Universidad, Ciencia y Tecnología
versión impresa ISSN 1316-4821
uct vol.20 no.81 Puerto Ordaz dic. 2016
Synthesis of calcium oxide by means of two different chemical processes
Miryam R-Joya1, Angela M. Raba2 y José José Barba-Ortega1
1Universidad Nacional de Colombia, Bogotá D.C., Colombia mrinconj@unal.edu.co; amrabap@unal.edu.co;
2Universidad Francisco de Paula Santander, Cúcuta, Colombia jjbarbao@unal.edu.co
Abstract:
The growth of samples of CaO (calcium oxide) under conditions of a high calcination temperature (800°C and 900°C) was studied. The CaO samples were prepared using the Pechini method with two different variations. In the first variation, HNO3 and NH4OH were added in order to control the pH. In the second, the pH was not controlled. By means of X-rays, it was seen that for method 1, the phases CaO, CaCO3, and Ca(OH)2 were present. For method 2, only the CaCO3 and Ca(OH)2 phases appeared. By means of Raman spectroscopy, significant changes in the intensity of the peaks were observed, these being greater for method 2. The peaks between 950 cm-1 and 1200 cm-1 also exhibited shifts at low frequencies for first strategy. With respect to photoluminescence, the intensity was greater for the samples prepared via second option. This photoluminescence is an indication that there are more defects in the structure of the samples using method 2. These results indicate the ability that the method of synthesis (Pechini) has for acquiring samples of high quality.
Keywords: Lime, Pechini, synthesis, Raman, photoluminescence.
Síntesis del óxido de calcio mediante dos procesos químicos diferentes
Resumen:
En este trabajo se estudió el crecimiento de muestras de CaO (óxido de calcio) en condiciones de altas temperaturas de calcinación (800 ° C y 900 ° C). Las muestras de CaO fueron preparadas por el método de Pechini en dos condiciones diferentes. En la primera variación, se adicionó HNO3 y NH4OH para regular el pH de las muestras. Para el segundo método de preparación, no se regulo el pH durante el crecimiento de las muestras. En la caracterización, por medio de los rayos x, se observó que para el método uno, están presenten las fases CaO, CaCO3, Ca(OH)2. No obstante, para él método dos solamente las fases CaCO3, Ca(OH)2 son observadas en las muestras. Por medio de la espectroscopia Raman, se determinaron cambios significativos en la intensidad de los picos, siendo mayores para el método dos. Los picos entre 950 cm -1 y 1200 cm- 1, en el caso del método uno, también muestran cambios para frecuencias bajas. En la fotoluminiscencia, la intensidad es mayor para las muestras preparadas por el segundo método. La fotoluminiscencia es un indicativo, que los defectos estructurales de las muestras son mayores, para el método dos. Estos resultados indican la capacidad que tiene el método de síntesis (Pechini) en la calidad de la obtención de las muestras.
Palabras claves: cal, Pechini, síntesis, Raman, fotoluminiscencia.
Recibido (19/09/16), aceptado (12/12/16)
I. INTRODUCTION
The production of calcium oxide by means of the use of CaCO3 (limestone) is one of the oldest known chemical processes in the world [1]. During the last few years, many researchers have worked on improving the yield, especially for multiple cycles of CaO, through various modifications, such as hydration, treatment with acid, and chemical doping [2–6]. Among the alkaline and alkaline earth oxides, CaO is one of the solids that have exhibited greater transesterification activity. Calcium oxide (CaO) is an important inorganic compound that is used across diverse industries as a catalyzing agent for cleaning up toxic waste [7–12]. Calcium oxide (CaO) also has the potential to be developed as an absorbent material for the capture of CO2 at high temperatures [13–15]. The participation of calcium and hydroxyl ions in the biological processes of tissue repair, as well as in antibacterial action, has been well studied in the literature, and this is one of many applications [16].
Accordingly, research on calcium peroxide is a good option in the study of how to improve the biodegradation of contaminants in the water that is contained in soil and earth, but the speed of the oxidation reaction of the calcium oxide and the contaminant is slow. If the calcium peroxide is administered correctly, it has the potential to deliver chemical oxidants to the contaminated parts of the environment, such as subterranean water and soil, at low cost [17].
The Pechini method has been of great help in the investigation of the photoluminescence of amorphous solids. The synthesis exhibits uniformity on the atomic scale and the formation of particles at the nanoscale [18,19,20]. As far as the optical properties, such as photoluminescence and Raman spectra, they depend on the structural and electronic properties, the stoichiometric composition, and the presence of impurities and defects. Photoluminescence is a technique known for providing information about the electronic structure and surface or deep defects in the material, depending on the length of the wave used in the process. Photoluminescence (PL) is an essential tool for research into the optical processes inside semiconductor samples. If the sample is excited by a laser with energy greater than the band gap, then the excess creates an electron-hole pair. These can recombine through various available recombination routes, some emitting a photon of energy hv. The Raman measurements give us information about the vibrational modes. Changes in the lattice parameter can be attributed to an anharmonic potential in the Raman spectra [18].
In the present paper, the effect of the annealing of the samples by means of two methods of synthesizing on the photoluminescent and Raman spectra was studied. Using either method of preparation, the samples did not exhibit pure phases. Using the first method, a mixture of three phases appeared, and with the second, two phases, which is not ideal and is not what one is trying to obtain, which induces us to propose another method for obtaining samples with pure phases.
In this work, a study of the structural and optical characterization of CaO, prepared by two different forms of synthesis. The samples were characterized by techniques, X-rays, Raman spectroscopy and photoluminescence. The work is divided into: abstract, introduction, development, results - discussion and conclusions.
II. DEVELOPMENT
For method 1, the powder was obtained via the Pechini method. Initially, 10.0305 ml of ethylene glycol (Panreac 99.8%) was heated to 70°C, and 9.4559 g of citric acid monohydrate (Panreac 99.8%) was slowly added, maintaining the temperature of the system constant at 70°C and continuously shaking the mixture until a transparent solution was obtained. Separately, a solution of the precursor was prepared with 0.3M (2.5356g) of calcium sulfate dihydrate (CaSO4·2H2O-Merck 98.2%) in an aqueous solution of 0.4M of nitric acid (HNO3 Panreac 65%). The precursor solution of calcium was added to the mixture of ethylene glycol and citric acid, and when it became semitransparent, the temperature was reduced to 25°C in order to begin adding NH4OH (Panreac 30%) until a solution at pH 5 was obtained. Taking care that no precipitate was formed in the now transparent system, the solution was heated to 90°C, with constant shaking, until a white resin was formed. The resulting resin was pre-calcinated at 300°C and the solid that resulted from this pre-calcination was macerated using an agate mortar. The resulting powders were subjected to final thermal treatments of 800°C and 900°C.
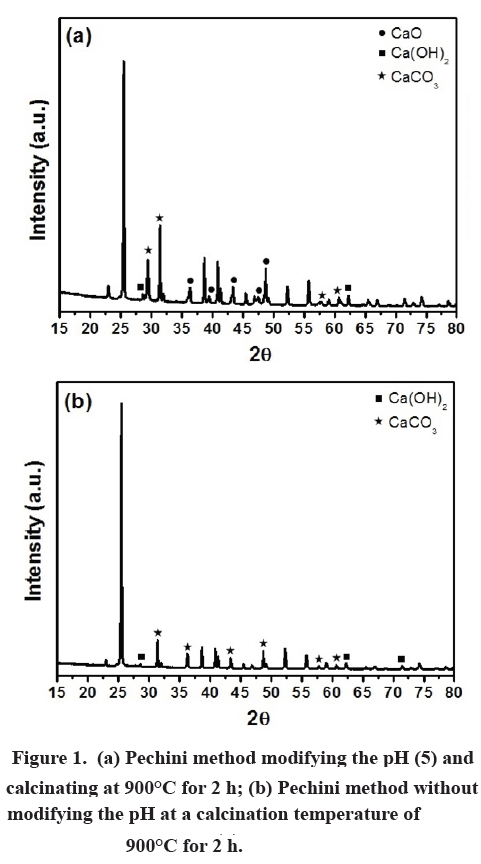
Method 2: The powder was obtained using the Pechini method. Initially, an aqueous solution of a 20% by mass calcium sulfate dihydrate (CaSO4 2H2O Merk 98.2%) was prepared, which contained 30.1435 g of solute. Separately, an aqueous solution of 20% by mass citric acid monohydrate (Panreac 99.8%) was prepared, which contained 112.3985 g of solute. The two solutions were mixed with constant shaking, maintaining a temperature of 80°C for half an hour. 67.3246 ml of ethylene glycol (Panreac 99.8%) was added to the mixture at 90°C. This temperature was maintained until the volume of water was reduced. The resulting resin was pre-calcinated twice at 300°C, and the solid that resulted from this pre-calcination was macerated using an agate mortar. The resulting material was sifted in a sieve no. 50 (pore size 300 μm). The powders that were obtained from the sifting were subjected to final heat treatments of 800°C and 900°C for two hours on the rise and two hours in plateau. X-ray analysis was carried out on the powders obtained via both Pechini methods in order to characterize the material. A representative result is shown in Fig. 1.
The XRD patterns of the samples synthesized via the two methods were obtained using Panalytical PW3373 equipment (Cu Kα1 radiation, λ= 1.540558 Å), operating at 40 mA with a step of 0.05° for 50 seconds. The 2θ angle range of 5-80° was used. The PL measurements were performed with a MonoSpec 27 monochromator (Thermal Jarrel AS, USA) coupled to an R446 photomultiplier (Hamamatsu Photonics, Japan). A Krypton ion laser (Coherent Innova 90 K, USA) (λ=350 nm) was used as an excitation source. Raman scattering spectra were obtained in the backscattering geometry with a Jobin-Yvon-T64000 spectrometer coupled to a N2-cooled CCD device, using as an excitation source the 532 nm laser line of a Verdi laser, operating at low power.
X-ray analysis was carried out on the powders obtained using the two Pechini methods in order to characterize the material. A representative result is shown in Fig. 1. In Fig. 1 (a), preparation method 1 is shown. The diffraction patterns confirm the coexistence of the phases CaO, Ca(OH)2, and CaCO3. The reason that the X-rays were analyzed for the 900°C temperature is that it was hoped that a pure phase would be obtained at this temperature. In the literature it was found that Ca(OH)2 is 100% directly converted to CaCO3 without the formation of intermediate phases above 600°C [21]. Nevertheless, we did not obtain a pure phase resulting from the preparation using method 1 at high temperature. In Fig. 1 (b) we show the X-ray diffraction for the sample calcinated at 900°C and prepared via Pechini method 2. Here the diffraction patterns present correspond to the phases Ca(OH)2 and CaCO3. Upon comparison with Fig. 1 (a), it can be seen that the intensity of the peaks is lower and the CaO phase is not present. The dominant phase in Fig. 1 (b) is CaCO3. This is an indication that the modification of the calcination temperature that was done with the same preparation method and under the same conditions led to significant changes in the production of pure phases.
The Raman spectra for Pechini method 2, the method in which the pH was not modified during the preparation of the samples, are shown in Fig. 2 (a) and (b). The calcination temperatures were 800°C and 900°C. The peaks are narrow and intense, and the peak at 1018 cm-1 is the most intense of all of them. According to the literature, there is some controversy over the assignation of vibrational modes for the phases CaO, Ca(OH)2, and CaCO3. Thomas et al. [22] wrote: "We were surprised by the number of open questions that still exist with respect to the interpretation of the Raman spectra of such common materials and of the phases of the lime cycle, especially the mistaken assignation of various luminescence bands and Raman modes, as well as the negligence in the analysis of the data in the literature" [22]. Seeking information about the Raman spectra of CaO, Ca(OH)2, and CaCO3 in the literature makes us believe that information is still scarce, in spite of their being among the oldest used and studied materials. In Fig. 2 (c) and (d), the Raman spectra are shown for method 1 for the samples at 800°C and 900°C. As can be seen, the intensity of the peaks is less in comparison with the spectra of method 1. Another significant difference is the shift of the peaks at low frequencies of 1018 cm-1 and 1086 cm-1, indicating the possibility of another phase in the material.
Fig. 3 (a) and (b) show the photoluminescence for the samples calcinated at 800°C for methods 2 and 1, respectively. As can be seen, the photoluminescence of greatest intensity is exhibited for method 2, peak centered at approximately 590 nm. The photoluminescence for the samples annealed at 900°C is shown in Fig. 3 (c) and (d) for methods 2 and 1, respectively. As can be seen in Fig. 1, the intensity of the photoluminescence in both cases is greater for method 2. According to the literature [18], in these oxides the photoluminescence is a phenomenon closely associated with the electronic transitions between the valence and the conduction bands. The structure of the band is determined by the coupling between the wave functions that define the energy of the electronic states of the atoms of the lattice. These states have very similar energy and are limited by the valence band and the conduction band, which in the theory of molecular orbitals correspond to the fundamental and the excited state, respectively. Photoluminescence, in the majority of inorganic solids, involves doping impurities or structural defects (vacancies). These imperfections are of the atomic and/or molecular type. Another important factor that influences the optical properties of many inorganic solids is the size of the radii of the cations in the lattice.
X-rays detect long-range order, that is, the total crystalline structure. Photoluminescence only gives information about ions with respect to their surrounding area, and so can investigate the short-range order. Photoluminescence supplies information about the material at short range. Here, the photoluminescence spectrum is more intense when the material is subjected to heat treatment at low temperatures. This is a strong indication that there is a disordered phase that is responsible for the intense photoluminescence in the spectrum [18]. With an increase in the calcination temperature, the order begins to increase at long range, so the electron-hole transitions undergo a decrease, which makes the photoluminescence decrease. Therefore, we can conclude that for Pechini method 2 the impurities or defects in the material are greater, and for that reason the material is more amorphous.
IV. CONCLUSIONS
Using the two Pechini methods, modifying and not modifying the pH, and subjecting the samples to calcination at high temperatures, combined phases were obtained in both cases. By means of the Raman and photoluminescence measurements of the samples, it can be concluded that the short-range order is greater for the method with a modification of the pH. Luminescence, for the majority of inorganic solids, involves impurities or structural defects that are called activators. These imperfections or impurities are of a structural nature and are due to the electronic states of the material. The Pechini method is shown to be a simple, cheap, and rapid method for obtaining samples with specific characteristics.
ACKNOWLEDGEMENT
The author would like to thank Professor Paulo de Tarso and Professor Maximo Siu Li of the Department of Physics of the Universidade Federal de Fortaleza and USP Sao Carlos, Brazil.
V. REFERENCES
1. Miskufova, A., Havlik, T., Bitschnau, B., Kielski, A., & Pomadowski, H., "Properties of CaO sintered with addition of active Alumina", Ceramics–Silikáty. Vol.59, No.2, p.115, 2015. [ Links ]
2. Joya, M. R., JH Bautista Ruiz, and A. M. Raba. "Quicklime as an alternative in the photodegradation of contaminants." Journal of Physics: Conference Series. IOP Publishing, Vol.687, No.1, p.012044, 2016. [ Links ]
3. Manovic, Vasilije, and Edward J. Anthony. "Lime-based sorbents for high-temperature CO2 capturea review of sorbent modification methods." International journal of environmental research and public health. Vol.7, No.8, p.3129, 2010. [ Links ]
4. Manovic, Vasilije, and Edward J. Anthony. "Improvement of CaO-based sorbent performance for CO2 looping cycles." Thermal Science. Vol.13, No.1, p.89, 2009. [ Links ]
5. Li, Yingjie, et al. "Cyclic CO2 capture behavior of limestone modified with pyroligneous acid (PA) during calcium looping cycles." Industrial & Engineering Chemistry Research. Vol.50, No.17, p.10222, 2011. [ Links ]
6. Tang, Zhen-Xing, et al. "Preparation of nano- CaO using thermal-decomposition method." Materials letters, Vol.62, No.14, p.2096, 2008. [ Links ]
7. Ramírez-Moreno, Margarita J., et al. "Alkaline and Alkaline-Earth Ceramic Oxides for CO2 Capture, Separation and Subsequent Catalytic Chemical Conversion", Chap.14, p.404, 2014. [ Links ]
8. Wendi, Wendi. "Effect of Reaction Temperature and Catalyst Concentration." Sriwijaya International Seminar on Energy-Environmental Science and Technology. Vol.1, No.1. 2014. [ Links ]
9. Suresh C. Ameta, Pinki B. Punjabi, Rakshit , Technology & Engineering, "Microwave- Assisted Organic Synthesis: A Green Chemical Approach". Edi. Amenta, p.297, 2014. [ Links ]
10. Roy, Arup, and Jayanta Bhattacharya. "Microwave-assisted synthesis and characterization of CaO nanoparticles." International Journal of Nanoscience, Vol.10, No.3, p.413, 2011. [ Links ]
11. Sadeghi, Meysam, and Mir Hassan Husseini. "A novel method for the synthesis of CaO nanoparticle for the decomposition of sulfurous pollutant." J. Appl. Chem. Res Vol.7, No.4 , p.39, 2013. [ Links ]
12. Tang, Zhen-Xing, et al. "Sonication-assisted preparation of CaO nanoparticles for antibacterial agents." Química Nova, Vol.36, No.7, p.933, 2013. [ Links ]
13. Arpin M T Yusup S " Enhancement of Calcium oxide (CaO) for carbon dioxide (CO2) capture". Canadian Journal of Pure and applied Science; Vol.5, No.1,p. 1391, 2011. [ Links ]
14. Yu, Cheng-Hsiu, Chih-Hung Huang, and Chung-Sung Tan. "A review of CO2 capture by absorption and adsorption." Aerosol Air Qual. Res Vol.12, No.5, p.745, 2012. [ Links ]
15. Arpin, MT; Yusup, S. "Enhancement of calcium oxide (CaO) for carbon dioxide (CO2) capture", Canadian Journal of Pure & Applied Sciences; Vol.5, No.1, p.1391, 2011. [ Links ]
16. Mohammadi, Z., S. Shalavi, and M. Yazdizadeh. "Antimicrobial activity of calcium hydroxide in endodontics: a review." Chonnam medical journal Vol.4, No.3, p.133, 2012. [ Links ]
17. Khodaveisi, J., Banejad, H., Afkhami, A., Olyaie, E., Lashgari, S., & Dashti, R. "Synthesis of calcium peroxide nanoparticles as an innovative reagent for in situ chemical oxidation." Journal of hazardous materials Vol.192, No.3, p.1437, 2011. [ Links ]
18. De Lucena, P. R., Pontes, F. M., Pinheiro, C. D., Longo, E., Pizani, P. S., Lázaro, S., & dos Santos, I. M. G. "Photoluminescence in disordered materials." Cerâmica, Vol.50, No.314, p.138, 2004. [ Links ]
19. Mubita, O. P, Márquez, J, Márquez, Y, Martínez, A, Mora E, "Síntesis electroquímica y evaluación micrográfica de electrocatalizadores Ru/Pd/Mo/ CV T", Universidad, Ciencia y Tecnología, vol.18, No.71, p.50, 2014. [ Links ]
20. Gil, Linda, Jiménez, Lorena, Liscano, Sugehis, Le Bourhis, Eric, & Staia, Mariana, " Efecto de la deposición de recubrimientos de TiN y ZrN sobre la resistencia a la corrosión de la aleación de aluminio AA7075T6", Universidad, Ciencia y Tecnología, Vol.14 No.56 p.157, 2010. [ Links ]
21. Molinder, R., Comyn, T. P., Hondow, N., Parker, J. E., & Dupont, V. "In situ X-ray diffraction of CaO based CO 2 sorbents." Energy & Environmental Science Vol.5, No.10, p.8958, 2012. [ Links ]
22. Schmid, Thomas, and Petra Dariz. "Shedding light onto the spectra of lime: Raman and luminescence bands of CaO, Ca (OH) 2 and CaCO3." Journal of Raman Spectroscopy Vol.46, No.1, p.141, 2015. [ Links ]












 uBio
uBio