Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos Venezolanos de Farmacología y Terapéutica
versión impresa ISSN 0798-0264
AVFT v.29 n.3 Caracas sep. 2010
Novel, broad-specrum antimycotic agents: the role of echinocandins today
Roberto Manfredi, MD
Department of Internal Medicine, Aging, and Nephrologic Diseases, Alma Mater Studiurum University of Bologna, S. Orsola-Malpighi Hospital, Bologna, Italy
Conflicts of interest, funding, sponsorship, acknowledgements: none
Correspondence: Prof. Roberto Manfredi. c/o Infectious Diseases, S. Orsola Hospital, Via Massarenti, 11 40138 Bologna
Telefono: 051-6363355. Cellulare: 329-3156697. Telefax: 051-343500. E-mail: Roberto.manfredi@unibo.it
Abstract
The echinocandins show comparable efficacy in the treatment of candidemia and invasive candidiasis. Caspofungin and micafungin appear to be similarly efficacious in salvage therapy in aspergillosis; anidulafungin has excellent in vitro activity against Aspergillus species but as yet there are no sufficient clinical data for anidulafungin in this disease state. Each drug has minor advantages and disadvantages compared to the others of the same classe; however, there are large differences in the approved indications for the different drugs. The formulary selection process should consider the direct and indirect costs of the single agents; the characteristics of the patient population at risk for invasive mycosis, such as frequent use of interacting drugs and the burden of monitoring plasma drug levels of drugs; and the implications of using products for indications which have not been still approved (off-label indications).
Key words: antifungal drugs, echinocandins, caspofungin, anidulafungin, micafungin, indications, clinical studies
Recibido: 07/06/2010 Aceptado: 01/09/2010
Introduction
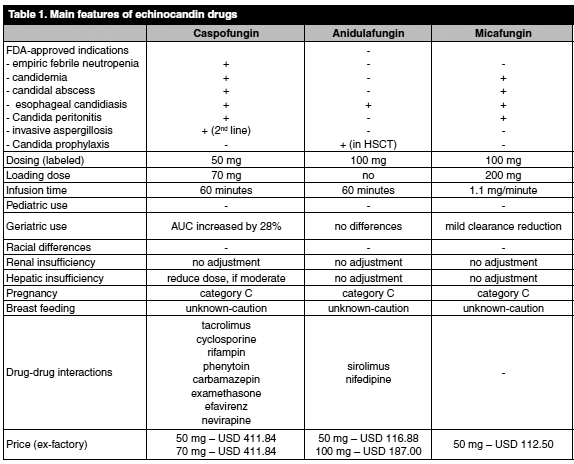
The echinocandins are a class of drugs that have made an enormous impact on the treatment of fungal infections. Less expensive than lipid formulations of amphotericin B, they have less toxicity than amphotericin products and fewer drug interactions than azoles. Efficacy for yeast/Candida species is comparable to amphotericin-based products, and they have activity against many mold species. Caspofungin was the first echinocandin approved by the FDA, coming on the market in 2001. Since then, two more products have been approved: micafungin (2005) and anidulafungin (2006). The development of competition in the echinocandin market has prompted a class review of these drugs in order to determine the choice with the most favorable balance of economics, safety and efficacy. The indications, dosing and costs are summarized in Table 1.
Pharmacological issues: an introduction
The echinocandins are large, semisynthetic, injectable lipopeptides derived from fungal fermentation products6,15,30. Their molecular weights range from 1,140 to 1,292 daltons. The echinocandins inhibit the growth of fungi by interfering with the synthesis of the fungal cell wall component 1,3- -Dglucan, a large polysaccharide that provides rigidity to the cell wall. The pharmacokinetic properties of the echinocandins are quite similar, and are summarized in Table 2.
Antimycotic activity
All three of the currently available echinocandins have in vitro activity against a variety of species of Candida, Aspergillus and other opportunistic fungi. The relationship between in vitro activity and clinical efficacy against fungal isolates is unclear; interpretive criteria have not yet been defined8. Tables 3 and 4 summarize the in vitro activity of the available echinocandins, according to consolidated literature evidences.

Caspofungin vs. Candida
The in vitro activity of caspofungin against Candida species has been well documented. In six studies using 7,109 clinical isolates of various species of Candida, caspofungin inhibited the overwhelming majority of isolates at concentrations ≤2 mcg/mL19,20,27,28,36,37. This held true even for fluconazole-resistant isolates. Candida parapsilosis isolates tended to have higher MICs than other species, but most were inhibited at or below 2 mcg/mL, and one study reported an MIC90 of >8 for 75 isolates of Candida guilliermondii28. Two studies reported overall MICs for all isolates in aggregate; for 751 isolates the MICs were 0.25-0.5 mcg/mL7,8.
Micafungin vs. Candida
Three studies evaluated the in vitro activity of micafungin against 551 clinical Candida isolates22,23,35. In one study of 315 fluconazole-resistant isolates, the overall MIC90 was 0.06 mcg/mL; C. glabrata isolates were the most sensitive to micafungin, with an overall MIC90 of 0.015 mcg/mL for 110 isolates22. A second study also found excellent activity against all species of Candida, although MICs for C. parapsilosis were among the highest, ranging from 0.5 to 2 mcg/mL23.The third study found micafungin to be the least active when compared to several azole antifungals, amphotericin B and flucytosine, with an overall MIC90 for 164 isolates greater than 8 mcg/mL, but this finding was primarily due to the high MIC90s of the 16 isolates of C. parapsilosis19.
Anidulafungin vs. Candida
The in vitro activity of anidulafungin against 3,251 clinical isolates of Candida species was evaluated in four studies1,5,30,37. Isolates of C. albicans and C. glabrata were highly susceptible to anidulafungin in all the four studies, with MIC90s of less than 2 mcg/mL. Higher MICs values were observed with isolates of C. parapsilosis in most of the studies, ranging from 2-8 mcg/mL. MICs for C. tropicalis, Candida dubliniensis, Candida famata and C. guilliermondii were also found to be higher in some studies1,30.
Caspofungin vs. Other Fungi
The in vitro activity of caspofungin against 700 isolates of Aspergillus species was evaluated in three studies6,11,12. The great majority of isolates were highly susceptible to caspofungin, although in one study, the range of MICs for 13 isolates of Aspergillus fumigatus was 0.5->16 mcg/mL, with a mean MIC of 2.15 mcg/mL11. The largest study included isolates from environmental sources as well as clinical sources; caspofungin was potently active with an MIC90 of less than 0.007 for all isolates regardless of the source12. Espinel-Ingroff et al. also evaluated the in vitro activity of caspofungin against other opportunistic fungi11. Caspofungin proved moderately active against Cladophiliophora bantiana, Bipolaris species, Scedosporidium prolifififi cans, Blastomyces dermatitidis and Histoplasma capsulatum, with MICs ranging from 2-8 mcg/mL. On the other hand, aspofungin tested inactive against Fusarium species, Rhizopus arrhizus, Cryptococcus neoformans and Trichosporon beigelii.
Micafungin vs. Other Fungi
The in vitro activity of micafungin against 596 environmental and clinical isolates of A. fumigatus was compared to that of five other antifungal agents21. Micafungin exhibited a very low MIC90 (<0.007 mcg/mL) for all isolates regardless of whether the organism was obtained from a clinical or environmental site. The in vitro activity of micafungin against 16 species of molds was evaluated by Nakai et al.25. Micafungin was highly active against all the six species of Aspergillus and had intermediate activity against Cladosporium trichoides, two Exophiala species and Fonsecaea pedrosoi. Micafungin was inactive against Absidia corymbifera, Cunninghamella elegans, two Rhizopus species, Fusarium solani and Pseudallescheria boydii.
Anidulafungin vs. Other Fungi
The in vitro antifungal activity of anidulafungin against Aspergillus spp. was initially compared to those of four other antifungal agents6. Anidulafungin was highly active against all 68 strains, with MICs of 0.03 mcg/mL for all strains tested. Later, Zhanel et al. evaluated the in vitro activity of anidulafungin against 64 clinical isolates of Cryptococcus neoformans, Blastomyces dermatitidis and Aspergillus species37. Anidulafungin potently inhibited all the five tested Aspergillus spp. It was ineffective against C. neoformans and B. dermatitidis.
Available clinical trials
Caspofungin in Candida infections
Caspofungin was compared to amphotericin B deoxycholate for invasive candidiasis in a double-blind, randomized trial in adult patients24. Eligible patients were adults with at least one positive culture for Candida from blood or another sterile site plus at least one sign of infection. Stepdown therapy with oral fluconazole was permitted, if clinically warranted, after 10 days of IV therapy with the study drug. Patients were stratified according to APACHE score and randomized to receive either caspofungin as a 70-mg loading dose followed by 50 mg per day or amphotericin B at a dose of 0.6 to 0.7 mg/kg/day for non-neutropenic patients and 0.7 to 1 mg/kg/day for neutropenic patients. The primary efficacy measure was overall response to therapy, with a favorable response defined as resolution of all symptoms and signs of the infection as well as microbiological eradication. Caspofungin would be considered non-inferior to amphotericin B if there was less than 20% difference between the two study groups once APACHE scores and neutropenia were accounted for. The rates of favorable response at the end of IV therapy did not differ significantly between the two groups (73.4% for caspofungin vs. 61.75 for amphotericin B; p=0.09). Among the 186 patients who met prespecified criteria for evaluation (inclusion in MITT analysis, no concomitant antifungal therapy, no protocol violations that could interfere with efficacy assessment, appropriate evaluation at the end of therapy and receipt of study drugs for five days or more), the respective response rates were 80.7% and64.9% (p=0.03); the criteria for non-inferiority were met. There were significantly more patients in the amphotericin group who had adverse events due to study drug (33 patients in the caspofungin MITT group; 28.9% vs. 73 patients in the amphotericin group; 58.4%; p=0.002). Significantly more patients in the amphotericin group withdrew from the study due to adverse events (23.2% vs. 2.6%; p=0.003). The authors concluded that caspofungin was as effective as amphotericin B for the treatment of invasive candidiasis and was less toxic than amphotericin B. Caspofungin was compared to amphotericin B deoxycholate for the treatment of endoscopically-confirmed esophageal candidiasisin a randomized, double-blind trial35. There were 128 patients enrolled in the study; they were randomized to receive caspofungin 50 mg/day, caspofungin 70 mg/day, or amphotericin B deoxycholate 0.5 mg/kg/day. A favorable response was defined as the resolution of symptoms plus either clearing of esophageal lesions or an improvement of two or more grade levels. The response rates at the end of therapy were high for all three treatments (85% for caspofungin 50 mg, 96% for caspofungin 70 mg, and 72% for amphotericin B). While the response rates were higher for the caspofungin groups, the study was not designed to show superiority, so no conclusions about the relative efficacy can be drawn. Response rates were similar regardless of the endoscopic grade of the lesions at enrollment.There was a significant difference in the proportion of patients who experienced adverse effects related to study drug (61% for caspofungin 50 mg, 68% for caspofungin 70 mg, and 93% for amphotericin; p<0.01 for each caspofungin group compared to amphotericin). The authors concluded that caspofungin was effective and well-tolerated in the treatment of esophageal candidiasis; the study was not designed to show non-inferiority. Villanueva et al. compared caspofungin to fluconazole for the treatment of esophageal candidiasis in a double-blind, randomized study36. One hundred seventy-seven adult patients with endoscopically-and microbiologically-confirmed candidal esophagitis were randomized to receive caspofungin 50 mg IV daily or fluconazole 200 mg IV daily. The primary efficacy endpoint was clinical plus endoscopic response. The combined clinical plus endoscopic response rates among the modified intent-to-treat population were 81% for the caspofungin group and 80% for the fluconazole group. There were no significant differences between the groups in the rates of endoscopic response, clinical response or microbiological response. Relapse rates at the two-week and four-week follow-up visits did not differ significantly between the two treatment groups. Adverse events occurred in over 30% of the patients in each group, but only one event, a cellulitis in a fluconazole-treated patient, was considered serious. There were no statistically significant differences between the groups in the incidence of individual adverse effects. The authors concluded that caspofungin was not inferior to fluconazole for the treatment of esophageal candidiasis, and that both drugs were well-tolerated. Kartsonis et al. evaluated the safety and efficacy of caspofungin in an open-label, compassionate-use study in adult patients with esophageal/pharyngeal or invasive candidiasis who had failed therapy with an IV formulation of amphotericin B due to either inability to tolerate the drug or to refractory infection14. The 37 patients enrolled received a 70-mg loading dose followed by 50 mg daily. The primary efficacy measure was a favorable response, defined for mucosal infections as resolution or significant improvement in symptoms; a normal followup oropharyngeal examination was also required in patients with oropharyngeal candidiasis. For invasive infections, a favorable response included resolution or significant improvement of signs and symptoms and radiographic studies and negative results of follow-up cultures. Among patients with mucosal infections there was a favorable response rate of 86%; the rate among patients with invasive infections was 87%. The favorable response rates were high (93% and 83%, respectively) among the 29 patients who had failed previous antifungal therapy. The response rates were similar regardless of the pathogen identified. The mean duration of therapy was 31.4 days and was similar for patients with mucosal and systemic infections. Six patients died during the study, although none of the deaths was attributed to the use of caspofungin or to the Candida infection. One patient experienced an adverse event attributed to caspofungin; a decreased platelet count was observed in a patient who was already thrombocytopenic due to an underlying HIV disease. The authors concluded that caspofungin is safe and effective in treating difficult Candida infections.
Micafungin in Candida infections
DeWet et al. compared micafungin to fluconazole in a randomized, double-blind, dose-ranging study in adult patients with endoscopically-confirmed esophageal candidiasis9. There were 251 patients randomized to receive either fluconazole 200 mg IV daily or micafungin 50 mg, 100 mg or 150 mg IV daily. The primary endpoint of the study was endoscopically confirmed cure, defined as a mucosal condition of zero (no evidence of lesions) on a 0-3 scale. The mean durations of therapy in the micafungin 50 mg, 100 mg and 150 mg groups were 16.3, 13.4 and 14.0 days, respectively, while in the fluconazole group it was 14.0 days. The cure rates among micafungin-treated patients were dose-related at 68.6%, 77.4% and 89.8% for the 50 mg, 100 mg and 150 mg doses and 86.7% for the fluconazole group in the ITT population. The two higher doses of micafungin had significantly higher cure rates than the 50 mg dose in the per-protocol population, and the 150 mg dose was significantly better than the 50 mg dose in the ITT population. Fluconazole also had a significantly higher cure rate than the 50 mg dose of micafungin, but the two higher doses of micafungin did not differ from fluconazole. For the analysis of secondary endpoint response rates, the authors combined the 100 mg and 150 mg doses of micafungin and compared the combined group to fluconazole, finding no significant difference between the combined group and fluconazole. Nine patients who received micafungin relapsed (one from the 50 mg group, five from the 100 mg group, and two from the 150 mg group); no patient from the fluconazole group experienced a relapse. Adverse events were common for patients receiving either drug, but these were generally mild or moderate and did not differ significantly in nature between the groups. The authors concluded that micafungin at 100 mg or 150 mg per day was comparable to fluconazole in the treatment of esophageal candidiasis in patients with HIV infection. A second study in 523 patients with esophageal candidiasis compared the efficacy and safety of micafungin 150 mg daily to that of fluconazole10. Patients at least 16 years old with symptomatic esophageal candidiasis that was confirmed by endoscopy were eligible for enrollment. Patients were randomized to receive either micafungin 150 mg IV daily or fluconazole 200 mg IV daily. The primary efficacy endpoint was a mucosal condition of zero on a 0-3 scale. The mean duration of therapy for both groups was 14 days. The rates of endoscopically-confirmed cures (mucosal condition of zero) were 87.7% for micafungin and 88.0% for fluconazole. The clinical success rates, which included patients with cures and with two-point improvements in mucosal condition, were 94.2% and 94.6%, respectively. Relapse rates did not differ significantly between the groups. Adverse event rates were similar and there was little difference between the groups in the type and frequency of events. The authors concluded that micafungin 150 mg daily was not inferior to fluconazole 200 mg daily for the treatment of esophageal candidiasis. Ostrosky-Zeichner et al. evaluated the use of micafungin in 126 adult and pediatric patients with candidemia, including cases refractory to at least five days of therapy with an alternate systemic antifungal27. Micafungin was dosed at 50 mg/day IV for C. albicans infections and 100 mg/day for non-albicans or germ tube-negative infections in patients weighing 40 kg or more; the dose could be increased in 50 mg increments as deemed necessary by the investigator. For patients weighing less than 40 kg, the dose was 1-2 mg/kg with the possibility of increasing the dose by 1 mg/kg increments. Micafungin was the sole therapy in patients with new infections; patients who had failed therapy could receive micafungin alone or in combination with their current therapy. The primary endpoint of the study was complete or partial response as determined by the investigators at the end of therapy. Among the 72 patients with new infections, 63 (87.5%) were treatment successes, with 55 (76.4%) complete responses; eight patients (11.1%) had partial responses. Seven patients (9.7%) had stable or progressive disease. Two patients were not evaluable. Among the patients who had failed other therapy or prophylaxis, there were 54 (77.8%) who had a complete response, two (3.7%) who had a partial response and 10 (18.4%) who had stable or progressive disease. In this group of patients, the results were similar regardless of whether the patients were treated with micafungin alone or received micafungin in addition to their previous therapy. Overall response rates were greater than 80% for patients with infections due to C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis. The response rate was lower (63.6%) with C. krusei infections. The highest response rates (≥90%) were seen in patients receiving 75-150 mg/day. The overall response rate among adult the patients was 84.9%, while in children, including 11 neonates, it was 75.0%. Adverse effects were generally mild and occurred in only 7.4% of patients, a rate far lower than that observed in other clinical trials for micafungin. The most common adverse event, occurring in three (2%) patients, was thrombocytopenia. The authors concluded that micafungin is safe and effective for use as a first-line agent and as salvage therapy in Candida bloodstream infections caused by a variety of species. In a randomized, double-blind, non-inferiority study presented in abstract form at the 46th ICAAC, micafungin 100 mg/day and 150 mg/day were found to be non-inferior to caspofungin as a 70 mg loading dose followed by 50 mg/day in the treatment of invasive candidiasis3. Patients received at least 10 days of study drug, after which they could be converted over to oral therapy. The overall success rates in the intent-to-treat population were 73.9% for micafungin 100 mg/day, 70.3% for micafungin 150 mg/day and 71.4% for caspofungin. There was no advantage in dosing micafungin at 150 mg/day over 100 mg/day. There were no differences in safety among the three treatment arms.
Anidulafungin in Candida infections
Krause et al. evaluated the use of anidulafungin in the treatment of esophageal candidiasis in a randomized, dose-ranging study in 123 adult patients17. Patients with positive blood or tissue cultures plus at least one sign or symptom of infection were randomized to receive 50 mg, 75 mg or 100 mg of IV anidulafungin once daily. The primary efficacy endpoint was global response, which included both clinical and microbiologic response. The global response rates were similar for all three doses (84%, 90% and 89% for the 50-mg, 75-mg and 100 mg-doses, respectively) at the end of therapy. The microbiological response rates were higher for the 75-mg and 100-mg doses (93% and 89%, respectively) than for the 50-mg dose (84%), but no statistical significance was reported for this difference. Just fewer than 30% of patients experienced an adverse event that was considered to be related to therapy. Most events were of mild or moderate severity. The most common of these events was hypokalemia, occurring in four patients (10%) in the 50-mg dose group. The authors concluded that anidulafungin at 100 mg/day was as effective as other treatment options for esophageal candidiasis, and that it was well-tolerated. A randomized, double-blind, double-dummy trial compared anidulafungin to fluconazole for the treatment of esophageal candidiasis18. Adult patients (n=601) with endoscopically- and microbiologically-confirmed esophageal candidiasis plus at least one sign or symptom of infection were randomized to receive either anidulafungin 100 mg IV on day one, followed by 50 mg/day plus oral placebo or fluconazole 200 mg PO on day one, followed by 100 mg/day plus IV placebo. The primary efficacy endpoint was endoscopic response at the end of therapy. The response rates among the intent-to-treat population were statistically similar (86.7% for anidulafungin and 88.0% for fluconazole). The two treatments were similar in the rates of clinical and mycologic responses as well. Among the 462 patients who were evaluated endoscopically two weeks after the end of treatment, significantly more patients in the fluconazole group had sustained endoscopic responses compared to the anidulafungin group (89.9% vs. 64.5%, respectively; p<0.001). Adverse events related to treatment occurred in 9.3% of patients in the anidulafungin group and 12.0% of patients in the fluconazole group. Few serious adverse events attributed to study drugs were reported. There were three patients in the fluconazole group and two in the anidulafungin group who withdrew due to adverse events. Th e authors concluded that the two drugs were similarly effective and well-tolerated in treating esophageal candidiasis, but that fluconazole produced more sustained responses. There were more patients in the fluconazole group who were taking antiretrovirals drugs, a factor that could confound this analysis, but the authors do not indicate whether this difference was statistically significant.
Caspofungin in Aspergillus infections
Maertens et al. evaluated caspofungin as salvage therapy for invasive aspergillosis (IA) in an open-label, noncomparative trial20. Ninety patients with probable or proven IA who had disease progression or lack of improvement with at least seven days of amphotericin B, lipid amphotericin B or itraconazole, or who had nephrotoxicity, increased serum transaminases or severe infusion reactions with those therapies were enrolled. The patients received a 70-mg loading dose IV and a 50-mg dose daily thereafter. The primary efficacy endpoint was clinical response. Among the modified intent-to-treat population, 44.6% of patients had a favorable response to therapy, 7% had stable disease, and 48% were considered treatment failures. Of those patients who had a favorable response, the great majority (89.2%) had a partial rather than a complete response. The response rates were significantly higher among patients with hematologic malignancies compared to those who had undergone HSCT (41.7% vs. 14.3%, respectively; p=0.01). Significantly higher response rates were seen among patients enrolled due to intolerance to conventional therapy compared to those with refractory infections (75.0% vs. 39.4%, respectively; p=0.03). Three of the 31 patients who had had a clinical response and were also evaluated at the four-week follow-up visit were found to have relapsed, despite receiving suppressive therapy with itraconazole. Most of the study participants (93.3%) experienced at least one adverse effect, but only 12.2% of the participants had an untoward effect that was considered to be related to caspofungin. All but one was considered to be mild or moderate in severity. The authors concluded that caspofungin was effective and welltolerated as salvage therapy in IA. A second study evaluated caspofungin as salvage therapy in 48 adult patients with IA using the same methods and enrollment criteria as the Maertens study15. The majority of the enrollees (90%) had IA refractory to conventional therapy. The primary efficacy endpoint was clinical, radiographic and bronchoscopic response. The rate of favorable responses to caspofungin was 44.4%; of the favorable responses, 55% were partial responses and 45% were complete responses. The rate of unfavorable responses was 44.4% and for stable disease the rate was 11.1%. Factors associated with a lower favorable response rate were underlying hematologic disease, extrapulmonary aspergillosis and infection refractory to multiple alternate agents, although the authors did not report p values for all these findings. Half of the patients enrolled in the study died during the study or follow-up period, with the majority of those (79%) dying as a result of IA. Five patients experienced adverse events associated with the use of caspofungin. Only one of these events (anaphylaxis) was considered serious enough to discontinue the study drug. The authors concluded that caspofungin was safe and effective as salvage therapy for IA.
Micafungin in Aspergillus infections
Kohno et al. evaluated micafungin in the treatment of deepseated Aspergillus and Candida infections16. Seventy adult patients with clinical and mycological evidence of invasive mycoses were treated with micafungin at doses ranging from 12.5 to 50 mg/day. The authors did not indicate how an initial dose was chosen; the daily dose could be escalated at seven-day intervals in aspergillosis and four-day intervals in candidiasis. The primary efficacy endpoint of the study was overall response. Study results were presented only for the 56 patients considered evaluable by the investigators. Four of the 14 patients not evaluated were eliminated because they received fewer than seven days of therapy, and 10 patients did not match the appropriate diagnostic criteria. Of the 56 evaluable patients, 42 had aspergillosis and 14 had candidiasis. Twenty-four (57%) of the patients with aspergillosis responded to therapy; response rates to the 150 mg dose were 80% for invasive pulmonary aspergillosis, 0% for disseminated aspergillosis; 75% for chronic necrotizing pulmonary aspergillosis and 67% for pulmonary aspergilloma. The corresponding response rates for the 75 mg dose were 33%, not available, 67%, and 63%. Among the patients with candidiasis, all patients who received 50 mg and 75 mg doses responded; the two patients with esophageal candidiasis who received 25 mg doses did not respond. The investigators did not differentiate between complete and partial responses. Adverse events related to micafungin were experienced by 30% of patients. The only event that was considered serious was neutropenia in a patient who withdrew from the study. The authors concluded that micafungin was safe and effective in the treatment of deep-seated fungal infections. Micafungin was evaluated in an open-label, non-comparative study in 331 patients with invasive aspergillosis who had failed or were intolerant to conventional therapy, or who had received less than 48 hours of other systemic antifungal therapy (the so-called primary patients)7. Patients with proven or possible invasive aspergillosis received micafungin 75 mg IV per day, or 1.5 mg/kg/day for patients weighing less than 40 kg. The dose could be increased in 75 mg/day or 1.5 mg/kg/day increments in 7-day intervals if cultures were persistently positive or if patients did not improve. Patients could continue to receive their prior therapy in addition to micafungin or could receive micafungin alone. The primary efficacy endpoint was favorable response to therapy based on clinical, radiologic and microbiologic evaluations. The rate of favorable (complete or partial) response among the modified intent-to-treat population was 35.6%, with another 11.1% of cases attaining stabilization of disease. The great majority of patients (85.3%) in this population were enrolled as refractory to their previous therapy. Among the refractory patients, 40.9% had a favorable response to micafungin as monotherapy (13.6% with a complete response and 27.3% with a partial response). The response rates for micafungin in combination were judged 34.5% favorable, 7.5% complete and 27.0% partial. Among the primary group, response rates were 50.0% favorable, 0% complete and 50% partial for patients receiving micafungin alone, and 29.4%, 17.6% and 11.8%, respectively for micafungin in combination. Lower response rates were seen in patients with neutropenia, HSCT and HIV/AIDS. The mean daily dose administered to adults was 111.4 ± 50.97 mg. Sixty-seven percent of patients required at least one dose escalation. Of the 145 patients seen for a six-week follow-up visit, 32.4% had a complete or partial response at that time. Adverse events considered to be attributable to study drug occurred in 31.9% of patients. The most commonly occurring effects were bilirubinemia, nausea, liver function test abnormalities and diarrhea. Moderate or severe adverse events occurred in 23.9% of patients and 3.1% of patients experienced a life-threatening adverse event. The authors concluded that micafungin is an effective treatment for invasive aspergillosis and is well-tolerated.
References
1. Arévalo MP, Carillo-Mu oz A-J, Salgado J, et al. Antifungal activity of the echinocandin anidulafungin (VER002, LY-303366) against yeast pathogens: a comparative study with M27-A microdilution method. J Antimicrob Chemother 2003; 51:163-166. [ Links ]
2. Barchiesi F, Schimizzi AM, Fothergill AW, et al. In vitro activity of the new echinocandin, MK-0991, against common and uncommon clinical isolates of Candida species. Eur J Clin Microbiol Inf Dis 1999; 18:302-304. [ Links ]
3. Betts RF, Rotstein C, Talwar D, et al. Comparison of micafungin and caspofungin for candidemia or invasive candidiasis. Presented as an abstract at the 46th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), September 27-30, 2006. [ Links ]
4. Cuenca-Estrella M, Rodriguez D, Almirante B, et al. In vitro susceptibilities of bloodstream isolates of Candida species to six antifungal agents: results from a population-based active surveillance programme, Barcelona, Spain, 2002-2003. J Antimicrob Chemother 2005; 55:194-199. [ Links ]
5. Cuenca-Estrella M, Gomez-Lopez A, Mellado E, et al. Head-to-head comparison of the activities of currently available antifungal agents against 3,378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrob Agents Chemother 2006; 50:917-921. [ Links ]
6. Del Carmen Serrano M, Valverde-Conde A, Chávez M, et al. In vitro activity of voriconazole, itraconazole, caspofungin, anidulafungin (VER-002, LY303366) and amphotericin B against Aspergillus spp. Diagn Microbiol Infect Dis 2003; 45:131-135. [ Links ]
7. Denning DW, Marr KA, Lau WM, et al. Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J Infect 2006; 53:337-349. [ Links ]
8. De Rosa FG, Garazzino S, Pasero D, Di Perri G, Rainieri VM. Invasive candidiasis and candidemia: new guidelines. Minerva Anestesiol 2008; Dec 17 (Epub ahead of print) [ Links ]
9. DeWet N, Llanos-Cuentas A, Suleiman J, et al. A randomized, doubleblind, parallel-group, dose-finding study of micafungin compared with fluconazole for the treatment of esophageal candidiasis in HIV-positive patients. Clin Infect Dis 2004; 39:842-849. [ Links ]
10. DeWet NTE, Bester AJ, Viljoen JJ, et al. A randomized, double-blind, comparative trial of micafungin (FK463) vs. fluconazole for the treatment of oesophageal candidiasis. Aliment Pharmacol Ther 2005; 21:899-907. [ Links ]
11. Espinel-Ingroff A. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol 1998; 36:2950-2956. [ Links ]
12. Guinea J, Peláez T, Alcalá L, et al. Antifungal susceptibility of 596 Aspergillus fumigatus strains isolated from outdoor air, hospital air, and clinical samples: Analysis by site of isolation. Antimicrob Agents Chemother 2005; 49:3495-3497. [ Links ]
13. Hebert MF, Smith HE, Marbury TC, et al. Pharmacokinetics of micafungin in healthy volunteers, volunteers with moderate liver disease, and volunteers with renal dysfunction. J Clin Pharmacol 2005; 45:1145-1152. [ Links ]
14. Kartsonis NA, Saah A, Lipka J, et al. Second-line therapy with caspofungin for mucosal or invasive candidiasis: results from the caspofungin compassionate-use study. J Antimicrob Chemother 2004; 53:878-881. [ Links ]
15. Kartsonis NA, Saah AJ, Lipka CJ, et al. Salvage therapy with caspofungin for invasive aspergillosis. Results from the Caspofungin Compassionate Use Study. J Infect 2005; 50:196-205. [ Links ]
16. Kohno S, Masaoka T, Yamaguchi H, et al. A multicenter, open-label clinical study of micafungin (FK463) in the treatment of deep-seated mycosis in Japan. Scand J Infect Dis 2004; 36:372-379. [ Links ]
17. Krause DS, Reinhardt J, Vasquez JA, et al. Phase 2, randomized, dose ranging study evaluating the safety and efficacy of anidulafungin in invasive candidiasis and candidemia. Antimicrob Agents Chemother 2004; 48:2021-2024. [ Links ]
18. Krause DS, Simjee AE, van Rensberg C, et al. A randomized, doubleblind trial of anidulafungin versus fluconazole for the treatment of esophageal candidiasis. Clin Infect Dis 2004; 39:770-775. [ Links ]
19. Laverdiere M, Hoban D, Restieri C, Habel F. In vitro susceptibilities of three new triazoles and one echinocandin against Candida bloodstream isolates from cancer patients. J Antimicrob Chemother 2002; 50:119-123. [ Links ]
20. Maertens J, Raad I, Petrikkos G, et al. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin Infect Dis 2004; 39:1563-1571. [ Links ]
21. Marco F, Pfaller MA, Messer SA, Jones RN. Activity of MK-0991 (L-743,872), a new echinocandin, compared with those of LY303366 and four other antifungal agents tested against bloodstream isolates of Candida species. Diagn Microbiol Infect Dis 1998; 31:33-37. [ Links ]
22. Messer SA, Diekema DJ, Boyken L, et al. Activities of micafungin against 315 invasive clinical isolates of fluconazole-resistant Candida spp. J Clin Microbiol 2006; 44:324-326. [ Links ]
23. Mikamo H, Sato Y, Tamaya T. In vitro activity of FK463, a new watersoluble echinocandinlike lipopeptide. J Antimicrob Chemother 2000; 46:485-487. [ Links ]
24. Mora-Duarte J, Betts R, Rotstein C, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. New Engl J Med 2002; 347:2020-2029. [ Links ]
25. Nakai T, Uno J, Otomo K, et al. In vitro activity of FK463, a novel lipopeptide antifungal agent, against a variety of clinically important molds. Chemother 2002; 48:78-81. [ Links ]
26. Ostrosky-Zeichner L, Rex JH, Pappas PG, et al. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob Agents Chemother 2003; 47:3149-3154. [ Links ]
27. Ostrosky-Zeichner L, Kontoyiannis D, Raffalli J, et al. International, open-label, noncomparative clinical trial of micafungin alone and in combination for treatment of newly diagnosed and refractory candidemia. Eur J Clin Microbiol Infect Dis 2005; 24:654-661. [ Links ]
28. Pfaller MA, Diekema DJ, Messer SA, et al. In vitro activities of caspofungin compared with those of fluconazole and itraconazole against 3,959 clinical isolates of Candida species, including 157 fluconazole-resistant isolates. Antimicrob Agents Chemother 2003; 47:1068-1071. [ Links ]
29. Pfaller MA, Messer SA, Boyken L, et al. Caspofungin activity against clinical isolates of fluconazole-resistant Candida. J Clin Microbiol 2003; 41:5729-5731. [ Links ]
30. Pfaller MA, Boyken L, Hollis RJ, et al. In vitro activities of anidulafungin against more than 2,500 clinical isolates of Candida spp., including 315 isolates resistant to fluconazole. J Clin Microbiol 2005; 43:5425-5427. [ Links ]
31. Product information. Cancidas (caspofungin). Whitehouse Station, NJ: Merck & Co.; 2005. [ Links ]
32. Product information. Eraxis (anidulafungin). New York, NY: Pfizer, Inc.; 2006. [ Links ]
33. Product information. Mycamine (micafungin). Deerfield, IL: Astellas Pharma US, Inc.; 2005. [ Links ]
34. Theuretzbacher U. Pharmacokinetics/pharmacodynamics of echinocandins. Eur J Clin Microbiol Inf Dis 2004; 23:805-812. [ Links ]
35. Villanueva A, Arathoon EG, Gotuzzo E, et al. A randomized, doubleblind study of caspofungin versus amphotericin for the treatment of candidal esophagitis. Clin Infect Dis 2001; 33:1529-1535. [ Links ]
36. Villanueva A, Gotuzzo E, Arathoon EG, et al. A double-blind, randomized study of caspofungin versus fluconazole for the treatment of esophageal candidiasis. Am J Med 2002; 113:294-299. [ Links ]
37. Zhanel GG, Karlowsky JA, Harding GAJ, et al. In vitro activity of a new semisynthetic echinocandin, LY-303366, against systemic isolates of Candida species, Cryptococcus neoformans, Blastomyces dermatitidis, and Aspergillus species. Antimicrob Agents Chemother 1997; 41:863-865. [ Links ]