium spp. showed mycelial and germination inhibition. SDS-PAGE electrophoresis of the partially purified extract revealed four bands of 70, 58, 45 and 31kDa and this extract showed activity of b-N-acetylglucosaminidase through zymogram analysis. Chitinases produced by L. lecanii are potentially useful against phytopathogenic fungi, insects and chitosan bioconversions.
Producción de b-n-acetilglucosaminidasa de lecanicillium (verticillium) lecanii ATCC 26854 por cultivo sólido fermentado utilizando caparazón de camarón
RESUMEN
Lecanicilium (Verticillium) lecanii produjo quitinasas mediante el uso de caparazón de camarón como inductor. La máxima producción de b-N-acetylglucosaminidasa se obtuvo a las 80h. Se observó estabilidad de la enzima en el intervalo de temperatura entre 30 y 40°C y su actividad máxima a los 50°C, pH 6,0. La actividad enzimática se incrementó con Ba2+, Co2+, Fe3+ and Zn2+. El bioensayo contra el hongo fitopatógeno Oidium spp. mostró inhibición micelar y de la germinación. La electroforesis SDS-PAGE del extracto parcialmente purificado mostró cuatro bandas de 70, 58, 45 y 31kDa y este extracto mostró actividad de b-N-acetylglucosaminidasa a través de un análisis de zimograma. Las quitinasas producidas por L. lecanii pueden ser potencialmente utilizadas contra hongos fitopatógenos, insectos y en la bioconversión del quitosán.
Produção de b-n-acetilglucosaminidasa de lecanicillium (verticillium) lecanii ATCC 26854 em fermentação em estado sólido utilizou exoesqueleto do camarão
RESUMO
Lecanicillium (Verticillium) lecanii producido quitinases utilizou exoesqueleto do camarão como inductor. Produção máxima de b-N-acetilglucosaminidasa foi obtido em 80h. A estabilidade de enzima estava em o intervalo de temperaturas de 30 - 40°C e os niveis máximos de atividade enzimática foram obtidos em 50°C, pH 6,0. A atividade de enzima foi aumentada com Ba2+, Co2+, Fe3+ e Zn2+. O Bioensaios contra fungo fitopatógenos Oidium spp. mostrou inibição miceliar e germinação. O electroforesis SDS-PAGE do extrato parcialmente purificado revelou quatro faixas de 70, 58, 45 e 31kDa e este extrato apresentou atividade b-N-acetilglucosaminidasa através de uma análise zimograma. O quitinases produzido por L. lecanii são potencialmente capaz de ser utilizado contra fungos fitopatógenos, insetos e bioconversions de quitosano
KEY WORDS / Antagonism / b-N-acetylglucosaminidase / Lecanicillium lecanii / Oidium ssp / Solid State Fermentation /
Received: 07/02/2007 Modified: 05/18/2009. Accepted: 05/19/2009.
Introduction
Agricultural practice has recently experienced a reorientation toward ecologically sustainable management. This has motivated the chemical industry to search for new technologies based on biological alternatives for pest control. As a result, an economic increment of 20% has been directed to explore biotechnological products of microbial origin, such as biofertilizers, biopesticides and microbial enzymes used for crop bioprocesses (Tergerdy and Szakács, 1998).
Companies such as Cyanamid, Ciba, Dupont, Monsanto, Sandoz and Zeneca have designed genetic engineering programs in order to develop crops resistant to insects, diseases and chemical herbicides (Froyd, 1997). These companies have evaluated natural products such as plant metabolites and microorganisms as an alternative practice for control. Strains of entomopathogenic fungi are the basis of diverse commercial products such as Mycotal, Biogreen, Mycotrol GH, Laginex (Butt et al., 2001). Among other applications, Lecanicillium (Verticillium) lecanii has been used to control whitefly and aphids (Steenberg and Humber, 1999) because L. lecanii synthesizes hydrolytic enzymes, such as proteases and chitinases. Chitinases have been used as mycopesticides (Deshpande, 1999) and, in the pharmaceutical industry, to convert chitin into chitosan (Nahar et al., 2004), an excipient that can be used for drug liberation (Alonso and Sánchez, 2003; Cerchiara et al., 2003).
Viniegra et al. (2003) have shown effective fungal growth and enzyme production in solid-state fermentation (SSF) systems, compared with liquid culture. Additional advantages of SSF are low water activity (aw), which reduces contamination problems; more thermostable enzymes and greater productivity (Matsumoto et al., 2004). In particular, L. lecanii ATCC 26854 has been used for the production of hydrolytic enzymes in solid-state culture with insect cuticle and shrimp waste silage as chitinases inducers (Barranco et al., 2002; Matsumoto et al., 2004). Shrimp shell wastes may be used as a disposable and economic substrate.
In this study the production and the biochemical characteristics of the b-N-acetylglucosaminidase produced by L. lecanii in SSF using shrimp shell as C source and inducer were investigated. The application of the Gompertz model is proposed to evaluate overall enzyme kinetic production.
Materials and Methods
Strain and stock medium
The entomopathogenic fungus L. lecanii ATCC 26854 and the phytopathogenic fungus Oidium spp. wild strain isolate in Morelos, Mex. were grown on potato/dextrose/agar (PDA) (Sigma) at 25ªC.
Solid-state fermentation
Solid-state cultivation of L. lecanii was performed in 250ml Erlenmeyer flasks. Culture medium was prepared as described by Barranco et al (2002). For chitinase induction, shrimp shell was used (60g·l-1) at pH 5.0. A concentration of 1×107 spores was inoculated per gram of moisture matter.
pH and protein determination
Two g of solid culture were added to 20ml of distilled water, mixed during 10min and the pH measured. Protein concentration was determined by Lowrys method (Lowry et al., 1951) using BSA as a standard.
Enzyme extraction
Once fermentation had taken place, water was added to the flask content 1:1 (w/v). It was pressed at 1500psi with a hydraulic press (ERKCO Aeroquip Mexican, S.A.) and centrifuged at 5000rpm for 10min. The extract was partially purified in a Spectrum Filtration System with a Micro-ProDiConTM membrane (MWCO 250kD), and stored at -20°C.
Bioassay
Oidium spp. was utilized as antagonist. For the mycelial inhibition test, L. lecanii and Oidium spp. were inoculated in PDA agar. They were incubated at 25°C and their radial growth was determined each 24h for 5 days. To determine the inhibitory effect of the antagonist fungus on germination, 200ml of Oidium spp. at concentration of 1×107 spores/ml were inoculated in the center of a Petri dish with PDA. Then, 500ml of the enzymatic extract was added and the dishes were incubated at 25°C during 5 days (Lorito et al., 1994). A culture with the antagonist Oidium spp. without extract was used as a control.
Enzymatic assays
b-N-acetylglucosaminidase activity was determined as described by Coudron et al. (1984), using p-nitrophenol N-acetyl-b-D-glucosamine (Sigma) as substrate. Fifty ml of the enzymatic extract were added to a mixture of 150ml of de-ionised water, 200ml of 0.2M citrate-phosphate buffer (pH 5.6) and 200ml of substrate (1mg·ml-1). The reaction mixture was incubated for 1h at 37°C and the reaction stopped with 1ml of a 0.02M NaOH solution. One unit of activity was defined as the amount of enzyme that releases 1mmole of p-nitrophenol per min at O.D of 400nm. All experiments were conducted in triplicate and the mean represents the number of enzyme units produced per gram of shrimp shell (Kunamneni et al., 2005).
Effect of metal ions
The effect of different metal ions on enzymatic activity was determined by the addition to the reaction mixture of 1mM of each of the following ions: Ba2+, Co2+, Fe2+, Fe3+, Mn2+, Zn2+, K+, Li+, Ag+, Ca2+ and Mg2+. Enzymatic activity was subsequently assayed under standard conditions.
Electrophoresis and zymogram
SDS-PAGE on 11% (w/v) polyacrylamide slab gels was performed according to Laemmli (1970). Gels were stained with silver nitrate and zymograms were run according to Guthrie et al. (2005). In brief, proteins were separated in polyacrylamide gel electrophoresis (PAGE) in native conditions lacking SDS. Crude protein samples were prepared in 125mmol·l-1 Tris-HCl (pH 6.8), 20% glycerol (v/v) and 0.2% bromophenol blue. Samples were loaded into 1.0mm gels with a 4% stacking gel and separated along a 10% resolving gel in a vertical electrophoresis system (OmniPAGE CVS10D, Cleaver Scientific Ltd). Native gel was simply washed in distilled water for 5min before being placed in the agarose-substrate solution previously prepared by heating 20ml of 100mmol·l-1 sodium acetate (pH 5.6), 1% agarose at 50ºC. The substrate 4-methyllumbelliferyl N-acetyl-b-D-glucosaminide [4-MU(GlcNAc)] (Sigma) was added to final concentration of 0.025mg·ml-1; gel was agitated gently in this solution for 5min at 37ºC prior to detection under UV light.
Statistical analysis
Data are reported as the arithmetic mean of three independent experiments ±SD and a One Way ANOVA (p<0.05) for Tukey HSD test was run for significant differences in program SPSS 13 for Windows.
Theoretical considerations of the model
Enzyme production was simulated using the Gompertz model. The integrated form of the Gompertz model allows for an algebraic relation of time-enzymatic activity (Saucedo et al., 1990) as follows:
P= Pmax exp[-b exp(-kt)]
where P: enzymatic activity, t: time, k: the enzymatic production rate constant, and Pmax: the highest enzymatic activity. The differential form of the Gompertz model can determine instant rates of enzymatic activity as:
dP/dt= k P ln(Pmax/P)
Parameters may be estimated using least squares in a nonlinear regression by the Marquardt method (Marquardt, 1963). The goodness of fit of the nonlinear regressions was evaluated by determination of correlation (R2), mean square error (MSE) and the residual values (Noguera et al., 2004).
Results and Discussion
b-N-acetylglucosaminidase activity of L. lecanii in solid culture
L. lecanii is a fungus that adapts to solid-state cultivation (Barranco et al., 2002). Barranco (2004) showed a correlation between enzymatic activity and increase of the CO2 produced during growth of the fungus, which indirectly reflects a biomass increment, and enzymatic activity was thus considered as an indirect measurement of fungal growth. As shown in Figure 1, enzyme production fitted the integrated form of the Gompertz model with the Marquardt algorithm (correlation coefficient= 0.997; MSE= 0.0038). Using the differential form, the instant rate of activity shows a maximum at 38h, during fermentation. Activity developed gradually; it began after the spore germination period (lag phase) during the first 20h of fermentation, reaching its maximum value after 70h and remaining at it for the 130h of cultivation. Previously, L. lecanii was used for chitinase production in two systems, solid-state fermentation with shrimp waste silage as carbon source (Matsumoto et al., 2004) and submerged fermentation with shrimp and crab shell powder (Bing-Lan et al., 2003). However, the high content of impurities in silage, in the first case, and the long cultivation time required in the second, demanded improvement of the chitinase production process, including an easier and cleaner enzyme-recovery operation. The present results of L. lecanii enzymatic activity are higher than those reported for Talaromyces flavus in liquid culture (Duo et al., 2005) and similar to those for Aeromonas schubertti (Shang et al., 2004). Throughout fermentation, the medium pH rose from 5.0 to 7.75 due to NH4+ liberation, resulting in a microenvironment modified by the fungus, which is a factor contributing to the pathogenic mechanism of the fungus (St-Leger et al., 1999). Total protein concentration increased during the fermentation process as a result of hydrolysis of the complex substrate and of the excreted extra cellular protein.
Figure 1. Fitted b-N-acetylglucosaminidase activity of L. lecanii. The symbols represent obtained experimental data, while the line is the result of data adjustment through the Gompertz model and the broken line is the enzymatic activity instant rate.
Antagonism of L. lecanii to Oidium ssp.
As is well known, the biodiversity of an ecosystem affects species survival. L. lecanii synthesizes metabolites that affect the growth and germination of phytopathogenic fungi. Figure 2 illustrates the antagonism between L. lecanii and the phytopathogen fungus Oidium ssp., a plague of peach plantations (Prunus persica). Although radial growth of Oidium was greater than that of L. lecanii after 5 days, this latter fungus initiated a mycoparasitic process by invading mycelia of Oidium ssp., thus inhibiting mycelium growth. This biological activity requires degradation of the cell wall of the phytopathogenic fungus, a process in which chitinases play a fundamental role, as reported for Trichoderma spp. (Kubicek et al., 2001). The enzymatic extract produced an inhibitory effect upon spore germination of the antagonist, as previously observed for Trichoderma harzianum and Gliocladium virens, which inhibit the germination of Botrytis cinerea spores (Lorito et al., 1994). Production of chitinases able to degrade the cell wall of other fungi would potentially allow the use of L. lecanii as a mycopesticide.
Figure 2. Interaction of L. lecanii with the phytopathogenic fungus Oidium spp. in the antagonism bioassay. 200µl of spores (1×107 spores/ml) of L. lecanii and Oidium spp. were inoculated in PDA agar and incubated at 25°C.
Effect of temperature and pH on b-N-acetylglucosaminidase stability and activity
The effect of temperature on stability of b-N-acetylglucosaminidase activity is shown in Figure 3a. Residual activity was determined in an enzyme solution. L. lecanii b-N-acetylglucosaminidase activity was most stable at 30-40°C. At 65°C residual activity was 15% and at 70°C the enzyme was inactive. Enzymatic thermostability was similar to that of the T. flavus chitinases CHIT41 and CHIT32, with maximum activity at 40°C, but with a higher resistance to heat inactivation (Duo et al., 2005). However, thermostability was lower than that reported for Pseudomonas aeruginosa (San and Wen, 1997). The effect of pH on enzymatic activity is shown in Figure 3b. Activity was highest at pH 5.0-6.0. At pH 3.0 and pH 9.0 residual activity was lower. Similar pH values have been found for several chitinases of other mycoparasitic fungi (Di Pietro et al., 1993; Harman et al., 1993; Duo et al., 2005). The enzyme showed maximum activity at 50°C, diminishing to approximately 20% at 60°C (Figure 3c).
Figure 3. a: Effect of temperature on b-Nacetylglucosaminidase stability; optimum temperature was determined by incubating at different temperatures ranging from 25°C to 70°C. Residual activity was assayed as described in Methods. b: Effect of pH on b-N-acetylglucosaminidase activity measured at various pH values at 50°C for 60 min; enzyme activity was defined as 100% which corresponds to 1.6U. c: Effect of temperature on enzymatic activity, activity measured from 30 to 60°C as described in Methods.
Effects of metal ions
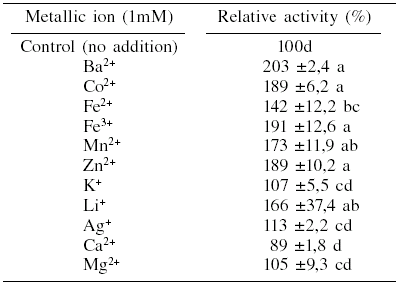
The enzymatic extract was incubated with different salt solutions (0.1mM) at 37°C for 1h. Table I shows significant increases in b-N-acetylglucosaminidase activity with the addition of Ba2+, Co2+, Fe3+, Zn2+, Mn2+, Li+ and Fe2+; and a decrease when Ca2+ was added. The enzyme activity was not affected by the presence of Ag+, K+ or Mg2+. These results show how diverse the effect of the metallic ions on the chitinases may be. Sutrisno et al. (2004) reported that the chitinase excreted by Ralstonia sp. A-471 was activated by Mn2+, Cu2+, Ca2+ and Mg2+, while Bacillus sp. 13.26 chitinase was slightly activated by Mg2+, inhibited by Ca2+ and significantly affected by Mn2+ and Co2+ (Yuli et al., 2004). Chitinase of Stenotrophomonas maltophilia was inhibited by Hg2+, while K+, Mg2+, Ca2+, Zn2+, Ni2+ and Co2+ did not affect this activity (Zhang et al., 2001). The variability of the effect of metallic ions possibly reflects different enzyme forms synthesized in order to hydrolyze the diverse chitin polymers existing in nature (Patil et al., 2000).
METALLIC ION EFFECT ON RELATIVE â-N-ACETYLGLUCOSAMINIDASE ACTIVITY PRODUCED BY L. lecanii ATCC 26854
Electrophoresis and zymogram
SDS-PAGE electrophoresis of enzyme extract is shown in Figure 4. The gel image consistently shows three protein bands of 31, 48 and 58kDa. Although chitinolytic activity with the chromogenic substrate was detected after 24h in the enzymatic assay, the bands only appeared at 48h, when activity increased. In addition, band intensity increased with fermentation time. These results coincide with previous reports. Krieger et al. (2003) identified a band of 31kDa, with exo and endochitinase activity in M. anisopliae after growing the microorganism on chitin; Baratto et al. (2003) reported a 42kDa protein with endochitinase activity in M. anisopliae, coinciding with other species such as T. harzianum (Carsolio et al., 1994) and Aspergillus nidulans (Baratto et al., 2003); Chul et al. (1998) reported a 58kDa protein in a cDNA library of M. anisopliae ATCC 20500, which presents homologous sequences to proteins from other microorganisms. Figure 4b shows the fluorescence of 4-MU from the zymogram with enzymatic extract visible under UV light, as a consequence of the activity of b-N-acetylglucosaminidase.
Figure 4. a: SDS-PAGE (11%) electrophoresis of enzyme extract from different fermentation times using shrimp shell as C source. M: standard marker, 1: 0h, 2: 24h 3: 48h, 4: 72h, 5: 96h. b: Zymogram of enzyme extract prepared from a 96h culture of L. lecanii; native gel was incubated in an agarose-4MU(GlcNAc) solution for 5min at 37ºC, with 5ìg (1) or 50ìg (2) of crude protein.
In conclusion, the solid-state fermentation system with shrimp shell as the substrate was suitable for L. lecanii b-N-acetylglucosaminidase production, since it induced enzyme activity and did not interfere with protein recovery. Moreover, the antagonistic assay showed that L. lecanii may potentially be used against phytopathogenic fungi on account of its chitinase activity and possibly also because of other metabolites produced by the fermentation process.
References
1. Alonso MJ, Sánchez A (2003) The potential of chitosan in ocular drug delivery. J. Pharm. Pharmacol. 55: 1451-1463. [ Links ]
2. Baratto CM, Da Silva MV, Santi L, Passaglia L, Silviera Schrank I, Henning VM, Schrank A (2003) Expression and characterization of the 42 kDa chitinase of biocontrol fungus Metarhizium anisopliae in Escherichia coli. Can. J. Microbiol. 49: 723-726. [ Links ]
3. Barranco JE (2004) Contribución al Estudio de las Actividades Enzimáticas Involucradas en el Mecanismo de Patogenicidad de Lecanicillium (Verticillium) lecanii Cultivado en Medio Sólido. Tesis. UAM-Iztapalapa, Mexico. 70 pp. [ Links ]
4. Barranco JE, Alatorre R, Gutiérrez M, Viniegra G, Saucedo G (2002) Criteria for the selection of strains of entomopathogenic fungi Verticillium lecanii. Enz. Microbiol. Technol. 30: 910-915. [ Links ]
5. Bing-Lan L, Pao-Min K, Yew-Min T, Kuo-Ching F (2003) Production of chitinase from Verticillium lecanii F091 using submerged fermentation. Enz. Microb. Technol. 33: 410-415. [ Links ]
6. Butt TM, Jackson C, Magan M (2001) Introduction of fungal biological control agents: progress, problems and potential. In Butt TM, Jackson C, Magan N (Eds.) Fungi as Biocontrol Agents. CABI. Publishing New York, USA. pp. 1-8. [ Links ]
7. Carsolio C, Gutiérrez A, Jiménez B, Van Montagu M, Herrera-Estrella A (1994) Characterization of ech-42, a Trichoderma harzianum endochitinase gene expressed during mycoparasitism. Proc. Natl. Acad. Sci. USA 91: 10903-10907. [ Links ]
8. Cerchiara T, Luppi B, Bigucci F, Zecchi V (2003) Chitosan salts as nasal sustained delivery systems for peptidic drugs. J. Pharm. Pharmacol. 55: 1623-1627. [ Links ]
9. Chul S, Park S, Gyu G (1998) Isolation and characterization of a chitinase cDNA from the entomopathogenic fungus Metarhizium anisopliae. FEMS Microbiol. Lett. 165: 267-271. [ Links ]
10. Coudron TA, Kroha MJ, Ignoffo CM (1984) Levels of chitinolytic activity during development of three entomopathogenic fungi. Compr. Biochem. Physiol. 79B: 339-348. [ Links ]
11. Deshpande MV (1999) Mycopesticide production by fermentation: Potential and challenges. Crit. Rev. Microbiol. 25: 229-243. [ Links ]
12. Di Pietro A, Lorito M, Hayes CK, Broadway RM, Harman GE (1993) Endochitinase from Gliocladium virens: Isolation, characterization and synergistic antifungal activity in combination with gliotoxin. Phytopathology 83: 308-313. [ Links ]
13. Duo LI, Chen S, Jing LU (2005) Purification and partial characterization of two chitinases from the mycoparasitic fungus Talaromyces flavus. Mycopathologia 159: 223-229. [ Links ]
14. Froyd JD (1997) Can synthetic pesticides be replaced with biologically based alternatives? –an industry perspective. J. Ind. Microbiol. Biotechnol. 194: 192-195. [ Links ]
15. Guthrie JL, Khalif S, Castle AJ (2005) An improved method for detection and quantification of chitinase activities. Can. J. Microbiol. 51: 491-495. [ Links ]
16. Harman GE, Hayes CK, Lorito M, Broadway RM, Tronsmo A, Woo SL, Di Pietro A (1993) Chitinolytic enzymes produced by Trichoderma harzianum: Purification of chitobiosidase and endochitinase. Phytopathology 83: 313-318. [ Links ]
17. Krieger C, Schrank A, Henning VM (2003) Regulation of extracellular chitinases and proteases in the entomopathogen and acaricide Metarhizium anisopliae. Curr. Microbiol. 46: 205-210. [ Links ]
18. Kubicek CP, Mach RL, Peterbauer CK, Lorito M (2001) Trichoderma from genes to biocontrol. J. Plant. Pathol. 83: 11-23. [ Links ]
19. Kunamneni A, Permaul K, Singh S (2005) Amylase production in solid state fermentation by the thermophilic fungus Thermomyces lanuginosus. J. Biosci. Bioeng. 100: 168-171. [ Links ]
20. Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685. [ Links ]
21. Lorito M, Peterbauer CK, Hayes CK, Woo SL, Harman GE (1994) Synergistic combination of cell wall degrading enzymes and different antifungal compounds enhances inhibition of spore germination. Microbiology 140: 623-629. [ Links ]
22. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 193: 265-275. [ Links ]
23. Marquardt DL (1963) An algorithm for the least squares estimation of non linear parameters. J. Soc. Ind. Appl. Math 2: 431-441. [ Links ]
24. Matsumoto Y, Saucedo-Castañeda G, Revah S, Shirai K (2004) Production of b-N-acetylhexosaminidase of Verticillium lecanii by solid state and submerged fermentations utilizing shrimp waste silage as substrate and inducer. Process Biochem. 39: 665-671. [ Links ]
25. Nahar P, Ghormade V, Deshpande MV (2004) The extracellular constitutive production of chitin deacetylase in Metarhizium anisopliae: possible edge to entomopathogenic fungi in the biological control of insect pests. J. Invertebr. Pathol. 85: 80-88. [ Links ]
26. Noguera RR, Saliba EO, Mauricio RM (2004) Comparación de modelos matemáticos para estimar los parámetros de degradación obtenidos a través de la técnica de producción de gas. Livestock Res. Rural Dev. From http://www.lrrd.org/lrrd16/11/nogu16086.htm [ Links ]
27. Patil RS, Ghormade V, Deshpande MV (2000) Chitinolytic enzymes: an exploration. Enzyme Microbiol Technol 26: 473-483. [ Links ]
28. San W, Wen Ch (1997) Purification and characterization of two bifunctional chitinases/lysozymes extracellularly produced by Pseudomonas aeruginosa K-187 in a shrimp and crab shell powder medium. Appl. Env. Microbiol. 63: 380-386. [ Links ]
29. Saucedo GC, González PB, Revah SM, Viniegra GG, Raimbault M (1990) Effect of lactobacilli inoculation on cassava (Manihot esculenta) silage: fermentation pattern and kinetic analysis. J. Sci. Food Agric. 50: 467-477. [ Links ]
30. Shang G, Jeen C, Wen L (2004) Purification and characterization of extracellular chitinase from Aeromonas schubertti. Enz. Microbiol. Technol. 35: 550-556. [ Links ]
31. Steenberg T, Humber RA (1999) Entomopathogenic potential of Verticillium and Acremonium species (Deuteromycotina:Hyphomycetes). J. Invertebr. Pathol. 73: 309-314. [ Links ]
32. St-Leger RJ, Nelson JO, Screen SE (1999) The entomopathogenic fungus Metarhizium anisopliae alters ambient pH, allowing extracellular protease production and activity. Microbiology 145: 2691-2699. [ Links ]
33. Sutrisno A, Ueda M, Abe Y, Nakazawa M, Miyatake K (2004) A chitinase with high activity toward partially N-acetylated chitosan from a new, moderately thermophilic, chitin-degrading bacterium, Ralstonia sp. A-471. Appl. Microbiol. Biotechnol. 63: 398-406. [ Links ]
34. Tergerdy RP, Szakács G (1998) Perspectives in agrobiotechnology. J. Biotechnol. 66: 91-99. [ Links ]
35. Viniegra G, Favela E, Aguilar CN, Romero-Gómez SJ, Díaz-Godínez G, Augur C (2003) Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem. Eng. J. 13: 157-167. [ Links ]
36. Yuli PE, Suhartono MT, Rukayadi Y, Hwang JK, Pyun YR (2004) Characteristics of thermostable chitinase enzymes from the Indonesian Bacillus sp.13.26. Enz. Microbiol. Technol. 35: 147-153. [ Links ]
37. Zhang Z, Yuen GY, Sarath G, Penheiter AR (2001) Chitinases from the plant disease biocontrol agent, Stenotrophomonas maltophilia C3. Phytopathology 91: 204-211. [ Links ]












 uBio
uBio 




