Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos Latinoamericanos de Nutrición
versión impresa ISSN 0004-0622
ALAN vol.66 no.2 Caracas jun. 2016
Review: dietary phenolic compounds, health benefits and bioaccessibility
Erick Paul Gutiérrez-Grijalva, Dulce Libna Ambriz-Pére, Nayely Leyva-López, Ramón Ignacio Castillo-López, José Basilio Heredia
Research Center for Food & Development (CIAD), AC., Functional Foods and Nutraceutical Laboratory, Culiacán, Sinaloa, 80110 México
SUMMARY Phenolic compounds are ubiquitous in plant-based foods. High dietary intake of fruits, vegetables and cereals are related to a decreased rate in chronic diseases. Phenolic compounds are thought to be responsible, at least in part, for those health effects. Nonetheless, the bioaccessibility of phenolic compounds is not often considered in these studies; thus, a precise mechanism of action of phenolic compounds is not known. In this review, we aim to present a comprehensive knowledge of the potential health promotion effects of polyphenols and the importance of considering the factors that affect their bioavailability on research projects.
Key words: Phenolic compounds, biotransformation, metabolism
Review: compuestos fenólicos dietéticos, beneficios a la salud y bioaccesibilidad.
RESUMEN Los compuestos fenólicos son ubicuos en alimentos de origen vegetal. La alta ingesta de frutas, vegetales y cereales está relacionada con un bajo índice en padecimientos crónicos. Se cree que los compuestos fenólicos son, en parte, responsables de este efecto benéfico. Sin embargo, la bioaccesibilidad y biotransformación de los compuestos fenólicos generalmente no es considerada en este tipo de estudios. Por lo tanto, no se ha podido obtener un mecanismo de acción de los compuestos fenólicos. En este trabajo, presentamos una revisión de literatura del potencial benéfico de los compuestos fenólicos y cómo diversos factores pueden afectar su absorción y metabolismo.
Palabras clave: Compuestos fenólicos, biotransformación, metabolismo
Recibido: 08-12-2015
Aceptado: 08-03-2016
INTRODUCTION
In recent years, there has been an increased awareness of the effect of food on health, thus leading to a rise in the consumption of fruit, vegetables, and cereal-based food. Many studies have approached the bioactive properties of bioactive compounds such as phenolic compounds. Nonetheless, bioactive claims are made without taking into consideration the further modifications to which phenolic compounds are subjected once ingested. This study is a comprehensive review of the health claims and bioavailability of phenolic compounds.
PHENOLIC COMPOUNDS IN CEREALS, FRUITS, AND VEGETABLES
Phenolic compounds constitute a substantial and an important group of phenylpropanoids produced by plants as secondary metabolites. Plants synthesize them to function as a chemicaldefense against predators and to participate in reproduction as well as in plant-plant interference (1). Phenolic compounds have an aromatic ring and several hydroxyl groups attached to it. Phenolic compounds can be classified into different groups. They are grouped as a function of the number of phenolic rings that they contain and the radicals that bind these rings to another one (2).
Recently, phenolic compounds have received considerable attention because their dietary intake is relatedtolowerincidenceof chronic degenerative diseases, such as cancer, diabetes, Alzheimers disease and cardiovascular diseases. Cereals, fruits, and vegetables are rich sources of phenolic compounds. In fact, the health benefits of their dietary intake have been related, at least in part, to their phenolic compounds content.
Effect of Consumption of Phenolics on Human Health
Epidemiological studies have related dietary intake of phenolic-rich food with lower incidence in the appearance of several chronic diseases (3, 4). In this section of the review, we will discuss the epidemiological evidence that supports phenolics health benefits.
Phenolics in Cereals and Their Relationship With Health
Phenolic compounds are among the health-promoting phytochemicals present in cereals. Phenolic compounds are receiving much attention because of their antioxidant properties. Phenolic acids and flavonoids are the most common types of phenolic compounds found in whole grains. In cereals, phenolic compounds can be present in the free or bounded form; bound phenolics are mostly attached to arabinosyl chains of cell wall arabinoxylans (5, 6). Most of these bound phenolic compounds are located in the aleurone layer, but can also found in seed and embryos (7-10).
Irakli, Samanidou (11) reported that phenolic acids such as coumaric, ferulic, gallic, hydroxybenzoic, vanillic, syringic, and sinapic acid are found in both, free and bounded form in durum wheat, bread wheat, barley, oat, rye, rice, corn, and triticale. In cereals, the free phenolic acids constitute a small portion of the total phenolic content while bound phenolic acids are the most predominant. In this case, it was proven that the total of bound phenolic acids constitutes from 88 % (rye) to 99.5 % (corn) of the total phenolic acids. Regardless phenolic acids being the most predominant in cereals, flavonoids are also present in grains. 20 genotypes of small grains cereals, including bread wheat, durum wheat, rye, hull-less barley, and hull-less oat were analyzed for total phenolic and flavonoid content. The highest content of these phytochemicals was found in hull-less barley, followed by hull-less oat, rye, durum wheat, and bread wheat. Nevertheless, monomeric phenolic compounds like catechin and epicatechin were only detected in hull-less barley genotypes (12). So it is concluded that the phenolic composition depends greatly on the type and variety of cereal.
It has been suggested that phenolic compounds play a significant role in the prevention of many chronic diseases due to their antioxidant, anti-inflammatory and anti-carcinogenic properties (7). In this sense, Hole, Grimmer (13) analyzed the anti-inflammatory action of ferulic, caffeic, ρ-coumaric and sinapic acids found in extracts of free and bound phenolic acids from oat, barley, and wheat flour by studying the modulation of NF-κB activity.NF-κB is a transcription factor involved in the regulation of pro-inflammatory genes that plays a critical role in the control of innate immunity processes and whose increased activation has been detected in several human cancers (14). The results of this study indicated that modulation of NF-κB activity exposed to cereal extracts containing phenolic acids is the result of phenolic acids synergic action. Moreover, a combination of ferulic, caffeic, ρ-coumaric and sinapic acid in low concentrations had a significant synergistic effect on NF-κB activity; while higher concentrations had better effect suppressing NF-κB activity (13). An important thing to remark is that in this study, the concentrations of extracts from cereal grains are similar to those found in the human diet, so their results increase the knowledge about the health-promoting effect of phenolic acids from cereal consumption.
Whole cereal grains are an excellent source of phenolic acids, and its consumption is associated with lower incidence of chronic diseases. Giacco, Costabile (15) reported that a diet based on whole-grain cereal products reduces postprandial insulin, and plasma triglyceride concentrations in individuals with metabolic syndrome, in 29 and 43 % respectively. The effects of whole-grain cereals on postprandial insulin and plasma triglyceride concentrations might explain the relationship between consumption of cereals and a reduced risk of type 2 diabetes and cardiovascular diseases. Nevertheless, the responsible compounds of these effects were not reported.
Phenolics in Fruits and Their Relationship With Health
Apples are one of the most popular fruits whose health benefits are attributed to phenolic compounds. The four polyphenol groups predominant in apples are flavan-3-ols, phenolic acids, dihydrochalcones and flavonols(16). Some phenolics such as chlorogenic acid, phloretin, epicatechin, quercetin and procyanidin B2 have been identified as major antioxidants in apples (17). In this sense, some in vitro properties of apple polyphenols have been elucidated, among these: enhancement of glutathione S-transferases, reduced formation of H2O2, protection against oxidative-induced DNA damage, inhibition of intestinal glucose absorption (which could help against metabolic syndrome) (18-20).
Mango fruits contain several bioactive compounds, such as vitamins, carotenoids, terpenoids and phenolic compounds. Phenolic acids like gallic, protocatechuic, chlorogenic and vanillic acids are predominant in mango pulp (21). On this subject, Noratto, Bertoldi (22) reported the presence of hydrolysable tannins and mangiferin in mango pulp of different varieties. Additionally, mango peel is a rich source of these bioactive compounds. Furthermore, gallic, protocatechuic, syringic and ferulic acids were phenolic acids identified in the bound phenolic fraction of mango peel dietary fiber. Among the bound flavonoids identified, kaempferol and quercetin were predominant, but traces of rutinwere also present (23). Amongst the in vitro health properties of mango polyphenols, it has been suggested that they can inhibit adipogenesis (24), also, anti-cancer properties of mango extracts have been proven, this anti-cancer bioactivity was mainly attributed to polyphenolics compounds in mango (22, 25, 26); also, in vivo studies have shown anti-cancer and antioxidant capacity of mango (27).
Citrus fruits are rich in various nutrients, such as vitamins A and C, folic acid and dietary fiber. Furthermore, these fruits are a source of bioactive compounds, being cinnamic acid derivatives, coumarins, and flavonoids the major groups of phenolic compounds (28). Citrus fruit has considerable amounts of flavonoids like, flavones, flavonols, and anthocyanins; however the main flavonoids are flavanones, which the most frequently found are hesperidin, naringin, nariturin and eriocitrin (29, 30). Other phenolics often found in citrus are ρ-coumaric, ferulic, caffeic and sinapic acids (30). The daily consumption of grapefruit and orange juice has shown to decrease diastolic blood pressure (31, 32)
It is always important to remember that phenolic composition and concentration are dependent on the variety and ripeness stage of the fruit, as of the part of the fruit that is being analyzed.
There is epidemiological and experimental evidence that consumption of fruits has a positive effect on health; this effect has been, in part, attributed to their content of phenolic compounds (33-36). In this regard, McCann, Gill (37) investigated the ability of a phenolic extract of apple waste (material left after juice extraction) to affect a range of colon cancer biomarkers, namely: DNA damage, colonocyte barrier function, cell cycle progression and invasion in vitro using HT29, HT115 and CaCo-2 cell lines as models. Flavan-3-ols, phloretin glycosides, quercetin glycosides, cyanidin glycoside and hydroxycinnamic acids were among the phenolic compounds present in apple phenolic extracts. Phenolic compounds from apples proved to help to decrease DNA damage in HT29 cells significantly, to enhance the colonic barrier function of CaCo-2 cells and to reduce the invasive potential of HT115 cells. These authors concluded that apple consumption may serve to protect against colon cancer by protecting gut cells against DNA damage and abnormal extracellular behavior. Additionally, some studies relate apple consumption to lower plasma cholesterol and reduction of risk of cardiovascular disease (38).
Studies on yuzu (Citrus junosSiebold ex Tanaka) show that peel and pomace exerted anti-obesity effects in zebrafish with diet-induced obesity. Yuzu peel suppressed the rise in plasma triacylglycerol and liver lipid accumulation (39). Yuzu peel and pomace are rich in flavonoids, such as hesperidin, naringin, and eriocitrinthat are recognized to have the ability to lower total blood cholesterol (40). In this sense, a recent study by Wu, Jiang (41), high-fat-diet-induced obese mice were fed from 50-200 mg/kg of blueberry anthocyanins, found that supplementation at high dose of anthocyanins decreased serum glucose, attenuated epididymal adipocytes, improved lipid profiles and down-regulated expression levels of inflammation-related genes TNFα, IL-6 PPARγ and FAS; their results suggest that anthocyanins could help to reduce obesity.
Phenolics in Vegetables and their relationship with health
Phenolic acids and isocoumarins were the predominant phenolics in carrots (42, 43). Among the most common phenolic compounds found in vegetables are flavonoids, phenolic acids and isocoumarins. For example, most of the compounds detected in black carrot roots and black carrot juice are composed of ρ-coumaric, caffeic and ferulic acids; 5-caffeoylquinic acid was the predominant phenolic acid. Besides, some phenolic glycosides like dihydroxybenzoic acid hexoside and quercetin-3-O-galactoside were detected (43). Alasalvar, Grigor (44) examined the phenolic content of carrots of four different colours: orange, purple, yellow and white. The four colored carrots contained mainly hydroxycinnamic acid derivatives, namely 3'-caffeoylquinic acid, 5'-caffeoylquinic acid, 3'-ρ-coumaroylquinic acid, 3'-feruloyquinic acid, 3',4'-dicaffeoylquinic acid, 5'-feruloyquinic acid, 5'-ρ-coumaroylquinic acid, 4'-feruloylquinic acid, 3',5'-dicaffeoylquinic acid, 3',4'-diferuloylquinic acid and 3',5'-diferuloylquinic acid. Anthocyanin content, a type of flavonoids, has also been reported in carrots. The anthocyanins found in purple carrots roots are cyanidin-3-xylosyl (glucosyl)-galactoside, cyanidin-3-xylosylagalactoside, cyanidin-3-xylosyl-(sinapoylglucosyl)-galactoside, cyanidin-3-xylosyl (feruloylglucosyl)-galactoside, cyanidin-3-xylosyl (coumaroylglucosyl)-galactoside, pelargonidin-3-xylosyl (feruloylglucosyl)-galactoside and peonidin-3-xylosyl (feruloylglucosyl)-galactoside(45).
In this sense another vegetable that has been widely studied are tomatoes, which are a key component in the Mediterranean diet and its dietary intake is associated with lower risk of chronic degenerative diseases, such as cancer and cardiovascular diseases (46). Vallverdu-Queralt, Regueiro (47) conducted an extensive study to identify the number of phenolic compounds extracted from tomato samples. They identified a total of 38 phenolic compounds, among which gallic acid, protocatechuic acid, caffeic acid derivatives, ferulic acid derivatives, kaempferol, rutin, naringenin, phloridzin, and quercetin were present.
Lettuce might be relevant as a dietary source of phenolic compounds. Several phenolicswere identified in five varieties of lettuce (iceberg, romaine, continental, red oak leaf and lollorosso). The phenolic compounds identified were 5-O-caffeoylquinic acid, caffeoylmalic acid, dicaffeoyltartaric acid, and 3,5-dicaffeoylquinic acid, flavonoid-malonyl glycosides (quercetin-3-malonylglucoside-7-glucuronide, quercetin-3-malonylglucoside-7-glucoside, quercetin-3-malonylglucoside) and flavonoid glycosides (quercetin-3-glucuronide, quercetin-3-glucoside, quercetin-3-rutinoside, luteolin-7-glucuronide, and luteolin-7-glucoside, luteolin-7-rutinoside) groups.Caffeic acid derivatives were the main phenolic compounds in green varieties; while flavonols were present in red varieties in higher quantities; besides, anthocyanins were present only in red-leafed varieties (48).
Onions, spinach and pepper fruits are a rich source of flavonoids (5). A blended mix of juice and pulp of carrot, parsley, beet, kale, broccoli, cabbage, tomato, and spinach, as well as sugar beet fiber, garlic powder, oat bran fiber, and rice bran was analyzed in order to identify the phenolic composition of this vegetable mix. Among the variety of phenolic compounds found, flavonols were the most abundant. A total of 28 compounds were identified which belong to the dihydrochalcones, flavone, flavonols, hydroxycinnamic acids, lignans and glucosinolategroups(49).
Reactive oxygen species (ROS) is a collective term that describes O2-derived free radicals, such as superoxide anion (O2-), hydroxyl (HO), peroxyl (RO2), and alkoxy (RO) radicals; O2-derived nonradical species such as hydrogen peroxide (H2O2) are also included. Reactive species of oxygen (ROS) are relevant mediators and influential factors in the development of colorectal cancer (50). Phenolic compounds with antioxidant potential are shown to play an important role in modulating the ROS level in the intestinal contents. Olejnik, Kowalska (45) studied the effect of digested purple carrot extract, rich in anthocyanins, on ROS generation and oxidative DNA damage in colon cells. Digested purple carrot extract exhibited intracellular ROS-inhibitory capacity, with 1 mg/mL showing the ROS clearance of 18.4 %. Digested purple carrot extract showed a 20.7 % reduction in oxidative DNA damage in colon mucosa. These findings indicate that purple carrot extract is capable of colonic cells protection against the adverse effects of oxidative stress. For this reason,phenolics contained in purple carrot extract may have a protective capacity of colonic cells (45).
Treatment of rats with CCl4 plus lettuce extract ameliorated the toxic effects of CCl4. This plant contains flavonoids that scavenge the oxidative damage to different cells and organs. These results demonstrate that ethanol lettuce extract treatment increases the antioxidants defense mechanism against CCl4-induced toxicity and provides evidence that it may have a therapeutic role in free radical mediated diseases(51).
Nevertheless, sole dietary consumption of plantbased foods allows us to utilize fully the phenolic compounds present in those foods. There are a lot of factors that affect the bioactivity of phenolic compounds present in plant foods. This subject will be further discussed.
DIETARY PHENOLIC COMPOUNDS
Phenolic compounds are derived from the secondary metabolism of plants. Phenolics are chemical compounds that have at least one aromatic ring to which one or more hydroxyl groups are bonded to aromatic or aliphatic structures (52). There is a wide variety of phenolic compounds. Nonetheless, this study will focus only in 2 groups:
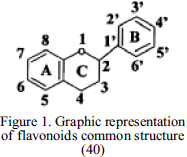
1) Flavonoids, composed of two aromatic rings linked through an oxygen heterocycle and depending on the degree of hydrogenation (Figure 1) and the replacement of the heterocycle they can be sub-classified as flavonols, flavones, isoflavones, anthocyanins, proanthocyanidins, flavanones, etc. Flavonoids are mostly found in the form of glycoside, the main sugars to which they are linked are glucose, rhamnose, galactose and xylose.
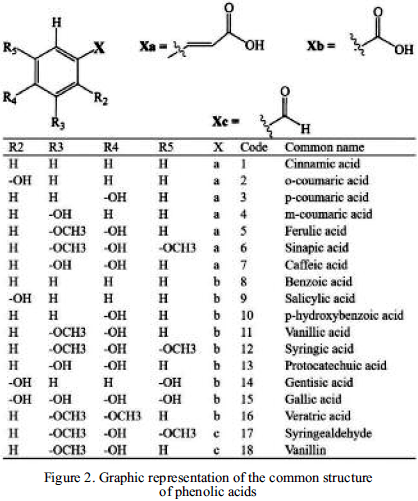
2) Non-flavonoids, like benzoic and cinnamic compounds, which are commonly called phenolic acids, these contain an aromatic ring that can be attached to different functional groups or esterified to organic acids (Figure 2). Some other phenolic compounds are stilbenes, tannins, lignins, and lignans. Properties, like color, flavour and astringency are caused by the presence of such compounds.
In recent years, phenolic compounds have been of increasing interest to science and industry for their beneficial health effects, especially because of its antioxidants properties. Halliwell and Gutteridge (53) defined an antioxidant as any substance that, when present at low concentrations compared with that of an oxidizable substrate, significantly delays or inhibits oxidation of that substrate. An antioxidant should also have the ability that after scavenging the radical, to form a new radical that is stable enough intramolecular hydrogen bonding on further oxidation (54). Free radicals, in turn, are molecules with an unpaired electron and seek electrons from other molecules to gain stability. Molecules as proteins, lipids or DNA, are known to function as a target for these free radicals, which may lead to a deteriorative process called oxidation (2, 55).
Antioxidant activity of phenolic compounds is attributed to their capacity to act as reducing agents to free radicals. Some common phenolic compounds found in nature are flavonoles, flavones, isoflavones, anthocyanins, flavonones, chatequines and proanthocyanidins(2, 35, 55). Additionally, the potential phenolic compounds have also been attributed, at least partially, to its anti-inflammatory properties (56).
The main factor on antioxidant activity of phenolic compounds is its number and position of hydroxyl groups. Flavonoids possess more hydroxyl groups thus present higher antioxidant activity.
Moreover, the solubility and stearic effects of each molecule may be affected by the structure the molecule; for example, the presence of glycosylated derivatives of other adducts, can increase or decrease the antioxidant activity of phenolic compounds. Flavonoid compounds are commonly present in plants as glycosides, but can be released by the action of enzymes to its corresponding aglycone. The antioxidant activity of phenolic acids is also based on the binding of these compounds to organic acids and sugars. The mechanisms by which these compounds act may vary depending on the concentration and types of compounds present in foods (52).
FACTORS AFFECTING THE BIOAVAILABILITY OF DIETARY PHENOLIC COMPOUNDS
A balanced diet provides many different phenolic compounds. Thus their bioavailability may vary, besides the diet changes in every country and every season. To consider phenolic compounds nutraceutical potential it is important to know how much of a phenolic is present in specific food or dietary supplement; also, it is necessary to know how much of it is bioavailable.
Bioavailability is defined as that fraction of an ingested nutrient or compound that reaches the systemic circulation and the specific sites where it can exert its biological action.Bioavailability depends on proper absorption, the release of a dosage form and presystemic elimination. Therefore, bioavailability also depends on the route of administration and dosage form used, but can vary from one individual to another, especially when factors that alter the absorption (57).
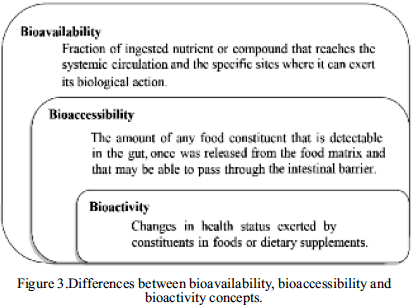
Bioavailability is related to other two concepts: bioaccessibility and bioactivity. In this sense, bioaccessibility is described as the amount of any food constituent that is released from the food matrix, detectable in the gut, and that may be able to pass through the intestinal barrier (21). This is crucial because only the compounds that released from the matrix or absorbed in the small intestine are potentially bioavailable and bioactive (Figure 3) (58).
Furthermore, recently it was proposed that once a compound is absorbed it is inevitably bioactive, because of this was suggested that the concept of bioavailability includes bioactivity (59). Nonetheless, it is important to note that the fact that a compound being bioavailable does not always imply its bioactive (Figure 1).
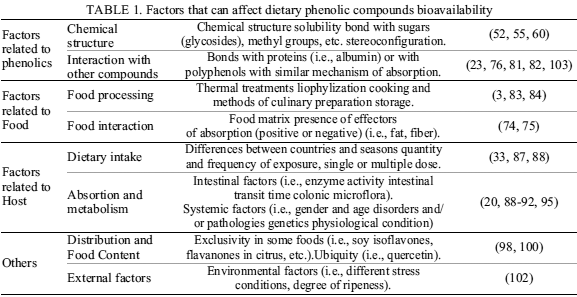
Phenolic bioavailability varies over a wide range from 0.3% estimated for anthocyanins to 43% in the case of isoflavones(55). In this sense, bioavailability is influenced by phenolic structure, food processing and matrix, host, among others; besides all these factors can interact with each other and influence phenolic compounds bioavailability (Table 1).
Factors Related to Phenolic Chemical Structure
-Solubility
The degree of solubility is given by the chemical structure of a molecule. It has been observed in both in vivo and in vitro studies, using phenolic compounds with different solubility, that there is variation in susceptibility to digestion, fermentation, and absorption from the gastrointestinal tract (52). These lead to suggest a classification of phenolic compounds that distinguishes between extractable and nonextractable phenols. Extractable polyphenols have low-intermediate molecular mass, and they can be extracted using different solvents such as water, methanol, aqueous acetone, etc. Aamong the major extractable polyphenols, we can find some hydrolysable tannins and proanthocyanidins. In the other hand, nonextractable polyphenols are high molecular weight compounds that are mostly bound to dietary fiber or protein that remains insoluble in the usual solvent and requires an extra step of hydrolysis during extraction to make them soluble and bioavailable (60). Also, it has been observed that the specific absorption of various extractable phenolics, depends on their extractability with different solvents (61).
-Glycosylation
Phenolic compounds exist as free aglycones and glycoside forms, the last ones can be as O-glycosides or as Cglycosides, with a number of sugars, glucose is the most commonly encountered, followed by galactose, rhamnose, xylose and arabinose, while mannose, fructose, glucuronic and galacturonic acids are unusual (62).
Aglycones and polyphenols bound to glucose, galactose or xylose are absorbed in the small intestine after deglycosylation by β-glucosidase and lactase phlorizin hydrolase (4), these enzymes releasing the aglycone into the intestinal lumen for absorption by a diffusion mechanism. Phenolic compounds bound to rhamnose must reach the colon to be hydrolysed by bacterial ramnosidasesbefore its absorption (55).
Flavonoids bound to sugars as β-glycosides are considered non-absorbable, only aglyconescan pass through the gut wall. The major sites of flavonoid metabolism are the liver and the colonic flora. In the liver occurs O-methylation, sulphation, and glucuronidation of hydroxyl groups improving flavonoid absorption; moreover, flavonoid glycosides are hydrolysed only by colon microorganisms, after this they can be absorbed (63).
Most of in vivo studies show gastric absorption of aglycones as quercetin and daidzein while glycosides are poorly absorbed (55). However, Hollman and Katan (64) observed that quercetin glycosides from onions were absorbed far well than the pure aglycone. Isoflavonesaglycones are absorbed in the stomach while their glycosides are absorbed in the intestine (4)
Within the glycosylated polyphenols, anthocyanins appear to be an exception, since the predominant forms in blood are their intact glycosides. Some authors have suggested the existence of a particular mechanism of anthocyanins absorption at the gastric level, which could involve transport via gastric bilitranslocase (2, 55, 65).
-Acetylation and Methylation
The acylated flavonoids such as epicatechin and epicatechingallateare absorbed without prior hydrolysis or deconjugation(4, 66). Studies have shown that approximately 50% of the amount of (–)-epicatechin that reaches the intestinal cells is absorbed, and a percentage of metabolites (especially sulfate conjugates) eliminated by efflux into the intestinal lumen, and there exists a relatively modest elimination of (–)-epicatechin by bile; it also has been observed a potential absorption of (–)-epicatechin and elimination by efflux in another segment of the gut lumen (67).
The O-methylation of flavonoids is a natural xenobiotic transformation by the O-methyl transferases, they are high selective enzymatic systems in plants, microbes, and mammalians (68). Methylation of phenolic compounds significantly increases their ability to be transported across biological membranes, making them more stable to metabolic changes also increase biological efficacy, particularly its antitumor activity. In this sense, O-methylated flavonoids exhibited a superior anticancer activity than the corresponding hydroxylated derivatives, being more resistant to the hepatic metabolism and showing a higher intestinal absorption (69). Moreover, methylated flavonoids showed effects on transport proteins that play a central role in the defense of organism against toxic compounds (multidrug resistance proteins) (70). It has been suggested that increasing the degree of methylation and decreasing the number of free hydroxyl groups that are available for conjugation with glucuronic acid and sulfate groups, stability and ability to transport across biological membranes is increased (71).
-Polymerization
Bioavailability and metabolism of monomeric phenols have been extensively studied, but little is known about the bioavailability of polymeric phenolics such as tannins, and the results are still controversial. Tannins bioactivity mostly depends on their grade of polymerisation and solubility. For example, highly polymerized tannins exhibit low bioaccessibility in the small intestine and low fermentability by colonic microflora(72).
Factors Related to Food
-Food Matrix
The biological properties and bioavailability of some phenolicsdepends largely on their release from the food matrix and their subsequent interaction with target tissues. Today, the food matrix is considered as the factor most decisive in the bioavailability and absorption of dietary polyphenols (73).
Most cereal phenolics have covalent interactions with glycosides from the cell wall, forming ester linkages which are not hydrolysed by Phase I and II biotransformation enzymes, this limiting their release into the colon to be metabolized by intestinal microbiota (74, 75).Such interactions depend on the specific porosity and surface properties of the cell wall that can measure between 4 and 10 mm diameter which restricts the penetration of molecules with high molecular weight polyphenols (>10 kDa) (76). These bound phenolics are denominated conjugated.
Free and some conjugated phenolic acids are thought to be readily available for absorption in the human small and large intestines; however, those covalently bound to indigestible polysaccharides can only be absorbed after being released from cell structures by digestive enzymes or microorganisms in intestinal lumen. The bound phenolic acids have very low bioavailability because the bran matrix severely hinders their access to the necessary enzymes (such as ferulate esterases, xylanases) that contribute to their release in the human gastrointestinal tract(55, 77).
Also, during the mastication of plant foods, the cells are disrupted, and polyphenols are released from the cell; this can cause phytochemicals interact with components of dietary fiber as cellulose, hemicellulose, and pectin, which affects bioavailability, increasing or decreasing (78-80).
Furthermore, some phenolic acids such as chlorogenic and caffeicacids, can form interactions with proteins, however, these interactions proved to be slightly disrupted during an in vitro digestion process and does not affect its bioavailability and absorption (81, 82).
-Food Processing
Food processing can either increase or decrease the phenolic content,it depends on the process. For example, milling processes have shown to increase the solvent-extractable phenolic content, because this processes increase the specific surface area of the particle and thus increase the accessibility of phenolic compounds to extraction solvents (77). Thermal processing techniques such as steaming, autoclaving, drying, roasting, and microwave heating are widely used in cereal processing for improving sensorial characteristics, stability and safety of the products, also, some of these techniques have shown the potential to increase the extractability of phenolic compounds in the materials, especially autoclaving (increase ≈ 50%) (83).
On the other hand, some processes tend to decrease the extractable phenolic content. For example, extrusion cooking causes decomposition of heat-labile phenolic compounds (3).
The storage is included as a food process that can or cannot cause-effect, increase or decrease the phenolic content. Broccoli and lettuce were stored at modified atmospheres (argon, helium and nitrogen atmosphere containing 2% oxygen) during9days, the authors observed that the content of total phenolics was reduced in relation to the control sample (stored in air) (84). Oppositely, frozen red raspberries were stored at 4 °C for 3 days, and then at 18 °C for 24 h, after this, the authors observed that anthocyanin levels were unaffected while elligitannins increased (85).
Factors Related to Host
-Dietary Intake
Phenolics dietary intake it is affected by the food availability, every country and seasons offer a different wide range of possibilities; besides, the quantity and frequency of exposure to particular compounds is determinant. Additionally, the diet of each host is diverse, because of this every host responds in a different way to phenolics. There are reports demonstrating that subjects with low circulating levels of antioxidants respond promptly to a rich phenolic diet. However, once plasma levels reach a certain concentration, there is no significant increase (86). This could be a defense mechanism of the human body homeostasis, avoiding accumulation in tissues where antioxidants might be potentially dangerous (87).
-Absorption and Metabolism
Renouf et al. (88) performed a dose–response study with ten healthy subjects, measuring plasma bioavailability of coffee phenolics and their metabolites; they observed significantinterindividual variability in plasma appearance, and this variability was greater for the most abundant metabolites. Multiple factors could explain this variability, from genetic background to gut microbial composition. However, they affirm that to identify the cause(s) of this variability of the metabolic fate of coffee metabolites remains very difficult.
Mechanism of Absorption of Phenolic Acids
Nowadays, the precise mechanism of absorption of phenolic compounds is being studied. In this study, the mechanism in which ferulic acid is absorbed will be explained as a model of all phenolic acids.
A study by Konishi and Shimizu (89), on the transepithelial transport of ferulic acid, using Caco-2 cell monolayers, reported that ferulic acid transport was dependent on pH, in the apical-basolateral direction, and that the permeation rate of ferulic acid was concentration-dependent; they also reported that ferulic acid uses various substrates for monocarboxylic acid transporters (MCTs); and it is suggested that other phenolic acids could be recognized and transported by MCT by intestinal absorption. In contrast, a study published by Poquet, Clifford (90) used co-cultured Caco-2 and mucus-producing HT29-MTX cells to study ferulic acid uptake, they found that ferulic acid was permeated by passive diffusion suggesting a transcellular transport. On the other hand, phenolic acids could have different transport mechanisms since they possess different chemical structures and properties. This is the case of rosmarinic acid, a study by Konishi and Kobayashi (91), reported that rosmarinic acid could be absorbed via paracellular, and that it could be further metabolized and degraded into m-coumaric and hydroxylatedphenylpropionic acids by gut microflora, and then absorbed and distributed by the MCT. In a similar way, the transport mode of ρ-coumaric acid and gallic acid, under a Caco-2 cell monolayer was studied. The authors reported that while the transepithelial transport of ρ-coumaric acid is via the MCT, the permeation of gallic acid appears to be via paracellular(92).
Additionally, once absorbed some phenolic acids may undergo through phase II metabolism. As it is the case of caffeic and dihydrocaffeic acids, both possess the catechol group, this makes it a target for sulfation at the 3-OH group by human liver S9, intestinal S9 and SULT1A1; the latter has been suggested as the most active enzyme in the sulfation of caffeic and dihydrocaffeic acids. Furthermore, glucuronidation can also occur in caffeic acid, UGT1A1 and UGT1A9 are the active enzymes reported in this process. While UGT1A1 catalyses the formation of caffeic acid-4-Oglucuronide, UGT1A9 conjugated the 3-OH and 4-OH groups of caffeic acid (93).
Moreover, in vivo studies
As it can be observed, the absorption of phenolic acids is not well known nor its mechanisms. The information on this subject is yet quite limited, and more studies are needed to understand the exact mechanisms of the most important phenolic acids in regard of their beneficial health effect.
Mechanism of Absorption of Flavonoids
In 2015, Actis-Goretta, Dew (94) reported a study on the absorption of hesperetin-7-O-rutinoside (hesperidin) using in vivo models (humans); they report that hesperidin was hydrolysed by brush border enzymes without involvement of pancreatic, stomach, or other enzymes, additionally no hesperidin metabolites were detected in blood, only amount traces were excreted in urine. In this sense, epicatechin is suggested to be absorbed in the jejunum and that its conjugation is a major determinant of the metabolic fate of epicatechin in the body (67). A study using Caco-2 cell monolayers found that quercetin and naringeninare poorly absorbed by Caco-2 cells; quercetin was absorbed by passive diffusion and a pH-dependent mechanism mediated by the organic anion transporting protein B, and that intestinal permeability was higher for naringenin than for quercetin. It is also mentionedthat, naringenin transport is somewhat ATP-dependent (95). Moreover, genistein absorption was studied by Liu and Hu (96) using Caco-2 cell monolayers and rats; in Caco-2 cells they reported that aglycones of genistein are 5 times more permeable than their corresponding glycosides; besides in rats, aglycones of genistein underwent phase II transformations of glucuronidation and sulfation.
The wide variety of flavonoids, their different molecular size, chemical structure, and chemical properties can vary the way in which they are absorbed, transported, metabolized and excreted. For this reason, it is highly important that the mechanisms of absorption are studied for each phenolic compound of interest, without assuming any general behaviour on their absorption and further metabolism.
Other Factors
-Distribution and Food Content.
Plant polyphenol composition is highly variable both qualitatively and quantitatively; while some phenolics are ubiquitous, as quercetin, others are restricted to specific families or species (i.e. isoflavones in legumes, flavanones in citrus). Some phenolics as anthocyanins are very common,they can be found in all parts of the plant, although they are accumulated mostly in flowers and fruits, but are also present in leaves, stems and storage organs. In general, the level of anthocyanins in fruits is much higher than in vegetables, among fruits the richest in anthocyanins are various berries and black currants (97).
It has been observed that genetic factors, environmental conditions, and growth or maturation stages are determinant influences in phenolic content, causing large variations even within single species (52).
For example, in grapes and apples, anthocyanins are found only in the red varieties (because of the color) and accumulate toward the end of ripening. Pinot noir grapes contain only anthocyanin 3-glucosides, whereas other grape cultivars also contain acylatedanthocyanins(98). Most of the phenolics in apples are hydroxycinnamic acids, flavanols and dihydrochalcones, these compounds are very common in most of the fruits, but dihydrochalcones like phloridzin and phloretin are unique to apples and apple trees (17, 38, 99).
In the case of tannins, hydrolysable tannins are characterized by a restricted taxonomic distribution and are mainly associated with dicotyledonous plants; it has also been observed that most of the plants that can synthesize hydrolysable tannins are unable to synthesize condensed and vice versa (100).
-External Factors
There several factors related to polyphenolics production in plants, among this there are external factors as environmental conditions, UV light exposure, insect and pathogens attack. Plants responses to light UV radiation include increased content of phenolic compounds, such as of hydrolysable tannins, flavonol, and anthocyanin compounds. Flavonols have been implicatedin providing photoprotection against UV irradiation through a screening function. Furthermore, the total phenol content increased proportionately with the dose of UV radiation (101). These increase in phenolic content is bigger especially with UV-C light, exposure of C. nivalis cells to UV-A light (365nm) for 5 days resulted in a 5–12% increase in total phenolics, whereas exposure to UV-C light (254 nm) led to a 12–24% increase in phenolics after 7 days of exposure (102).
Among all phenolic compounds, tannins have been major associated to insect attack. It has been observed that insect damage and wounding can have strong stimulatory effects on tannin production in some plants, suggesting that tannin synthesis contributes to induceddefense by deterrence and/or toxicity (100, 103).
CONCLUSIONS
Epidemiological studies relate a decreased rate of chronic diseases in individuals with higher intake of phenolic-rich foods. Nevertheless, little is known about the biological activities of phenolic compounds that have gone through the digestion process and its relation to the factors affecting its bioaccessibility. In this regard, it has been argued that the phenolic compounds once ingested, are oxidized during digestion and lose their biological properties. On the other hand, epidemiological evidence suggests that consumption of polyphenolrich foods reduces the incidence of chronic diseases.
There have been efforts to study phenolics metabolism; nonetheless, the main limiting factor in this kind of research is the lack of chromatographic standards of each metabolite produced. Obtaining these standards may be difficult considering the wide spectrum of phenolic compounds that exist in nature and the high number of metabolites that are produced during digestion and metabolism.
Finally, it is recommended as highly critical that each factor that affects polyphenols bioavailability must be considered to be properly used in the pharmacological industry. In this sense, it is important to obtain the pharmacokinetic of phenolic compounds, their interaction with other drugs, interaction with other food constituents and their effective doses. Additionally, to create further dietary recommendations, it is relevant to understand phenolic compounds relationship with food matrix and how it affects their bioaccessibility.
REFERENCES
1. Velderrain-Rodríguez G, Palafox-Carlos H, Wall-Medrano A, Ayala-zavala J, Chen CO, Robles-Sánchez M, et al. Phenolic compounds: their journey after intake. Food Funct. 2014;5(2):189-97. [ Links ]
2. Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1):230S-42S. [ Links ]
3. Sharma R. Polyphenols in health and disease: practice and mechanisms of benefits. Polyphenols in human health and disease, Academic, San Diego 2014. p. 757-78. [ Links ]
4. Rein MJ, Renouf M, Cruz Hernandez C, Actis Goretta L, Thakkar SK, da Silva Pinto M. Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. Br J Clin Pharmacol. 2013;75(3):588-602. [ Links ]
5. Naczk M, Shahidi F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J Pharm Biomed Anal. 2006 8/28/;41(5):1523-42. [ Links ]
6. Vieno P, Anna-Maija L, Päivi E, Marjatta S-M, Kirsi-Helena L. CHAPTER 7: Micronutrients and Phytochemicals in Wheat Grain. WHEAT: Chemistry and Technology: AACC International, Inc.; 2009. p.179-222. [ Links ]
7. Bondia-Pons I, Aura A-M, Vuorela S, Kolehmainen M, Mykkänen H, Poutanen K. Rye phenolics in nutrition and health. J Cereal Sci. 2009;49(3):323-36. [ Links ]
8. Vitaglione P, Napolitano A, Fogliano V. Cereal dietary fibre: a natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci Technol. 2008;19(9):451-63. [ Links ]
9. Arranz S, Saura-Calixto F, Shaha S, Kroon PA. High contents of nonextractable polyphenols in fruits suggest that polyphenol contents of plant foods have been underestimated. J Agric Food Chem. 2009;57(16):7298- 303. [ Links ]
10. Wang W, Guo J, Zhang J, Peng J, Liu T, Xin Z. Isolation, identification and antioxidant activity of bound phenolic compounds present in rice bran. Food Chem. 2015;171:40-9. [ Links ]
11. Irakli MN, Samanidou VF, Biliaderis CG, Papadoyannis IN. Development and validation of an HPLC-method for determination of free and bound phenolic acids in cereals after solid-phase extraction. Food Chem. 2012;134(3):1624-32. [ Links ]
12. ilić S, ukalović VH-T, Dodig D, Maksimović V, Maksimović M, Basić Z. Antioxidant activity of small grain cereals caused by phenolics and lipid soluble antioxidants. J Cereal Sci. 2011;54(3):417-24. [ Links ]
13. Hole AS, Grimmer S, Jensen MR, Sahlstrøm S. Synergistic and suppressive effects of dietary phenolic acids and other phytochemicals from cereal extracts on nuclear factor kappa B activity. Food Chem. 2012;133(3):969-77. [ Links ]
14. Escarcega R, Fuentes-Alexandro S, Garcia-Carrasco M, Gatica A, Zamora A. The transcription factor nuclear factor-kappa B and cancer. Clin Oncol. 2007;19(2):154-61. [ Links ]
15. Giacco R, Costabile G, Della Pepa G, Anniballi G, Griffo E, Mangione A, et al. A whole-grain cereal-based diet lowers postprandial plasma insulin and triglyceride levels in individuals with metabolic syndrome. Nut Metab Cardiovasc Dis. 2014;24(8):837-44. [ Links ]
16. Ceymann M, Arrigoni E, Schärer H, Bozzi Nising A, Hurrell RF. Identification of apples rich in health-promoting flavan-3-ols and phenolic acids by measuring the polyphenol profile. J Food Compost Anal. 2012 5//;26(1–2):128-35. [ Links ]
17. Lee KW, Kim YJ, Kim D-O, Lee HJ, Lee CY. Major phenolics in apple and their contribution to the total antioxidant capacity. J Agric Food Chem. 2003; 51 (22): 6516-20. [ Links ]
18. Miene C, Klenow S, Veeriah S, Richling E, Glei M. Impact of apple polyphenols on GSTT2 gene expression, subsequent protection of DNA and modulation of proliferation using LT97 human colon adenoma cells. Mol Nutr Food Res. 2009;53(10):1254-62. [ Links ]
19. Petermann A, Miene C, SchulzRaffelt G, Palige K, Hölzer J, Glei M, et al. GSTT2, a phase II gene induced by apple polyphenols, protects colon epithelial cells against genotoxic damage. Mol Nutr Food Res. 2009;53(10):1245-53. [ Links ]
20. Schulze C, Bangert A, Kottra G, Geillinger KE, Schwanck B, Vollert H, et al. Inhibition of the intestinal sodium coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans. Mol Nutr Food Res. 2014;58(9):1795-808. [ Links ]
21. Palafox-Carlos H, Yahia EM, González-Aguilar GA. Identification and quantification of major phenolic compounds from mango (Mangifera indica, cv. Ataulfo) fruit by HPLC–DAD–MS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012 11/1/;135(1):105-11. [ Links ]
22. Noratto GD, Bertoldi MC, Krenek K, Talcott ST, Stringheta PC, Mertens-Talcott SU. Anticarcinogenic Effects of Polyphenolics from Mango (Mangifera indica) Varieties. J Agric Food Chem. 2010 2010/04/14;58(7):4104- 12. [ Links ]
23. Ajila C, Rao UP. Mango peel dietary fibre: Composition and associated bound phenolics. J Funct Foods. 2013;5(1):444-50. [ Links ]
24. Taing M-W, Pierson J-T, Hoang VL, Shaw PN, Dietzgen RG, Gidley MJ, et al. Mango fruit peel and flesh extracts affect adipogenesis in 3T3-L1 cells. Food Funct. 2012;3(8):828-36. [ Links ]
25. Pierson J-T, Curry MC, Shaw PN, Dietzgen RG, Gidley MJ, Roberts-Thomson SJ, et al. Polyphenolic contents and the effects of methanol extracts from mango varieties on breast cancer cells. Food Sci Biotechnol. 2015;24(1):265-71. [ Links ]
26. García-Solís P, Yahia EM, Morales-Tlalpan V, Díaz-Muñoz M. Screening of antiproliferative effect of aqueous extracts of plant foods consumed in Mexico on the breast cancer cell line MCF-7. Int J Food Sci Nutr. 2009;60(sup6):32-46. [ Links ]
27. García-Solís P, Yahia EM, Aceves C. Study of the effect of Ataulfomango (Mangifera indica L.) intake on mammary carcinogenesis and antioxidant capacity in plasma of N-methyl-N-nitrosourea (MNU)-treated rats. Food Chem. 2008;111(2):309-15. [ Links ]
28. Turner T, Burri BJ. Potential nutritional benefits of current citrus consumption. Agric. 2013;3(1):170-87. [ Links ]
29. Benavente-García O, Castillo J, Marin FR, Ortuño A, Del Río JA. Uses and Properties of Citrus Flavonoids. J Agric Food Chem. 1997 1997/12/01;45(12):4505-15. [ Links ]
30. Sun Y, Qiao L, Shen Y, Jiang P, Chen J, Ye X. Phytochemical Profile and Antioxidant Activity of Physiological Drop of Citrus Fruits. J Food Sci. 2013;78(1):C37-C42. [ Links ]
31. Morand C, Dubray C, Milenkovic D, Lioger D, Martin JF, Scalbert A, et al. Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. Am J Clin Nutr. 2011;93(1):73-80. [ Links ]
32. Reshef N, Hayari Y, Goren C, Boaz M, Madar Z, Knobler H. Antihypertensive Effect of Sweetie Fruit in Patients With Stage I Hypertension*. Am J Hypertens. 2005;18(10):1360-3. [ Links ]
33. Wang Y, Li F, Wang Z, Qiu T, Shen Y, Wang M. Fruit and vegetable consumption and risk of lung cancer: A dose–response meta-analysis of prospective cohort studies. Lung Cancer. 2015;88(2):124-30. [ Links ]
34. Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly) phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. 2013;18(14):1818-92. [ Links ]
35. Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45(4):287-306. [ Links ]
36. Feliciano RP, Pritzel S, Heiss C, Rodriguez-Mateos A. Flavonoid intake and cardiovascular disease risk. Curr Opin Food Sci. 2015;2:92-9. [ Links ]
37. McCann M, Gill C, OBrien G, Rao J, McRoberts W, Hughes P, et al. Anti-cancer properties of phenolics from apple waste on colon carcinogenesis in vitro. Food Chem Toxicol. 2007;45(7):1224-30. [ Links ]
38. Serra AT, Rocha J, Sepodes B, Matias AA, Feliciano RP, de Carvalho A, et al. Evaluation of cardiovascular protective effect of different apple varieties–correlation of response with composition. Food Chem. 2012;135(4):2378-86. [ Links ]
39. zang L, Shimada Y, Kawajiri J, Tanaka T, Nishimura N. Effects of Yuzu (Citrus junos Siebold ex Tanaka) peel on the diet-induced obesity in a zebrafish model. J Funct Foods. 2014;10:499-510. [ Links ]
40. Bok S-H, Lee S-H, Park Y-B, Bae K-H, Son K-H, Jeong T-S, et al. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and acyl CoA: cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J Nutr. 1999;129(6):1182-5. [ Links ]
41. Wu T, Jiang Z, Yin J, Long H, Zheng X. Anti-obesity effects of artificial planting blueberry (Vaccinium ashei) anthocyanin in high-fat diet-treated mice. Int J Food Sci Nutr. 2016:1-8. [ Links ]
42. Babic I, Amiot M, Nguyen-The C. Changes in phenolic content in fresh ready-to-use shredded carrots during storage. Physiol Bas Posth Technol 1992;343:123-8. [ Links ]
43. Kammerer DR, Kammerer J, Valet R, Carle R. Recovery of polyphenols from the by-products of plant food processing and application as valuable food ingredients. Food Res Int. 2014;65:2-12. [ Links ]
44. Alasalvar C, Grigor JM, Zhang D, Quantick PC, Shahidi F. Comparison of volatiles, phenolics, sugars, antioxidant vitamins, and sensory quality of different colored carrot varieties. J Agric Food Chem. 2001;49(3):1410-6. [ Links ]
45. Olejnik A, Kowalska K, Olkowicz M, Rychlik J, Juzwa W, Myszka K, et al. Anti-inflammatory effects of gastrointestinal digested Sambucus nigra L. fruit extract analysed in co-cultured intestinal epithelial cells and lipopolysaccharide- stimulated macrophages. J Funct Foods. 2015;19:649-60. [ Links ]
46. Paran E, Novack V, Engelhard YN, Hazan-Halevy I. The effects of natural antioxidants from tomato extract in treated but uncontrolled hypertensive patients. Cardiovasc Drug Ther. 2009;23(2):145-51. [ Links ]
47. Vallverdú-Queralt A, Regueiro J, Martínez-Huélamo M, Alvarenga JFR, Leal LN, Lamuela-Raventos RM. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014;154:299-307. [ Links ]
48. Llorach R, Martínez-Sánchez A, Tomás-Barberán FA, Gil MI, Ferreres F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008;108(3):1028-38. [ Links ]
49. Bresciani L, Calani L, Cossu M, Mena P, Sayegh M, Ray S, et al. (Poly) phenolic characterization of three food supplements containing 36 different fruits, vegetables and berries. PharmaNutrition. 2015;3(2):11-9. [ Links ]
50. Afanasev I. Reactive oxygen species signaling in cancer: comparison with aging. Aging Dis. 2011;2(3):219. [ Links ]
51. Hefnawy HTM, Ramadan MF. Protective effects of Lactuca sativa ethanolic extract on carbon tetrachloride induced oxidative damage in rats. Asian Pacific Journal of Tropical Disease. 2013;3(4):277-85. [ Links ]
52. Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56(11):317-33. [ Links ]
53. Halliwell B, Gutteridge JM. The definition and measurement of antioxidants in biological systems. Free Radic Biol Med. 1995;18(1):125-6. [ Links ]
54. Carocho M, Ferreira IC. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 2013;51:15-25. [ Links ]
55. Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727-47. [ Links ]
56. Ambriz-Pérez DL, Leyva-López N, Gutierrez-Grijalva EP, Heredia JB, Yildiz F. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016;2(1):1131412. [ Links ]
57. Chen M-L, Shah V, Patnaik R, Adams W, Hussain A, Conner D, et al. Bioavailability and bioequivalence: an FDA regulatory overview. Pharm Res. 2001;18(12): 1645-50. [ Links ]
58. Tagliazucchi D, Verzelloni E, Bertolini D, Conte A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010;120(2):599-606. [ Links ]
59. Stahl W, van den Berg H, Arthur J, Bast A, Dainty J, Faulks RM, et al. Bioavailability and metabolism. Mol Aspects Med. 2002. p. 39-100. [ Links ]
60. Bravo L, Abia R, Saura-Calixto F. Polyphenols as dietary fiber associated compounds. Comparative study on in vivo and in vitro properties. J Agric Food Chem. 1994;42(7):1481-7. [ Links ]
61. Jimenez-Ramsey LM, Rogler JC, Housley TL, Butler LG, Elkin RG. Absorption and distribution of 14C-labeled condensed tannins and related sorghum phenolics in chickens. J Agric Food Chem. 1994;42(4):963-7. [ Links ]
62. Di Carlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65(4):337-53. [ Links ]
63. Stalmach A, Edwards CA, Wightman JD, Crozier A. Colonic catabolism of dietary phenolic and polyphenolic compounds from Concord grape juice. Food Funct. 2013;4(1):52-62. [ Links ]
64. Hollman PCH, Katan MB. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed Pharmacother. 1997;51(8):305-10. [ Links ]
65. McGhie TK, Walton MC. The bioavailability and absorption of anthocyanins: towards a better understanding. Molecular nutrition & food research. 2007;51(6):702-13. [ Links ]
66. Karakaya S. Bioavailability of phenolic compounds. Crit Rev Food Sci Nutr. 2004;44(6):453-64. [ Links ]
67. Actis-Goretta L, Lévèques A, Rein M, Teml A, Schäfer C, Hofmann U, et al. Intestinal absorption, metabolism, and excretion of (–)-epicatechin in healthy humans assessed by using an intestinal perfusion technique. Am J Clin Nutr. 2013;98(4):924-33. [ Links ]
68. Das S, Rosazza JP. Microbial and Enzymatic Transformations of Flavonoid. J Nat Prod. 2006;69(3):499-508. [ Links ]
69. Bernini R, Crisante F, Ginnasi MC. A convenient and safe O-methylation of flavonoids with dimethyl carbonate (DMC). Molecules. 2011;16(2):1418-25. [ Links ]
70. van Zanden JJ, Wortelboer HM, Bijlsma S, Punt A, Usta M, van Bladeren PJ, et al. Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. Biochem Pharmacol. 2005;69(4):699-708. [ Links ]
71. Walle T. Methylation of dietary flavones increases their metabolic stability and chemopreventive effects. Int J Mol Sci. 2009;10(11):5002-19. [ Links ]
72. Serrano J, PuupponenPimiä R, Dauer A, Aura AM, Saura Calixto F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res. 2009;53(S2):S310-S29. [ Links ]
73. Adom KK, Sorrells ME, Liu RH. Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. J Agric Food Chem. 2005;53(6):2297- 306. [ Links ]
74. Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. 2010;23(01):65-134. [ Links ]
75. Pekkinen J, Rosa NN, Savolainen O-I, Keski-Rahkonen P, Mykkänen H, Poutanen K, et al. Disintegration of wheat aleurone structure has an impact on the bioavailability of phenolic compounds and other phytochemicals as evidenced by altered urinary metabolite profile of diet-induced obese mice. Nutr Metab. 2014;11(1):1. [ Links ]
76. Hanlin R, Hrmova M, Harbertson J, Downey M. Review: Condensed tannin and grape cell wall interactions and their impact on tannin extractability into wine. Aust J Grape Wine Res. 2010;16(1):173-88. [ Links ]
77. zhou K, Laux JJ, Yu L. Comparison of Swiss red wheat grain and fractions for their antioxidant properties. J Agric Food Chem. 2004;52(5):1118-23. [ Links ]
78. Alminger M, Aura AM, Bohn T, Dufour C, El S, Gomes A, et al. In Vitro Models for Studying Secondary Plant Metabolite Digestion and Bioaccessibility. Compr Rev Food Sci Food Saf. 2014;13(4):413-36. [ Links ]
79. Padayachee A, Netzel G, Netzel M, Day L, zabaras D, Mikkelsen D, et al. Binding of polyphenols to plant cell wall analogues–Part 2: Phenolic acids. Food Chem. 2012;135(4):2287-92. [ Links ]
80. Padayachee A, Netzel G, Netzel M, Day L, zabaras D, Mikkelsen D, et al. Binding of polyphenols to plant cell wall analogues–Part 1: Anthocyanins. Food Chem. 2012;134(1):155-61. [ Links ]
81. Bennick A. Interaction of plant polyphenols with salivary proteins. Crit Rev Oral Biol Med. 2002;13(2):184- 96. [ Links ]
82. Bordenave N, Hamaker BR, Ferruzzi MG. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014;5(1):18-34. [ Links ]
83. Dona AM. Enhancing antioxidant activity and extractability of bioactive compounds of wheat bran using thermal treatments: University of Manitoba; 2011. [ Links ]
84. Jamie P, Saltveit ME. Postharvest changes in broccoli and lettuce during storage in argon, helium, and nitrogen atmospheres containing 2% oxygen. Postharvest Biol Technol. 2002;26(1):113-6. [ Links ]
85. Mullen W, Stewart AJ, Lean MEJ, Gardner P, Duthie GG, Crozier A. Effect of freezing and storage on the phenolics, ellagitannins, flavonoids, and antioxidant capacity of red raspberries. J Agric Food Chem. 2002;50(18):5197-201. [ Links ]
86. Riso P, Pinder A, Santangelo A, Porrini M. Does tomato consumption effectively increase the resistance of lymphocyte DNA to oxidative damage? Am J Clin Nutr. 1999;69(4):712-8. [ Links ]
87. Porrini M, Riso P. Factors influencing the bioavailability of antioxidants in foods: A critical appraisal. Nut Metab Cardiovasc Dis. 2008;18(10):647-50. [ Links ]
88. Renouf M, Marmet C, Giuffrida F, Lepage M, Barron D, Beaumont M, et al. Dose–response plasma appearance of coffee chlorogenic and phenolic acids in adults. Mol Nut Food Res. 2014;58(2):301-9. [ Links ]
89. Konishi Y, Shimizu M. Transepithelial transport of ferulic acid by monocarboxylic acid transporter in Caco-2 cell monolayers. Biosci Biotechnol Biochem. 2003;67(4):856-62. [ Links ]
90. Poquet L, Clifford MN, Williamson G. Transport and metabolism of ferulic acid through the colonic epithelium. Drug Metabol Dispos. 2008;36(1):190-7. [ Links ]
91. Konishi Y, Kobayashi S. Transepithelial transport of rosmarinic acid in intestinal Caco-2 cell monolayers. Biosc Biotechnol Biochem. 2005;69(3):583-91. [ Links ]
92. Konishi Y, Kobayashi S, Shimizu M. Transepithelial transport of p-coumaric acid and gallic acid in Caco-2 cell monolayers. Biosc Biotechnol Biochem. 2003; 67(11):2317-24. [ Links ]
93. Wong CC, Meinl W, Glatt H-R, Barron D, Stalmach A, Steiling H, et al. In vitro and in vivo conjugation of dietary hydroxycinnamic acids by UDP-glucuronosyltransferases and sulfotransferases in humans. J Nutr Biochem. 2010;21(11):1060-8. [ Links ]
94. Actis Goretta L, Dew TP, Lévèques A, Pereira Caro G, Rein M, Teml A, et al. Gastrointestinal absorption and metabolism of hesperetin 7 O rutinoside and hesperetin 7 O glucoside in healthy humans. Mol Nutr Food Res. 2015;59(9):1651-62. [ Links ]
95. Chabane MN, Ahmad AA, Peluso J, Muller CD, Ubeaud Séquier G. Quercetin and naringenin transport across human intestinal Caco 2 cells. J Pharm Pharmacol. 2009;61(11):1473-83. [ Links ]
96. Liu Y, Hu M. Absorption and metabolism of flavonoids in the caco-2 cell culture model and a perused rat intestinal model. Drug Metab Dispos. 2002;30(4):370-7. [ Links ]
97. Andrés-Lacueva C, Medina-Remon A, Llorach R, Urpi-Sarda M, Khan N, Chiva-Blanch G, et al. Phenolic compounds: Chemistry and occurrence in fruits and vegetables. Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value and Stability: Wiley-Blackwell: Iowa; 2010. p. 53-80. [ Links ]
98. Cheynier V. Polyphenols in foods are more complex than often thought. Am J Clin Nutr. 2005;81(1):223S-9S. [ Links ]
99. Kalinowska M, Bielawska A, Lewandowska-Siwkiewicz H, Priebe W, Lewandowski W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol Biochem. 2014;84: 169-88. [ Links ]
100. Haslam E. Vegetable tannins–Lessons of a phytochemical lifetime. Phytochem. 2007;68(22):2713-21. [ Links ]
101. Younis ME-B, Hasaneen MNA-G, Abdel-Aziz HMM. An enhancing effect of visible light and UV radiation on phenolic compounds and various antioxidants in broad bean seedlings. Plant Signal Behav. 2010;5(10):1197-203. [ Links ]
102. Duval B, Shetty K, Thomas WH. Phenolic compounds and antioxidant properties in the snow alga Chlamydomonas nivalis after exposure to UV light. J Appl Phycol. 1999;11(6):559-66. [ Links ]
103. Barbehenn RV, Constabel CP. Tannins in plant–herbivore interactions. Phytochem. 2011;72(13):1551-65. [ Links ]