I. INTRODUCTION
Biosensors are analytical devices with the capability of combining a physiochemical transducer with an element of biological recognition for the detection of specific analytes and transforming this interconnection into a quantifiable signal 1. This form of sensors represents a fundamental tool for modern medical diagnostics due to their high precision, Immediate respond and ability to provide real time and point of attention monitoring. Recent advancements in nanotechnology have caused an increment in the development of nanobiosensors, which incorporate nanomaterials mainly less than 100nm in size to strengthen the biosensors execution by enhancing the electron transfer rates, biomolecule immobilization efficiency and surface area 1.
Different types of nanomaterials like graphene, gold nanoparticles (AuNPs), carbon nanotubes (CNTs), and metal oxide nanostructures have shown remarkable benefits in biosensing applications 2. These materials allow lower detection limits, a faster reaction time and higher sensitivity in comparison to traditional bulk materials. The nanoscale dimensions for these devices enable a productive transduction of biological signals and support the miniaturization process for the incorporation of wearable or portable diag- nostic system devices. More precisely, such forms of nanotechnology have led to signifi- cant advancements in optical, piezoelectric and electrochemical biosensors 2.
In comparison to the large range of nanomaterials on the market, zinc oxide (ZnO) has proven to be a potential candidate for nanobiosensing technologies. ZnO provides a mixture of valuable characteristics, including a large bandgap (∼ 3.3 eV), chemical stabil-ity, a high isoelectric point (∼ 9.5)) and an excellent biocompatibility, contributing to its status as suitable for the immobilization of low isoelectric point (IEP) biomolecules like antibodies and enzymes 2. In addition, ZnO can be engineered into a wide range of nanostructures like nanoflowers, nanowires, nanorods and nanosheets, using financially sustainable and industrially scalable methods for example: sol-gel processes, sputtering and hydrothermal synthesis 3 4 Furthermore, the variation on ZnO structures mor- phologically speaking helps with the increment of the surface area and promote stronger affinities with analytes, contributing to greater selectivity and sensitivity in biosensors made of ZnO nanostructures 4.
This review aims to provide an overview of recent advances (2022-2024) in ZnO-based biosensors used for medical diagnostics. On top of that, the review underscores proper- ties that make ZnO-based biosensors highly favorable in a unique way, highlights key uses for glucose monitoring, cancer biomarker sensing, infectious disease detection, and explores methods for device fabrication and biomolecule functionalization. Lastly, this document describes current challenges in integration, reproducibility and stability, and addresses future scenarios for real-world implementation and clinical technology trans- fer of ZnO-based biosensing devices.
II. STRUCTURAL AND FUNCTIONAL BASIS OF ZNO NANOBIOSENSORS
A. Physiochemical properties of ZnO for biosensing
ZnO nanostructures have a unique mixture of electrical, chemical, and morphological properties that make them suitable for biosensor applications.
High isoelectric point and biomolecule adsorption - exhibits an isoelectric point of approximately 9.5, making its surface positively charged under physiological pH conditions 5. This favors the electrostatic adsorption of enzymes and proteins re- sulting in a stable microenvironment, facilitating both preservation of biomolecular activity and efficient electron transfer.
High surface-to-area-to-volume ratio - this enables the anchoring of biological molecules, leading to enhanced detection of a specific target analyte 5. The ability to modify morphology and surface chemistry plays a critical role in modulating the biosensor’s selectivity, sensitivity, and operational range.
Table 1 presents the different morphologies of ZnO in biosensing applications. Each ZnO morphology offers unique structural and functional features that enhance biosen- sor performance. These structures vary in dimensionality, surface characteristics, and electron transport capabilities.
B. Electrical and optical properties for sensor stability
ZnO is a wide-bandgap semiconductor with a bandgap energy of 3.37 eV, which means it can operate at high voltages and under strong electric fields without undergoing electri- cal breakdown 6. This property ensures the long-term electrical stability of biosensors, especially those used in biological fluids or high-impedance environments.
ZnO also exhibits strong photoluminescence for sensitive optical detection due to its high exciton binding energy (60meV) 6. This photoluminescence emission typically occurs in the UV and visible blue range and is highly sensitive to changes at the surface of the nanostructure enabling real-time monitoring of biomolecular interactions.
C. Signal transduction mechanismss
1. Electrochemical transduction - electrochemical biosensors operate by converting biochemical interactions into electrical signals. The ZnO nanostructures act as elec- trode materials or electrode modifiers due to their moderate conductivity, high sur- face area, and ability to stabilize redox-active biomolecules. They facilitate efficient electron transfer between the enzyme’s active site and the electrode 7.
2. Optical transduction - the ability to interact with light and produce measurable changes in their optical emission properties upon biomolecular recognition. When a target biomolecule binds to the ZnO surface, it modifies the surface electronic structure or local environment affecting the photoluminescence behavior 4.
Tabla 1 Morphologies of ZnO in biosensing applications.
| Morphology | Dimensio- nality | Structure Description | Key Functional Features | Biosensor Application |
|---|---|---|---|---|
| Nanoparti- cles (NPS) 4 | 0D (zero- dimensional) | Spherical or quasi- spherical particles with nanoscale diameters | High density of surface atoms; excellent for maximizing surface interactions | Antibody /aptamer- based sensors; electrochemi- cal detection |
| Nanorods/ Nanowires 4 | 1D (one- dimensional) | Vertically aligned or elongated rod-shaped structures | Direct, continuous electron transport paths; fast redox reactions | Enzyme- based glucose, uric acid, and cholesterol sensors |
| Nanosheets 4 | 2D (two- dimensional) | Thin, flat plate-like layers with polar surface exposure | Enhanced binding of charged biomolecules; flexible integration | Wearable patch sensors; DNA/protein electrochemi- cal sensors |
| Nanoflowers / Porous structures 4 | 3D (three- dimensional) | Branched assemblies with high surface area and inner voids | High target-binding molecule loading capacity; improved analyte diffusion and transport | SARS-CoV-2 gene sensors; cancer biomarker im- munosensors |
3. Piezoelectric / acoustic transduction - thin films or nanostructures act as mass- sensitive platforms causing an analyte (such as a protein or virus) when bonded to a functionalized ZnO surface, it causes a measurable mass change 9. Due to ZnO’s strong piezoelectric coefficient and high acoustic velocity, even minute changes in mass lead to detectable frequency shifts, enabling highly sensitive, label-free detec- tion of biomolecules in real time.
4. Field-effect transistor (FET) transduction - ZnO nanowires or thin films form the semiconducting channel between the source and drain electrodes 6 10. These channels are functionalized with biorecognition molecules. When a charged an- alyte (such as a protein, ion, or DNA) binds to the surface, it modifies the local electric field, affecting the charge carrier density in the ZnO channel and the con- ductivity of the device 6. This results in a modulation of the drain-source current, which serves as the detection signal 11 12 13 14.
III. METHODOLOGY
This review is based on an extensive analysis of various scientific articles released be- tween 2022 and 2024, directing attention to the applications of ZnO nanotechnologies in biosensors disease detection. Importantly, it covers both peer-reviewed reviews and open-access articles focusing on surface modification approaches for ZnO-based biosen- sors surface, key aspects of biosensing, and recent experimental investigations into its capabilities in optical and electrochemical sensors. The articles used in this review were selected based on their consistency with the study’s focus 15, clinical relevance, and uniqueness, relying on sources like Springer, ScienceDirect, ELsevier and literature databases including IEEE Xplore and Scopus.
Tabla 2 Key contributions in ZnO-based biosensors
| Ref. | Year | Key Contribution |
|---|---|---|
| 9 | 2024 | Development of a self-powered lactate biosensor based on the piezoelectric effect of ZnO nanowires |
| 10 | 2023 | Non-enzymatic glucose sensor using Ag-decorated ZnO nanorods, ≈ 1.3 µM, high sensitivity |
| 14 | 2023 | Electrochemical immunosensor for anti-PSA employing spherical ZnO structures, achieving nanomolar detection |
| 16 | 2023 | Microfluidic platform with ZnO nanorods for point-of-care dengue (DENV-3) immunofluorescent detection |
| 6 | 2022 | Review of 2D ZnO nanostructure-based biosensors, cover- ing synthesis through device fabrication and performance metrics |
IV. RESULTS
Recent studies have shown that ZnO structures can be adapted into several mor- phologies - from one-dimensional (1D) nanowires and nanorods to two-dimensional (2D) nanosheets and hierarchical nanoarchitectures - to improve the bio detection per- formance 11. These modified forms of ZnO offer a large surface area and a high iso- electric point (favorable surface chemistry) for the immobilization of bioreceptors, which translates into high sensitivity and low limits of detection (LOD) in medical diagno- sis 11. This section in specific examines the experimental findings from 2022 to 2024 on ZnO-based biosensing devices targeted at a wide range of medically relevant ana- lytes, including pathogens, glucose, cancer biomarkers and hormones. The emphasis of this section is placed on new ZnO-based nanostructures (e.g., 2D nanosheets, ver- tically aligned nanorods, flower-like hierarchical structures), various sensor configura- tions (optical, field-effect transistor, electrochemical), key performance indicators (LOD, linear range, sensitivity), and the demonstrated potential for real-life applications (point- of-care devices and wearable devices). Table 3 outlines exemplary findings from recent open-access studies.
Tabla 3 Performance comparison of ZnO-based biosensors
| Analyte | ZnO Nanos- tructure | Detection Method | Linear Range | LOD | Sensitivity |
|---|---|---|---|---|---|
| H2O2(en- zymatic) | Waxberry-like ZnO micro- spheres 12 | Amperometric enzyme biosensor | 0.15-15 mM | 0.115 µM | - |
| H2O2 (non- enzymatic) | ZnO nanopar- ticles on MWCNTs 13 | Amperometric enzyme-free sensor | 1-20 mM | - | - |
| Glucose | High-aspect- ratio ZnO nanorods 5 | Amperometric enzyme biosensor | - | - | Fastest re- sponse (∼5s) |
| Glucose | Ag-decorated ZnO nanorods 10 | Amperometric enzyme-free biosensor | 50-175 µM | 1.3 µM | 2792µA /(mM·cm2) |
| Anti-PSA antibody | Spherical ZnO nanostruc- tures 14 | Electrochemical immunosen- sor | - | ∼1-2 nM | Higher with spherical ZnO |
| CA-125 | ZnO-Au hybrid nanorods 15 | Immunosensor (DPV) | - | ∼2.5 ng/µL | ∼100× better than ELISA |
| Dengue virus anti- gen | ZnO nanorods in microfluidic chip 16 | Immuno- fluorescence detection | ∼3.1×10−4 - 3.1×103 ng/mL | 3.1×10−4 ng/mL | 2.7× higher signal vs flat glass |
| Lactate | ZnO nanowire array on Ti substrate 9 | Piezoelectric enzymatic biosensor | Up to 27 mM | ∼1.3 mM | Self-powered signal from LOx reaction |
A. ZnO Nanobiosensors for Glucose and H 2 O 2 Monitoring
Significant advances in biosensing have been led by ZnO-based nanostructures, espe- cially in the detection of hydrogen peroxide (H2O2) and glucose. ZnO-based micro- spheres resembling waxberries and with a large surface area for enzyme immobilization, making possible a horseradish peroxidase (HRP) biosensor showing a linear range of 0.15-15 mM and a low detection limit of ∼0.115 µM for hydrogen peroxide 12. In an- other study, it was showed that ZnO nanoparticles anchored onto carbon nanotubes pro- duced stable, enzyme-free amperometric sensing of hydrogen peroxide, with the perfor- mance largely dictated by the ZnO structural form (spherical or rod-shaped) 13. These investigations underscore how nanostructures design contributes to enhanced biosensor performance.
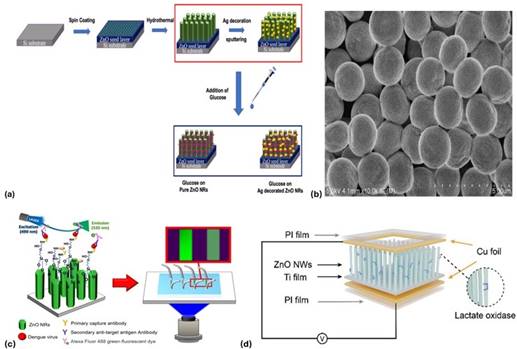
In addition, the morphology of ZnO nanorods for glucose sensing was modified, revealing that thinner, longer structures facilitated higher enzyme immobilization and resulted in the fastest amperometric response (5s) and the highest performance 5. In recent studies, an enzyme-independent glucose sensor with the use of vertically aligned ZnO-based nanorods was modified with silver nanoparticles. Their device demonstrated an outstanding sensitivity (2792 µA/(mM·cm2)), with a linear range from 50 µM to 175 µM and a low LOD of 1.3 µM 10. These findings validate that ZnO surface and structure modification (e.g., noble metal decoration) play a critical role in biosensors efficiency.
B. ZnO-Based Sensors for Cancer Biomarkers
In a recent investigation, a ZnO-based sensor made for the detection of the anti-prostate- specific antigen (anti-PSA) antibodies was developed and this device achieved nanomo- lar detection limits, while evaluating and contrasting different types of ZnO morphology to enhance sensitivity 14. Their results demonstrated that the morphology exhibiting superior sensitivity was the spherical nanostructures, outperforming rod-like forms. A design of ZnO-Au nanohybrid immunosensor for CA-125 (a biomarker for ovarian can- cer) achieved a detection limit of 2.5 ng/µL, close to 100× more sensitive than conven- tional immunoblots 15. These novel findings have shown the ZnO’s growing impact in non-invasive cancer diagnosis.
C. ZnO Nanobiosensors for Infectious Disease Diagnostic
A notable example that illustrates the potential of ZnO nanomaterials in infectious dis- ease diagnostics is developing a ZnO nanorod-integrated microfluidic immunofluores- cence platform for the detection of Dengue virus serotype 3 (DENV-3) 16. This study is especially relevant for low-resource settings and point-of-care diagnostics due to its sensitivity, speed, and minimal sample requirements.
The ZnO nanorods were synthesized via a seed-assisted hydrothermal growth pro- cess, resulting in vertically aligned structures with a predominant (002) crystal orienta- tion. The ZnO nanorods functioned as an immobilization surface for specific monoclonal antibodies (mAbs) to the DENV-3 envelope protein. Functionalization was achieved using 3-glycidyloxypropyl trimethoxysilane (GPTMS) which is a silane linker that co- valently bonds the antibodies to the ZnO surface. Among different surface treatments tested, ZnO modified with 4% GPTMS yielded the highest fluorescence intensity 16.
The mechanism of signal generation is based on optical transduction, where the amount of fluorescent signal directly correlates to the concentration of antigen present on the ZnO surface. The ZnO nanorods play a critical role by:
Providing a high surface area for mAb immobilization
Enhancing binding efficiency and signal amplification
Supporting stable, reproducible fluorescence output
This study is a strong example of how ZnO nanorod morphology can be strategically manipulated to improve biosensor performance through surface chemistry optimization and microfluidic integration. The work demonstrates that ZnO nanostructures not only enhance biomolecular binding but also enable label-based fluorescence detection at ex- tremely low analyte concentrations.
D. Hormone and Metabolite Sensors with Nanostructures
A recent study presents a novel approach to lactate detection using a self-powered ZnO nanowire-based biosensor that represents the multifunctionality of ZnO in wearable di- agnostics 9. The authors developed a biosensing device in which ZnO nanowire arrays were hydrothermally grown on a titanium substrate. The ZnO surface was then func- tionalized with lactate oxidase (LOx), an enzyme that catalyzes the conversion of lactate into pyruvate and hydrogen peroxide 9. What distinguishes this work is its use of the piezoelectric properties of ZnO to enable energy-autonomous sensing. The detection mechanism is based on the piezo-enzymatic coupling effect. Mechanical deformation (e.g., pressure or bending) of the ZnO nanowires generates a piezoelectric voltage due to lattice polarization. In the presence of lactate, the enzyme-catalyzed reaction alters the local ionic environment at the ZnO surface, which modulates the piezoelectric signal out- put. The magnitude of the output voltage correlates with lactate concentration, allowing real-time, quantitative measurement without external power or labeling agents.
This study represents an important advancement in self-powered biosensing and demonstrates the potential of ZnO as a material platform for mechanically responsive, label-free, and wearable diagnostics. The concept of piezo-enzymatic sensing offers a path forward for battery-free devices in personalized healthcare. Furthermore, the au- thors demonstrated a clear integration strategy between material design (ZnO nanowires), biofunctionalization (LOx), and mechanical actuation.
E. Toward Real-World Implementation
ZnO has been positioned as a leading material in next generation biosensors due to its compatibility with chemical stability, biocompatibility and scalable fabrication tech- niques (e.g., screen printing, hydrothermal growth). These ZnO-based nanobiosensors are more frequently tailored for wearables and point-of-care deployment. A thorough review by Zhou et al described ZnO-based enzyme biosensors and substantiated the ma- terial’s broad relevance to clinical diagnostics.
CONCLUSIONS
ZnO nanobiosensors demonstrate exceptional physicochemical properties that position them as a root for the future development of advanced biosensing platforms. Their wide bandgap, high isoelectric point, and morphological diversity provide versatility for engi- neering medical diagnostic devices. The reviewed studies validate that strategic manip- ulation of ZnO nanostructures can significantly expand their applicability beyond tradi- tional laboratory settings into real-time, portable, and point-of-care diagnostic settings.
The adaptability of ZnO opens pathways for developing next-generation biosensors capable of multiplex detection, self-powered operation, and miniaturization. Future ex- ploration should focus on overcoming challenges related to long-term stability, scalabil- ity, and clinical validation to ensure successful translation into healthcare practice.
ZnO-based nanobiosensors not only offer promising routes for disease diagnosis but also inspire broader applications in personalized medicine, continuous health monitor- ing, and environmental sensing.