Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos Latinoamericanos de Nutrición
versión impresa ISSN 0004-0622versión On-line ISSN 2309-5806
ALAN v.51 n.1 supl.1 Caracas mar. 2001
Bioavailability of iron bis-glycinate chelate in water
Manuel Olivares G., and Fernando Pizarro A.
Instituto de Nutrición y Tecnología de los Alimentos (INTA), Universidad de Chile
SUMMARY .
Iron amino acid chelate is being increasingly considered in programs for iron fortification of foods. The bioavailability of iron bis-glycinate chelate given in water was studied using a double-isotopic method in a group of 14 women. Iron absorption from aqueous solutions of 15 mg/L of elemental iron as either iron bis-glycine chelate or ferrous ascorbate was not significantly different (34.6% and 29.9% respectively). Standardized iron absorption of the iron bis-glycinate was 46.3% (standardized to 40% absorption of the reference dose). There was a significant correlations between (In) iron absorption of iron bis- glycinate chelate with (In) serum ferritin (r= -0.60, p <0.03) and with (In) iron absorption from ferrous ascorbate (r= 0.71, p<0.006), suggesting that iron bis-glycinate chelate absorption is indeed regulated by the iron stores of the body.
Key words: Ferrochel, absorption, bioavailability, regulation.
RESUMEN.
Biodisponibilidad del hierro bis-glicinato quelado dado en agua. El hierro aminoácido quelado está siendo considerado en forma creciente para programas de fortificación de alimentos. La absorción del hierro del bisglicinato dado en agua the estudiada en un grupo de 14 mujeres utilizando la técnica doble isotópica. Las absorciones de soluciones acuosas de 15 mg/L de hierro elemental como bis-glicinato o como ascorbato ferroso no theron significativamente distintas (34,6% y 29,9% respectivamente). La absorción estandardizada del bis-glicinato de hierro the 46,3% (estandarizado a 40% absorción de la dosis de referencia de ascorbato ferroso). Existió una correlación significativa entre el logaritmo de la absorción del bis-glicinato y el logaritmo de la ferritina sérica (r= -0,60, p <0,03) y el logaritmo de la absorción del ascorbato ferroso (r= 0,71, p<O,O06), sugiriendo que la absorción del bis-glicinato de hierro es regulada por los depósitos de hierro del organismo.
Palabras clave: Ferrochel, absorción, biodisponibilidad, regulación.
INTRODUCTION
Iron deficiency continues to be one of the most prevalent nutritional deficiencies in the world. For physiological reasons the most commonly affected groups are infants, children, adolescents and women of childbearing age (1).
Fortification of foods with iron is considered the best sustainable way of preventing iron deficiency (2). The sequential steps that should be followed in establishing an iron fortification program have been well defined. Perhaps the most difficult technical hurdle is finding the adequate combination of iron compound and food vehicle. Consequently, the use of an iron compound that is less influenced by inhibiting dietary ligands is an appealing strategy.
Iron amino acid chelate is a compound formed by two glycine molecules bound to an iron atom, resulting in a double heterocyclic ring compound. It has been proposed that this configuration protects the iron from dietary inhibitors and intestinal interactions (3). Recent studies have shown that iron bis-glycinate chelate is well absorbed when it is added to foods with a predominance of inhibitors (4,5).
The aim of the study was to etablish the bioavailability of iron bis-glycinate chelate when given in water.
SUBJECTS AND METHODS
Subjects
Iron absorption studies were performed in a group of 14 women between the ages of 27 and 51 years. None were pregnant, all used contraceptive intrauterine devices and were in apparent good health. Written informed consent was obtained from each volunteer before participation in the study. The protocol was reviewed and was found in accordance with the standards set by the Institute of Nutrition and Food Technology's Ethics Committee on Human Research. Radioactive doses were approved by the Chilean Nuclear Energy Commission.
Isotope studies
Iron isotopes (55Fe and 59Fe) of high specific activity were used as tracers. Both isotopes are iron (III) chlorides as purchased (Du Pont de Nemours & Co. Inc., Wilmington, DE). Isotopes were mixed with water immediately before administration. Iron bis-glycinate chelate (Ferrochel® , Albion Laboratories, Ine., Clearfield, Utah) was intrinsically labeled during the synthesis of iron amino acid chelates. This process was performed by the manufacturer. The specific activity of the labeled iron bis-glycinate chelate was 37 kBq of 59Fe/mg elemental iron. The preparations were consumed after an overnight fast, and no food or beverages other than water were permitted for 4 hours following consumption. The amounts of aqueous solutions ingested were calculated by differential weight of the glasses. For the calculation of total radioactivity ingested, aliquots of the aqueous solutions were counted in sextuplicate as standards. Measurement of blood radioactivity was performed in duplicate venous samples according to the method of Eakins and Brown (6), and were counted for sufficient time to ensure less than 3% counting error. A liquid-scintillation counter (Beckman LS 5000 TD, Beckman Instruments, Inc., Fullerton, CA) was used for the double isotope measurements. Percent absorption was calculated based on the blood volume estimated from height and weight, assuming an 80% red cell utilization of radio iron (7).
On day 1 the subjects received 200 mL of an aqueous solution of 15 mg/L of elemental iron as iron bis-glycinate chelate labeled with 111 kBq of 55Fe and on day two 200 mL of a reference dose of 15 mg/L of elemental iron as ferrous ascorbate (molar ratio 1:2 iron to ascorbic acid) labeled with 37 kBq of 59FeC13. A venous blood sample was obtained two weeks later (day 16) to measure the circulating radioactivity and to determine the iron status of the subjects.
Hemoglobin, mean cell volume, free erythrocyte protoporphyrin, serum iron, total iron binding capacity and serum ferritin were determined in the venous blood obtained on day 16 (8).
For comparative studies of iron bioavailability, the absorption of 3 mg of elemental iron as ferrous ascorbate is used to offset the effect of differences in iron status among individuals (9). For purposes of comparison all studies currently refer to 40% absorption of the reference dose of ferrous ascorbate. This absorption percentage is used because it corresponds to that which is obtained in borderline iron deficient populations.
Because the percentages of iron absorption and serum ferritin concentrations have a skewed distribution, these values were converted to natural logarithms before performing mean and SD calculations, and the results were transformed back using antilogarithms to recover the original units, and expressed as geometric means ± SD (7). Statistical analyses included paired Student t test and Pearson correlation. Statistical analyses was performed on logarithmically transformed data using the program SPSS for Windows, release 6.0, SPSS Inc., Chicago, IL, 1993.
RESULTS
The iron bioavailability of the aqueous solutions of iron bis-glycinate chelate and ferrous ascorbate were not significantly different (34.6% and 29.9% respectively; t = 0.80, p NS). When iron absorption was standardized to 40% absorption of the reference dose using the data shown in Table 1, the corresponding percentage of iron absorption for the iron bis-glycinate chelate given in water was 46.3%.
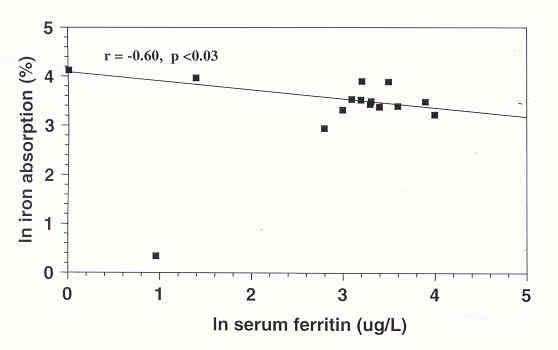
There was a significant inverse correlation between (In) serum ferritin and (In) iron absorption from iron bis-glycinate chelate given with water (r= -0.60, p <0.03). The correlation between the absorption of (In) ferrous ascorbate and (In) iron bis-gIycine chelate given with water was (r=0.71, p < 0.006).
DISCUSSION
The efficacy of an iron compound for supplementation or food fortification interventions can be predicted from iron bioavailability studies of the supplement or the fortified food. Bioavailability of iron is influenced by the characteristics of the iron compound, total amount of iron in the diet, iron status of the individual, rate of erythropoiesis, and the presence of inhibitors or enhancers of iron absorption present in the intestinal lumen or in the diet (9,10). If soluble inorganic iron salts are given with water solutions without food, absorption of iron is high, but, absorption of iron salts decreases markedly when given with foods (11).
Our results showed that the iron bioavailability of iron bis-glycinate chelate given in water is high, compared to the absorption of the reference dose of ferrous ascorbate. The iron absorption of this compound was calculated to be 46.3% in a population with low iron stores (a population absorbing 40% of the reference dose of iron ascorbate).
It has been proposed that the iron amino acid chelate is absorbed in the jejunum rather than as inorganic iron in the duodenum (3). Therefore, there has been some concern about the role of iron stores on the regulation of iron absorption from the chelate. The results of ibis study, suggest that the absorption of the iron bis-glycinate chelate is likely to be controlled by the iron stores of the subjects. There was an inverse relationship between the iron stores of the body, as reflected by serum ferritin, and the absorption of the iron bis-glycinate chelate. This holds true as well when we compare the absorption of the iron bis-glycinate chelate with that of ferrous ascorbate which showed an excellent correlation (r=0.71). However, this correlation may not necessarily prove causality. Another study performed in adult women also showed an inverse correlation between serum ferritin and the absorption of iron bis-glycinate chelate (12). The demonstration that regulation of iron absorption by iron stores occurs with the iron bis-glycinate chelate should dispel the risk of iron overload if this compound was to be used in food fortification programs for the population at large. Further research is needed to elucidate the mechanism of absorption of iron bis-gIycine chelate.
We can conclude that in water the absorption of iron bis-glycine chelate is high. Studies to compare the bioavailability of the iron amino acid chelate when added to different foods are needed.
Iron absorption from iron bis-glycinate chelate1 Iron Absorption % Ratio Subject Age y Hb g/L FEP ug/dL Rbc Sat % SF ug/L Iron bisglucinate 55 (A) Iron Ascorbate 59 (B) I A/B GR EM AC BM LM GR MA YR XM MLG GM PG PA LA Mean SD 48 43 51 38 49 33 43 45 46 40 42 43 45 43 43.5 4.4 133 150 140 132 142 138 148 144 147 131 143 144 143 118 139.5 8.2 57.0 42.8 60.0 48.6 37.1 54.3 42.9 57.2 88.6 45.6 65.7 62.9 49.4 191.4 64.5 37.3 26.7 15.4 23.1 20.4 18.1 34.3 17.1 27.1 23.9 15.2 25.2 27.8 26.1 9.9 22.2 6.2 24 29 53 36 21 23 48 16 26 25 27 32 4 1 19 2 7-53 33.8 29.3 25.1 29.8 27.6 34.3 32.6 19.0 31.0 49.5 32.8 49.0 52.9 61.8 34.6 2 25.4-47.2 6.0 8.5 14.4 17.6 28.4 29.1 30.3 32.7 35.0 36.5 46.2 72.6 79.4 124.0 29.9 2 13.4-66.6 5.63 3.45 1.74 1.69 0.97 1.18 1.08 0.58 0.89 1.36 0.71 0.67 0.67 0.50 1.16 2 0.60-2.25 1
2
Geometric mean and range 1 SDFIGURE 1
Relationship between natural logarithm (In) iron absorption of iron amino acid chelates given in water and ln setum ferritin
REFERENCES
1. DeMayer E, Adiels- Tegman M. The prevalence of anemia in the world. World Health Statist Q 1985;38:302-316. [ Links ]
2. International Nutritional Anemia Consultative Group (INACG). Guidelines for the eradication of iron deficiency anemia: a report of the International Nutritional Anemia Consultative Group. The Nutrition Foundation, Inc. Washington, DC, 1977:1-29. [ Links ]
3. Ashmead HD, Graff DJ, Ashmead HH. Intestinal absorption 0f metal ions and chelates. Charles C. Thomas Publisher, Springfield, 11, 1985: 1-251. [ Links ]
4. Olivares M, Pizarro F, Pineda O, Name JJ, Hertrampf E, Walter T. Milk inhibits and ascorbic acid favors ferrous bis-glycine chelate bioavailability in humans. J Nutr 1997;127:1407-1411. [ Links ]
5. Bovell-Benjamin AC, Allen LH, Viteri FE. Iron is well absorbed from ferrous bisglycinate (Ferrochel) added to a high phytate whole-maize meal. FASEB J 1997; 11 :A606 (abstract) [ Links ]
6. Eakins JD, Brown DA. An improved method for the simultaneous determination of iron-55 and iron-59 in blood by liquid scintillation counting. Int J Appl Radiact Isotopes 1966;17:191-197. [ Links ]
7. Cook JD, Layrisse M, Finch CA. The measurement of iron absorption. Blood 1969;33: 421-429. [ Links ]
8. International Anemia Consultative Group (INACG). Measurement of iron status: a report of the International Anemia Consultative Group. The Nutrition Foundation, Washington, DC, 1985:1-49. [ Links ]
9. Cook JD, Bothwell TH. Availability of iron from infant foods. In: Iron nutrition in infancy and childhood. Stekel A, ed., pp. 119-145. Nestlé/Raven Press, Vevey/NewYork. 1984:119-145. [ Links ]
10. Bothwell TH. Iron absorption. Ann Rev Med 1983;34: 55-68. [ Links ]
11. Hurrell RF. Bioavailability of different iron compounds used to fortify formulas and cereals: technological problems. In: Stekel A, ed. Iron nutrition in infancy and childhood. Nestlé/ Raven Press, Veyey/New York, 1984:147-178. [ Links ]
12. Allen LH. Properties of iron amino acid chelates as iron fortificants for maize. International Conference on Human Nutrition. Salt Lake City, TU, January 24-25, 1998, pp 96- 108. [ Links ]














