Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Zootecnia Tropical
versión impresa ISSN 0798-7269
Zootecnia Trop. vol.32 no.2 Maracay jun. 2014
Evaluation of crude glycerine inclusion in beef cattle diet: apparent nutrient digestibility and microbial protein synthesis
Evaluación de la inclusión de glicerina cruda en dietas para ganado de carne: digestibilidad aparente de los nutrientes y síntesis de proteína microbiana
Roman D. Castañeda Serrano1, Antonio Ferriani Branco2, Silvana Teixeira2 y Milene Osmari2
1 Universidad del Tolima. Ibagué, Tolima, Colombia. Grupo de investigación en sistemas agroforestales pecuarios. Correo electrónico: rcastaneda@ut.edu.co
2 Universidade Estadual de Maringá. Programa de Pós-graduação em Zootecnia. Grupo de pesquisa em nutrição de ruminantes. Brasil.
ABSTRACT
Crude glycerine (CG) is an abundant biodiesel byproduct and an alternative material for livestock diets. The objective of this study was to evaluate the effects of glycerol supplementation on the digestibility and microbial protein synthesis in Nelore steers. Five ruminally cannulated Nelore breed steers (522±43 kg) were used in a replicated 5×5 Latin Square design to evaluate 0 (control), 3, 6, 9 and 12% of CG in total dry matter of diets during five experimental periods of 21 days. The dry matter intake (DMI), ruminal apparent digestibility (RAD), intestinal apparent digestibility (IAD), total apparent digestibility coefficient (TAD) of dry matter (DM), organic matter (OM), crude protein (CP), neutral detergent fiber (NDF) and non-fiber carbohydrates (NFC) were not affected (P > 0,05) by CG inclusion. Fecal flow of ether extract (EE) decreased linearly (P < 0.05) as the level of CG in the diet increased. However, the CDI and CDT of EE increased linearly (P < 0.05) as the level of CG in the diet increased. The inclusion of different levels of CG in the diet showed no significant (P > 0.05) effect on microbial protein synthesis. It is concluded that CG may be used in the diet of beef cattle up to 12% and can be considered a good alternative energy.
Key words: biodiesel, co-products, glycerol, ruminants.
RESUMEN
La glicerina cruda (GC) es un subproducto del procesamiento del biodiesel que se produce abundantemente y puede ser utilizado como un ingrediente alternativo en la nutrición animal. El objetivo de este trabajo de investigación fue evaluar el efecto de la suplementación con glicerina cruda sobre la digestibilidad de los nutrientes y síntesis de proteína microbiana en dietas para novillos Nelore. Cinco novillos raza Nelore canulados (522±43 kg) fueron utilizados en un diseño experimental cuadrado latino 5x5 para evaluar los tratamientos: 0 (control), 3, 6, 9 y 12% of GC en base a la materia seca total de la dieta, durante cinco periodos experimentales de 21 días. La ingestión de material seca (IMS), digestibilidad ruminal aparente (DRA), digestibilidad aparente intestinal (DAI), digestibilidad aparente total (DAT) de la materia seca (MS), materia orgánica (MO), proteína cruda (PC), fibra en detergente neutro (FDN) y carbohidratos no fibrosos (CNF) no fueron afectados (P > 0,05) por la inclusión de GC en las dietas. El flujo fecal del extracto etéreo (EE) disminuyó linealmente (P < 0,05) a medida que el nivel de GC en la dieta aumentó. Sin embargo, la DAI y DAT del EE aumento linealmente (P < 0,05) a medida que aumentó el nivel de GC en la dieta. La inclusión de diferentes niveles de GC en las dietas no afectó (P > 0,05) la síntesis de proteína microbiana. Se concluye que la GC puede ser utilizada en las dietas para ganado de carne hasta en un 12% y puede ser considerada como un buen ingrediente energético en las raciones.
Palabras clave: biodiesel, subproductos, glicerol, rumiantes.
Recibido: 21/04/14 Aprobado: 01/12/14
INTRODUCTION
Crude glycerine CG is an impure form of glycerine and a major and abundant biodiesel by-product that is being explored for alternative uses such as in livestock diets given its nutritional value. Expansion of biodiesel industry in recent years has lead to a glycerin´s saturated market, thus efforts directed to use its high energy content in animal feed, is an environmentally friendly strategy.
Feedlot cattle or beef feedlot in Brazil has been experiencing major changes in the last years, particularly in the size of enterprises, management and feeding industry. This industry is characterized by the use of diets high in corn. However, corn and another forages are intended for ethanol production and the tendency is a continue increase of corn price and limited use of corn in cattle feeding sector, mainly in the United States. To face those limitations in beef feedlot economy, CG has emerged as a valuable opportunity to reduce feeding costs given its ability to substitute conventional energy foods such as corn grain (Mach et al., 2009). Since the cost of gain for feedlot cattle is considered high, there is great interest in the use of alternative foods that may eventually replace part of grains used in the commercial diets without significant detriment in the physiological, metabolic processes and animal performance (Lage et al., 2010).
Initial studies demonstrated that the inclusion of CG in ruminant´s feed was safe and lead to the complete fermentation of glycerol to propionate (Johns, 1953), and those findings are consistent with recent work (Wilbert et al., 2013). These observations are interesting in the nutrition and beef cattle feeding industry and have been proven by studies on the kinetics using C14 – labeled glycerol (Bergner et al., 1995).
According to Trabue et al. (2007), CG supply in diets tends to reduce the quantity of available carbon and hydrogen ions for methane production in the rumen, a process that occurs by the reduction of acetate, with the benefits of an improvement in energy use by the animal. Furthermore, glycerol present in CG may increase water retention by the rations, particularly in low humidity environments and increase the palatability of commercial feed because of their sweet fragrance and flavor (Elam et al., 2008). However, evaluation and testing those advantages in vivo experiments in needed to construct solid scientific basis for its correct use and potential impact in animal nutrition and beef cattle industry. This study was conducted to evaluate the effects of CG addition in beef cattle diets on the apparent nutrient digestibility and microbial protein synthesis.
MATERIAL AND METHODS
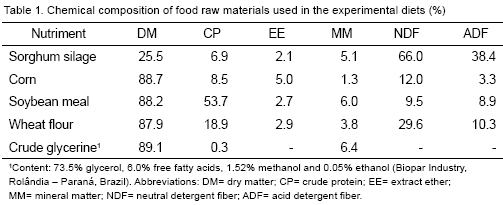
The experiment was performed in the Iguatemi Experimental Farm and the Food and Animal Nutrition Analysis Laboratory, in the Universidad Estadual de Maringá,Brazil. Five Nelore steers with 565±45 kg body-weight and a rumen cannula were housed in individual pens. The diets were formulated to allow 1.10 to 1.20 kg d-1 daily weight gains. A 5 x 5 latin square design was used with five treatments, five periods and five steers per treatment. The experimental periods were of 21 d each with the first15 d intended for adaptation to the experimental diets (Table 1) and the following 6 d for sample collection (foods offered, rejections, feces, rumen liquid, omasaldigesta and urine). Food intake was adjusted to obtain 5-10 % of rejections from the total food offered. Daily food intake was calculated as the difference between food supplied and food rejected, based on the dry matter DM. The rate between fodder and commercial feed was 40:60. The chemical composition of food raw materials used in the experimental diets are shown in Tables 1 and 2.
The GC inclusion in feedlot diets consisted of 0, 3, 6, 9 and 12% based on the total diet´s dry matter. The diets were provided ad libitum a complete mixture twice at day (at 08:00 h and at 16:00 h), and the CG was completely mixed with the other ingredients during feed preparation.
In order to determinate total and partial digestibility of dry matter (DM), total dry matter intake (DMI), organic matter (OM), crude protein (CP), ether extract (EE), neutral detergent fiber (NDF) and non-fiber carbohydrates (NFC), six samples of omasal chime (~500 ml) were collected daily, throughout the reticulo-omasal orifice by suction, based on the technique described by Leão et al. (2005), six samples of feces (~200 g) were collected directly from the rectum. Omasal chyme and feces were collected from day 14, for 6 consecutive days and at 0, 2, 4, 6, 8 and 10 h after the feeding time 8:00 h daily.
To measure the omasal flow and fecal production, 10 g d-1titanium dioxide (TiO2) was administered directly in the rumen, from day 7 of each period. Collected omasalchyme and feces samples were placed in marked plastic bags and frozen at -20 °C. The samples were thawed, pre-dried for 72 h in an air circulation stove at 55 °C and ground in Willey type mills (1 mm mesh). The samples were mixed based on the percentage of dry weight to obtain samples made up of omasal digest and feces per animal/ treatment/period.
Samples of foods used in experimental diets, rejections, omasalchyme and feces were analyzed to determine DM, OM, PC, EE and ashes (AOAC, 1990), NDF and ADF (Van Soest et al., 1991). The values of non-fiber carbohydrates (NFC) and total digestible nutrients TDN were calculated with the Sinffen et al. (1992) equations. Samples of omasalchyme and feces were analyzed for titanium concentration (Myers et al., 2004).
Four urine samples per steer were collected during spontaneous urination at 3-4 h after feeding at 08:00 h of each experimental period (days 17 -20). The urine samples were homogenized and filtered through cloth filters, and aliquots of 10 mL were immediately diluted in 40 mL of H2SO4 0.036 N (Chen et al., 1995). The pH in the samples was adjusted to values lower than 3 to avoid the bacterial degradation of purine derivatives and the precipitation of uric acid; later, they were stored at -20 °C for analysis of allantoin and uric acid. One subsample of urine without acid was taken on the same day to determine the concentration of creatinine in the urine through the method of final point, using picrate and an acidifying agent following the manufacturer´s recommendations (GoldAnalisa®). Daily volume of urine were calculated based on the creatinine concentration in urine samples and the average levels of creatinine excreted (27.4 mg kg-1) by castrated Nelore reported previously (Barbosa et al., 2006). Those values of urine volume were used to calculate daily excretion of allantoin and uric acid by each animal.
Allantoin in urine was performed by a colorimetry method based on the original technique reported by Fujihara et al. (1987), with minor modifications reported later (Chen & Gomes, 1992). The concentration of uric acid was estimated by using a commercial kit (Labtest®). The total excretion of purine derivatives was the result of urinary excretion of allantoin and uric acid.
Absorbed microbial purines (X, mmol d-1) were calculated from the excretion of purine derivatives (Y, mmol d-1), according to the following ecuation: Y=0.85X + 0.385 PV0.75, where 0,85 is the recovery of purines absorbed as purine derivatives and 0,385 PV0.75 is the endogenous contribution for purine excretion (Verbic et al., 1990).
The intestinal flow of microbial nitrogenous compounds (Nmic, g N d-1) was calculated from the absorbed microbial purines (Pabs, mmol d-1) according to the following formula: Nmic=(70*Pabs)/ (0.83*0.116*1000), where 70 corresponds to the N content in purines (mg N-1mmol) and 0,83 is the digestibility of microbial purines; 0,116 is the N purine: N total rate of microorganisms in the rumen (Chen & Gomes, 1992).
The data were analyzed by an ANOVA test using the GLM procedure and when there was a significant effect (P ≤ 0.05) for the treatment, a polynomial regression analysis was performed using the SAS software (SAS, 2004). The experimental design had the statistical model:
Yijk=μ + Ai + Pj + Tk + eijk
Where: Yijk= responses to treatment k, animal i, in period j; μ = treatments mean; Ai=effect of the animal i, (1...5); Pj=effect of the period j, (1...5); Tk=effect of the treatment k, (1...5); eijk= residual error.
RESULTS AND DISCUSSION
Intake of DM, OM, NDF, CP and NFC, expressed as kg/day were similar among treatments (P > 0.05). DMI ranged from 9.53 to 10.11 kg/day and occurred in treatments with 9 and 3% GC inclusion, respectively (Table 2).
Similar results were observed by Mach et al. (2009), who evaluated four levels of CG inclusion with 85.7% glycerol, on the performance of Holstein bulls fed with high-concentrate diets and found DMI values of 8.18, 8.19, 8.53 and 8.19 kg/ day for 0, 4, 8 and 12% CG levels, respectively. The same authors reported that the existing methanol in CG can have potentially negative effect on the DMI, but the presence of 0.09% of this compound did not affect the intake of animals; and concluded thatCG may be included as an alternative energy source to replace grain up to the level of 12%. In the present study, 1.5% methanol in CG did not result in significant changes on DMI. Indeed, Lage et al. (2010), reported that ruminants do not have health risks associated with methanol in CG inclusion in the diet because methanol is naturally produced in the rumen as result of pectin fermentation.
Inclusion of 16% vs. 0% CG in feedlot steers was reported to decrease DMI in 8.84 and 7.80 kg/ day, respectively (Parsons et al., 2009). However, the authors did not report the percentage of glycerol or methanol in CG. In the other hand, Gunn et al. (2010) observed that sheep treated with CG (87.5% glycerol), increased DMI in a linear manner and DMI rose from 1.01 to 1.41 kg/ day when animals were treated with 0 and 20% CG respectively.
The inclusion of CG in the diet of Nelore steers had no effect (P > 0.05) on the CRD, TCD of CID and the DM, OM, CP, NDF and NSC. In this regard, Donkin et al. (2009) found that the TCD of the DM and OM increased with the inclusion of CG (0-15%), however, the TCD of the NDF was not affected in our study. Several studies have shown that inclusion of CG in the diet may decrease fiber digestibility. Schröeder & Südekum, (1999) worked with dairy cows receiving diets with forage and 40 to 60% of commercial feed and reported that when 10% of CG are included in diets with high starch, there is a tendency of reducing the digestibility of cell wall, but it does not interfere with TCD of DM and OM.
In vitro experiments had demonstrated that 0.5 to 5.0% pure glycerol inhibit cellulose degradation by cellulolytic bacteria and fungi, respectively (Roger et al., 1992). Although fungi do not play a vital role in ruminal fermentation of high commercial feed diets, the suppression of lytic activity by glycerol may alter the performance of animals or reduce the intake of dry matter and the TCD of the NDF in animals fed fi ber rich diets (Schneider, 2010). According to those observations Lage et al. (2010) reported that TCD of the NDF had quadratic effect with the inclusion of CG in sheep diets, getting values of 57, 38, 40, 43 and 43% for TCD when CG were 0, 3, 6, 9 and 12%, respectively.
In this study, the inclusion of CG in diets for Nelore steers decreases the flow of fecal EE (P < 0.05), results that reflect increased CID and TCD (P < 0.05) with higher inclusion of CG (Table 3). Lage et al. (2010) fed sheep with similar levels of CG, and noted that the TCD of the EE decreased linearly from 83 to 73% in animals treated with 0 and 12% CG, respectively. The percentage of glycerol in CG was 32.6%, and the authors claimed that the digestibility of EE was influenced by excess of fatty acids present in CG (46.5%). In the present study, the percentage of fatty acids in CG was only 6%, it could be assumed that the increase in CID and TCD can be due to decreased lipolysis in the rumen. Krueger et al. (2010) observed 48 and 77% reduction in lipolysis when 2 and 20% of glycerol, respectively is included in in vitro experiments. This fact may causes a greater flow of EE to the gut, thus improving the digestibility.
Our results also indicated that the inclusion of CG in the diet had no effect (P > 0.05) on the urinary volume allantoin, ureic acid, total purine, purines absorbed, microbial nitrogen and efficiency of microbial protein synthesis (Table 4).
According to Gurtler et al. (1987), the urinary volume of one adult bovine ranges from 5 to 10 L/day, in agreement with the values observed for urine production in all treatments. The average urine volume observed in this experiment (6.72 L/day) was superior to the volume reported by Barbosa et al. (2006), for castrated Nelore males (4,67 L/day) and lower those observed by Chizzotti et al. (2006), in heifers with 453 kg (17.47 L/day) mean body-weight.
The mean urinary excretion of allantoin and ureic acid obtained in this study (149 mmol/ day) were higher than those obtained by Wang et al. (2009), who evaluated different levels of (100, 200 and 300 g/day) glycerol in the diet of Simental steers and observed 58, 64 and 60 mmol/day of allantoin excretion, respectively, and 7.38, 7.44 and 7.32 mmol/day of ureic acid in diets of steers, respectively.
The percentage of uric acid in the purine derivatives (PD) ranges from 15 to 20% and it was very constant in the same animal, but variable between animals (Chen & Gomes, 1992). However, in this experiment the percentage of uric acid ranged from 3.9 to 5.3%, and it was lower than those observed by Castañeda et al. (2009), whom reported 6.4 to 10.4% in Holstein steers and the mean value of 8.25% in total purine in uric acid in heifers with 523 Kg mean body-weight reported by Chizzotti et al. (2006).
The average value for efficiency in microbial protein synthesis from protein microbial was 131.17 g/kg of TDN intake and this value was similar to that reported by Magalhães et al. (2005) whom reported 133.55 CP mic g/kg of TDN intake in steers fed diets with increasing levels of urea. However, Nascimento et al. (2010) obtained values between 85 and 103 g PBmic/ kg TDN intake in crossbred steers fed different energy sources. Those values are lower those observed in this study and indicate that. Finally, the values observed in Nelore steers in this study were close to those (130.0 g CP mic/kg TDN) proposed by NRC (2001) and higher to those reported by Valadares Filho et al. (2006) for cattle (120.0 g CP mic/kg of TDN) in tropical conditions. Thus, it can be inferred that the estimated values for microbial synthesis are between normal ranges and support the theory that CG inclusion does not adversely alter the population of microorganisms in the rumen micro-environment.
CONCLUSIONS
The inclusion of 12% crude glycerin in the diet of beef cattle, do not influence the ingestion of dry matter, partial and total digestibility of nutrients and microbial protein synthesis. The intestinal and total digestibility of lipids improved with the inclusion of dietary CG. It is concluded that crude glycerin with low ethanol levels could be a good alternative energy feedstuff, and may be added up to 12% of total DM to achieve a partial replacement of ground corn grain in the diet of beef cattle.
LITERATURA REVIEW
1. A.O.A.C. Association of Official Agricultural Chemists 1990. Official Methods of the Association of the Agricultural Chemists. Washington. 15ta Ed. [ Links ]
2. Barbosa, A. M., R. F. D. Valadares, S. C. Valadares Filho, R. M. L. Véras, M. I. Leão, E. Detmann, M. F. Paulino, M. I. Marcondes e M. A. Souza. 2006. Efeito do período de coleta de urina, dos níveis de concentrado e de fontes proteicas sobre a excreção de creatinina, de ureia e de derivados de purina e a produção microbiana em bovinos Nelore. Revista Brasileira de Zootecnia, 35(3):870-877.
3. Bergner, H., C. Kijora, Z. Ceresnakovae and J. Szakacs.1995. In vitro studies on glycerol transformation by rumen microorganisms. ArchivfürTierernaehrung, 48(3):245.
4. Castañeda, R. D., A. F. Branco, S. M. Coneglian, J. C. Barreto, F. Granzotto e S. Teixeira.2009. Substituição de uréia por cloreto de amônio em dietas de bovinos: digestibilidade, síntese de proteína microbiana, parâmetros ruminais e sanguíneos. ActaScientiarum: Animal Sciences, 31(3):271-277.
5. Chen, X. B., A. T. Mejia, D. J. Kyle and E. R. Ørskov. 1995. Evaluation of the use of the purine derivative: creatinine ratio in spot urine and plasma samples as an index of microbial protein supply in ruminants: studies in sheep. The Journal of Agricultural Science, 125(01):137-143.
6. Chen, X. B. and M. J. Gomes. 1992. Estimation of microbial protein supply to sheep and cattle based on urinary excretion of purine derivatives an overview of technical details. Bucksburnd: Rowett Research Institute. InternationalFeedResources Unit. 21 p.
7. Chizzotti, M. L., S. D. C. Valadares Filho, R. F. D. Valadares, F. H. M. Chizzotti, J. M. D. S. Campos, M. I. Marcondes, & M. A. Fonseca, 2006. Consumo, digestibilidade e excreção de uréia e derivados de purinas em novilhas de diferentes pesos. Revista Brasileira de Zootecnia, 35(4):1813-1821.
8. Donkin, S. S., S. L. Koser, H. M. White, P. H. Doane and M. J. Cecava. 2009. Feeding value of glycerol as a replacement for corn grain in rations fed to lactating dairy cows. Journal of dairy science, 92(10):5111-5119.
9. Elam, N. A., K. S. Eng, B. Bechtel, J. M. Harris and R. Crocker. 2008. Glycerol from Biodiesel Production: Considerations for feedlot diets. In Proceedings of the Southwest Nutrition Conference, Arizona, United States.
10. Fujihara,T., E. R. Ørskov, P. J. Reeds and D. J. Kyle. 1987. The effect of protein infusion on urinary excretion of purine derivatives in ruminants nourished by intragastric nutrition. The Journal of Agricultural Science, 109(01):7-12.
11. Gunn, P. J., M. K. Neary, R. P. Lemenager and S. L. Lake. 2010. Effects of crude glycerin on performance and carcass characteristics of finishing wetherlambs. Journal of animal science, 88(5):1771-1776.
12. Gürtler, H., E. Kolb, L. Schröder, H. A. Ketze y H. Seidel.1987. Fisiologia Veterinaria. Rio de Janeiro, Guanabara Koogan. 4ta Ed.
13. Johns, A. T. 1953. Fermentation of glycerol in the rumen of sheep. New Zealand Journal of Science and Technology, 35:262-269.
14. Krueger, N. A., R. C. Anderson, L. O. Tedeschi, T. R. Callaway, T. S. Edringtone and D. J. Nisbet. 2010. Evaluation of feeding glycerol on free-fatty acid production and fermentation kinetics of mixed ruminal microbes in vitro. Bioresource technology,101(21):8469-8472.
15. Lage, J. F., P. V. R. Paulino, L. G. R. Pereira, S. C. Valadares Filho, A. S. Oliveira, E. Detmann e J. C. M. Lima. 2010. Glicerina bruta na dieta de cordeiros terminados em confinamento. Pesquisa Agropecuária Brasileira, 45(9):1012-1020.
16. Leão, M. I., S. C. Valadares Filho, L. N. Rennó, P. R. Cecon, J. A. G. Azevedo, L. C. Gonçalves e R. F. D. Valadares. 2005. Consumos e digestibilidades totais e parciais de carboidratos totais, fibra em detergente neutro e carboidratos nãofibrosos em novilhos submetidos a três níveis de ingestão e duas metodologias de coleta de digestas abomasal e omasal. Revista Brasileira de Zootecnia, 34(2):670- 678.
17. Mach, N., A. Bach and D. Devant. 2009. Effects of crude glycerin supplementation on performance and meat quality of Holstein bulls fed high-concentrate diets. Journalof Animal Science, 87(2):632-638.
18. Magalhães, K. A., S. C. Valadares Filho, R. F. D. Valadares, M. L. Paixão, D. S. Pina, P. V. R. Paulino e M. O. Porto. 2005. Produção de proteína microbiana, concentração plasmática de uréia e excreções de uréia em novilhos alimentados com diferentes níveis de uréia ou casca de algodão. Revista Brasileira de Zootecnia, 34(4):1400-1407.
19. Myers, W. D., P. A. Ludden, V. Nayigihugu and B. W. Hess. 2004. Technical Note: A procedure for the preparation and quantitative analysis of samples for titanium dioxide. Journalof Animal Science, 82(1):179-183.
20. Nascimento, M. L. D., M. F. Paulino, E. Detmann, M. I. Leão, S. D. C. Valadares Filho e L. T. Henriques. 2010. Fontes de energia em suplementos múltiplos para novilhos em pastejo durante o período das águas. Revista Brasileira de Zootecnia, 39(4):861- 872.
21. N R C. National Research Council. 2001. Nutrient requirements of dairy cattle. Ed. National Academic. Washington, D. C. 7ma Ed. Parsons, G. L., M. K. Shelor and J. S. Drouillard. 2009. Performance and carcass traits of finishing heifers fed crude glycerin. Journal of Animal Science, 87(2):653-657.
22. Roger, V., G. Fonty, C. Andre and P. Gouet. 1992. Effects of glycerol on the growth, adhesion, and cellulolytic activity of rumen cellulolytic bacteria and anaerobic fungi. Current microbiology, 25(4):197-201.
23. SAS. Statistical Analysis System. 2004. Version 9.1. Cary, North Carolina: SAS Institute. Schneider, C. J. 2010. Crude glycerin in feedlot cattle diets and as a solvent in maillard reaction processes intended for manufacturing value-added protein meals. Thesis of Ph. D. Kansas State University, Department of Animal Sciences and Industry College of Agriculture, Manhattan, Kansas.
24. Schröder, A. and K. H. Südekum.1999. Glycerol as a by-product of biodiesel production in diets for ruminants. 10th International Rapessed Congress, Canberra, Australia.
25. Sniffen, C. J., J. D. Oconnor and P. J. Van Soest. 1992. A net carbohydrate and protein system for evaluating cattle diets. II. Carbohydrate and protein availability. Journal of Animal Science, 70(11):3562-3577.
26. Trabue, S., K. Scoggin, S. Tjandrakusuma, M. A. Rasmussen and P. J. Reilly. 2007. Ruminal fermentation of propylene glycol and glycerol. Journal of agricultural and food chemistry, 55(17):7043-7051.
27. Valadares Filho, S. C., P. V. R. Paulino e K. A. Magalhães. 2006. Exigências nutricionais de zebuínos e tabelas de composição de alimentos BR-CORTE. Ed. Suprema Gráfica Ltda., Viçosa, 2da. Ed.
28. Van Soest, P. J., J. B. Robertson and B. Lewis. A.1991. Symposium: carbohydrate methodology metabolism and nutritional implications in dairy cattle. Journal of Dairy Science, 74(10):3583-3597.
29. Verbic, J., X. B. Chen, N. A. MacLeod and E. R. Ørskov. 1990. Excretion of purine derivatives by ruminants. Effect of microbial nucleic acid infusion on purine derivative excretion by steers. The Journal of Agricultural Science, 114(03):243-248.
30. Wang, C., Q. Liu, W. Z. Yang, W. J. Huo, K. H. Dong, Y. X. Huang e D. C. He. 2009. Effects of glycerol on lactation performance, energy balance and metabolites in early lactation Holstein dairy cows. Animal feed science and technology, 151(1):12-20.
31. Wilbert, C. A., Ê. R. Prates, J. O. J. Barcellos e J. Schafhäuser. 2013. Crude glycerin as an alternative energy feedstuff for dairy cows. Animal Feed Science and Technology, 183(3):116-123.