Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Agronomía
versión impresa ISSN 0378-7818
Rev. Fac. Agron. v.21 n.2 Caracas abr. 2004
A new method for the isolation of betalaines by HPTLC.
Un nuevo método para el aislamiento de betalainas por HPTLC.
M. J. Moreno-Alvarez1, A. Viloria-Matos1, D. M. Hidalgo-Báez2.
1Universidad Simón Rodríguez. Valencia. Republica Bolivariana de Venezuela. Email: morenoalvarez@cantv.net
2Universidad de Los Andes. Mérida. Republica Bolivariana de Venezuela.
Abstract
Beta vulgaris L. roots were processed for to research the betalaines composition. Isolation and purification were carried out by means of high performance thin layer chromatography (HPTLC). They were eluted independently in one dimension with two solvent systems. Results showed red fraction and yellow fraction with maximum absorbance to 537 nm (betacyanin) and 465 nm (betaxantin) respectively. The HPTLC resolution was fine and route time was smaller fine that TLC, lowering oxidation hazard.
Key words: Beta vulgaris, betacyanin, betaxantin, HPTLC, purification.
Resumen
Raíces de Beta vulgaris L., fueron procesadas para investigar la composición de betalainas. La separación y purificación se llevo a cabo mediante cromatografía de capa fina de alta resolución (HPTLC). Fueron eluidos en dos sistemas de solventes en una sola dimensión independientemente. El estudio químico revela una fracción roja y otra amarilla, determinándose absorbancia máxima a 537 nm (betacianina) y 465 nm (betaxantina), respectivamente. La técnica cromatografica empleada fue de buena resolución y de menor tiempo de recorrido que las de TLC, evitando el riesgo de oxidación.
Palabras clave: Beta vulgaris, betacianinas, betaxantina, HPTLC, purificación.
Recibido el 2-9-2002 l Aceptado el 21-11-2003.
Introduction
Betalaines are derived from betalamic acid. The biological importance of these compounds includes theirs antiviral and antibacterial activities and they are also taxonomic and phylogenetic markers. They play an important role in the attraction of dispensers of seed and pollinators. They have a high potential as natural pigments for food applications. Betalaines have been used as a substitute of synthetic coloring in processing of gelatin, strawberry yogurt, ice cream, fruits cocktails, candies and biscuits. The European Economic Community has accepted (Cod. E162), dehydration and pulverization red beet from Beta vulgaris L. (3, 4, 5).
Several methods for isolation and purification of betalaines have been reported (1, 7, 8, 9) including ion exchange chromatography, electrophoresis, HPLC and TLC (2, 3, 6).
The Bilyk,s method (2) using cellulose preparative TLC is the most used technique. It includes three one-dimensional developments in two different solvents systems (Mixture I: isopropanol 55: ethanol 20: water 20: acetic acid 5, Mixture II: isopropanol 30: ethanol 35: water 30: acetic acid 5). The chambers to dry the plates are conditioned with nitrogen liquid. The total eluting time is higher for this method, the author did not report this data but our experiments using the conventional TLC method showed a total eluting time of 5h. These characteristics are disadvantages due to increase the risk of oxidation and the cost.
The present work reports a rapid, simple and high resolution method for the isolation of betalaines which is an important compounds for food applications.
Materials and methods
Samples
The roots of Beta vulgaris L. cultivate in the Andes region (145.0 ± 0.1 g) were acquired from a local commercial expense of the municipio Bejuma, Canoabo, Carabobo state, Venezuela. The harvest season was November 2000.
The samples shown ripeness for consumption, homogeneous color and absence of apparent physical damage. Containers conditioned with dry ice were used for samples transportation (temperature 7.0 ± 1.0 °C). The roots
were washed with water and dried in absorbent paper. After the skin was removed, 120.0 ± 0.1 g of roots were obtained and the samples were cut with stainless steel knife and extracted. The pH was evaluated.
Instrumentation
The extract was obtained using a juice extractor, Eastern electric® model JX5000. An Orion pHmeter was used for pH measurements. The HPTLC-Fertigschichten plates of cellulose, 10 x 10 cm, Merck, No Cat. 5632 were used for isolation of the compounds. An Emerson, Model 5A55JXGTD-4144 electric pump was connecting to a vacuum stove witch was used to dry the plates. The filtration process was done using a porcelain funnel Pyrex® USA, No 36060, 15 ml, ASTM 10-15 M.
Isolation and purification
The extract was applied with capillaries as continuous lines until saturation on cellulose HPTLC plates, which were previously activated during 30 min at the temperature of 90.0 ± 1.0 °C.
Two one-dimensional develop-ments were performed using two different solvents systems (table 1). The first development was in mixture II and the second one in the mixture I (2). The eluting time in the mixture II was 1.20 h. After this, the plates were dried into a conditioned vacuum stove connected an electric pump,
inside it 400.0 ± 0.1 g of dry ice were placed. The conditions of the drying cooling were: temperature of 5.0 ± 1.0 °C, pressure of 5 inch Hg and the time 25 min. The same procedure was done using the mixture I, the eluting time with this solvents system was 1.0 h.
The development and drying process were done in dark conditions. The Rf values (table 2) for each detecting compound were determined. The mayor compounds were removed using a spatula and placed into a porcelain funnel. Distillated water was used to wash (10 mL) it. The filtrated was directly collected in a spectronic cell and its visible spectrum was taken. The pH of the collecting compounds was 6.1. Removing and washing process were done in dark. Four plates were needed to get the visible spectrum. However, ten plates were use in order to study the reproducibility of this method.
Table 1. Composition and proportion of the two solvents systems used in HPTLC.
| Solvents | System I | System II |
| Isopropanol | 55 | 30 |
| Ethanol | 20 | 35 |
| Water | 20 | 30 |
| Acetic acid | 5 | 5 |
Table 2. Rf values and color of the major compounds detected by HPTLC.
| Compounds | Rf | Color |
| 1 | 0.48 | Yellow |
| 2 | 0.34 | Yellow1 |
| 3 | 0.22 | Red1 |
1major compounds detected
In order to compare the method using HPTLC with the conventional TLC technique, all experiments were done using these two types of plates under the same conditions.
Results and discussion
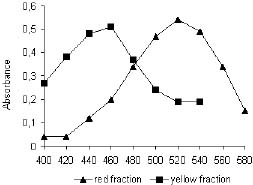
The Rf values and the colors for the major compounds are shown in table 2. Visual detection allowed the observation of three delimited fractions (figure1), the fraction with the high Rf value was not identified. Rf values were 0.22 and 0.34 for the red compound and the yellow, respectively. Both compounds were identified by theirs visible spectra (figure 2). The red compound showed a maximum absorbance at 537 nm and the yellow at 465 nm. These absorbances have been reported to betaxantin and betacyanin, respectively (1, 2, 3, 8).
Several advantages were observed in the HPTLC method described in this work. The reduction of the total eluting time from 5 h (TLC technique) to 2.20 h and the use of two developments diminished the risk of betalaines oxidation. The replacement of nitrogen liquid (2) by dry ice decreased the cost and it made the HPTLC method easy to handle. Because of it a special chamber to dry the plates was not needed.
Figure 1. HPTLC chromatoplate of the aqueous extract of B. vulgaris L.
Figure 2. Visible spectra of the betalaines of B. vulgaris L.
Conclusions
The HPTC method for the isolation and purification of betalaines showed high reproducibility, simplicity and celerity. The oxidation of betalaines was reduced because the eluting times are short. The uses of dry ice for transportation and drying process make this method more versatile with a low cost.
Acknowledgements
The authors thank to Dr. José Luis Burguera, of the Universidad de Los Andes and Dra. Carmen Saénz, Universidad de Chile, for theirs suggestions and critical revision of the manuscript. We are grateful to Lic. Nancy Cordero, Mrs. Marina Oliveros, Mrs. María de Lourdes Figueroa and Mrs. Yannet Silva, staff
of the Universidad Simón Rodríguez for their efficiency in the buying of reactives using in this investigation. Also we would like to thank to the staff of the Virtual Room of the Núcleo de Canoabo: TSU. Rafael Cabrera, TM. Francisco Fuentes and Br. Julio Ramirez for advising the searching of information.
Literature cited
1. Bilyk, A. 1979. Extractive Fractionation of Betalaines. J. Food Sci. 44:1249-1251. [ Links ]
2. Bilyk, A. 1981. Thin-Layer Chromatographic Separation of Beet Pigments. J. Food Sci. 46:298-299. [ Links ]
3. Delgado-Vargas, F., R. Jiménez, and O. Paredes-López. 2000. Natural Pigments: Carotenoids, Anthocyanins, and Betalains - Characteristics, Biosynthesis, Processing, and Stability. Crit. Rev. Food Sci. Nutr. 40:173-289. [ Links ]
4. Domínguez-López, A. 1995. Revisión: Empleo de los frutos y de los cladodios de la chumbera (Opuntia spp) en la alimentación humana. Food Sci. Tech. Int. 1:65-74. [ Links ]
5. García Barrera, F.A., C.R. Reynoso y E. González de Mejia. 1998. Estabilidad de las betalaínas extraídas del garambullo (Myrtillocactus geometrizans).Food Sci. Tech. Int. 4:115-120. [ Links ]
6. Huang, A.S. and J.H. A. Von Elbe. 1985. Kinetics of the degradation and regeneration of betanine. J. Food Sci. 50:1115-1120. [ Links ]
7. Sapers, G. M. and J.S. Horstein. 1979.Varietal Differences in Colorant properties and stability of red beet pigments. J. Food Sci. 44:1245-1248. [ Links ]
8. Viloria-Matos, A., M.J. Moreno-Alvarez and D. Hidalgo. 2001. Isolation and identification of Betacyanin from fruits of Opuntia boldinghii Br. et R. by HPTLC. Cienc. Tecnol. Aliment. 3(3):140-143. [ Links ]
9. Wiley, R. and Y. Lee. 1978. Recovery of Betalaines from red beets by a diffusion-extraction procedure. J. Food Sci. 43:1056-1058. [ Links ]