Investigación Clínica
versión impresa ISSN 0535-5133
Invest. clín v.45 n.3 Maracaibo set. 2004
Central nervous system paracoccidioidomycosis: case report and review.
Antonio G. Tristano, María Eugenia Chollet, María Willson, Julián Perez and Marcos Troccoli.
Department of Internal Medicine, Dr. Domingo Luciani Hospital, Caracas, Venezuela.
Abstract. Paracoccidioidomycosis is a systemic infection caused by a dimorphic fungus (Paracoccidioides brasiliensis). The most common lesions frequently occur in the bucopharinx mucosa. Other lesions occur in the adrenal glands, liver, bone, gastrointestinal tract, lungs and nervous system. We report here a case of neuroparacoccidioidomycosis. The patient was a 49 year-old male, who consulted due to neurological symptoms (cephalalgia, speech difficulty and one tonic clonic seizure with urinary incontinence) of eight months duration. Upon physical examination it was observed an emaciated male with nail clubbing, a skin ulcer with raised edges and a crusted bottom of 4 × 2 cm in diameter located in the right supraclavicular region and an ulcerated lesion in the left tonsil with edema. The rest of the physical examination reveled a discrete left side hemiparesis and pulmonary rales in the left hemitorax. The fungus was identified through direct examination of cerebrospinal fluid (CSF). The histopathology of suprarenal, lungs, brain and skin showed multiple paracoccidioidal granulomas. To the best of our knowledge, this is the third case reported in the literature. We review the literature on the pathogenesis and prevalence of neuroparacoccidioidomycosis.
Key words: Neuroparacoccidioidomycosis, paracoccidioidomycosis, central nervous system.
Paracoccidioidomicosis del sistema nervioso central: reporte de un caso y revisión de la literatura.
Resumen. La Paracoccidioidomicosis es una infección sistémica causada por un hongo dimorfo (Paracoccidioides brasiliensis). Las lesiones más comunes frecuentemente ocurren en la mucosa bucofaríngea. Otras lesiones ocurren en la glándula adrenal, hígado, hueso, tracto gastrointestinal, pulmones y sistema nervioso. Se presenta un paciente masculino de 49 años de edad, quién consultó con historia de ocho meses de duración caracterizada por síntomas neurológicos (cefalea, dificultad para hablar, un episodio de convulsión tónico-clónica generalizada con relajación del esfínter vesical). Al examen físico se encontró un paciente emaciado con dedos en palillo de tambor y una úlcera de bordes elevados y fondo costroso de 4 × 2 cm de diámetro en la región supraclavicular derecha. Además, se apreció una lesión ulcerada en la amigdala izquierda con edema. El resto del examen físico reveló una hemiparesia izquierda y crepitantes en el hemitorax izquierdo. Nosotros reportamos un caso de neuroparacoccidiodomicosis donde el hongo fue identificado a través del examen directo del líquido cefalorraquídeo. Este es el tercer caso reportado en la literatura. La histopatología de las suprarrenales, pulmones, cerebro y piel mostró múltiples granulomas paracoccidioidales. Hacemos una revisión de la literatura sobre la patogénesis y prevalencia de neuroparacoccidiodomicosis.
Palabras clave: Neuroparacoccidioidomicosis, paracoccidioidomicosis, sistema nervioso central.
Received: 02-07-2003. Accepted: 20-05-2004
INTRODUCTION
Paracoccidioidomycosis (South American Blastomycosis) is a systemic infection caused by a dimorphic fungus (Paracoccidioides brasiliensis) (1). It is common in the rural areas of Latin America (1, 2). The majority of the reported cases come from Brazil, Colombia and Venezuela (3).
Patients get infected by inhaling mycels found in the natural environment or rarely from traumatic inoculation via mucous membranes (1). The most common lesions frequently occur in the bucopharinx mucosa. Others lesions occur in the adrenal glands, liver, bones, gastrointestinal tract, lungs and nervous system (2-7).
The infection of the nervous system is always secondary, it was initially described by Pereira and Jacobs in 1919. Its frequency fluctuates between 9.9% and 27.7%. The two clinical presentations are meningeal and pseudotumoral, the latter taking the form of abcesses, granulomas, nodules or cysts (5-8). The P. brasilienses affect more frequently cerebral hemispheres (solitary or multiple granuloma) and could involve the cerebelum, pons, bulb and meninges, rarely the spinal cord (1).
The diagnosis of neuroparacoccidioidomycosis is difficult to establish. Neuroimaging studies such as Computarized Tomography (CT) and Magnetic Resonance Imaging (MRI) are helpful, but the definitive diagnosis is obtained only when the fungus is observed microscopically or isolated from biopsies or from cerebrospinal fluid (CSF) (9). CSF is usually normal or with slight pleocytosis, proteins may be normal or raised, reaching values of up to 200 mg/dl, glucose values are normal or reduced. Only four reported cases had abnormal CSF. Direct examination rarely shows the fungi (6, 8).
Amphotericin B either intravenously or intrathecally has been regarded as the best drug for the treatment of neuroparaccocidioidomycosis (10, 11). The prognosis is not good; even with adequate treatment, the mortality reaches up to 20% in disseminated forms. Certain sulfonamide- trimathroprim combinations, such as cotrimoxazol and cotrimazine, have also been used due to the high drug levels attained in the CFS (12). We present here the record of a Venezuelan patient with neuroparacoccidioidomycosis where the fungus was observed microscopically from cerebrospinal fluid.
CASE REPORT
The patient was a 49 year-old male patient, truck driver, admitted to the Dr. Domingo Luciani Hospital, Neurosurgery Service because of cephalalgia, weight loss of aproximately 50-60 pounds, dyspnea with cyanosis, speech difficulty and one tonic clonic seizure with urinary incontinence; all of which had a duration of eight months. He had a history of heavy drinking and smoking habits for more than thirty years. He had had cutaneous leishmaniasis in his left leg treated 25 years earlier.
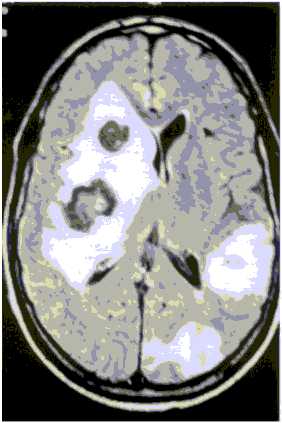
A computer tomographic scanning (C-T scan) revealed two hypodense lesions, one of them in the right parietal cerebral hemisphere with mass effect and perifocal edema and the other in the left parietal hemisphere respectively. The magnetic resonance imaging (MRI) performed showed multiple lesions predominantly in the right side with perifocal edema and mass effect that were enhanced in a ring like pattern with contrast (Fig. 1). A presumptive diagnosis of cerebral metastasis of unknown origin was made and dexamethasone (24 mg/day) and diphenilhidantoin (300 mg/day) was administered.
Fig. 1. Magnetic resonance finding: round lesion in both cerebral hemispheres with mass effect and edema.
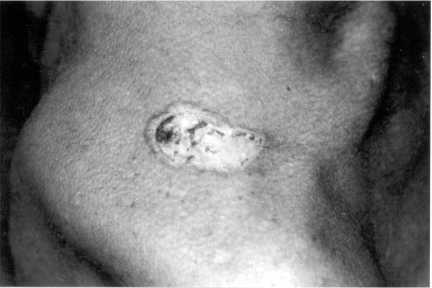
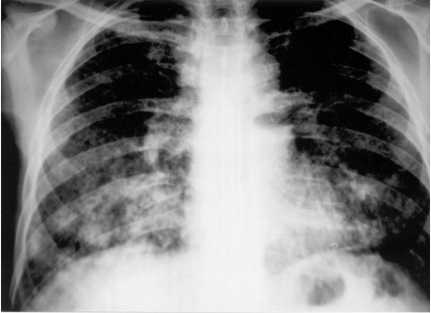
The patient was transfered to the Internal Medicine Service and physical examination revealed an emaciated male with nail clubbing, a skin ulcer with raised edges and a crusted bottom, of 4.0 × 2.0 cm in diameter located in the right supraclavicular region (Fig. 2) and an ulcerated lesion in the left tonsil with edema. The rest of the physical examination revealed a discrete left side hemiparesis and pulmonary rales in the left hemitorax. Laboratory studies including hemogram, ESR, urea, creatinine, glucose, liver tests, electrolites were normal and HIV was negative. A chest X-Ray film showed bilateral symetric reticular-nodular infiltrates (densities). Fourteen days later the radiodensities were enlarged with consolidation in the basal right lung (Fig. 3). Direct examination of a bronchioalveolar lavage and a sputum sample revealed multiple budding yeasts. The culture of tonsil biopsy disclosed P. brasiliensis as did the skin ulcer (Fig. 4).
Fig. 2. Ulcerative lesion with high borders and crusted center
Fig. 3. Chest X-Ray: diffuse reticulonodular interstitial pattern predominantly in both pulmonary bases.
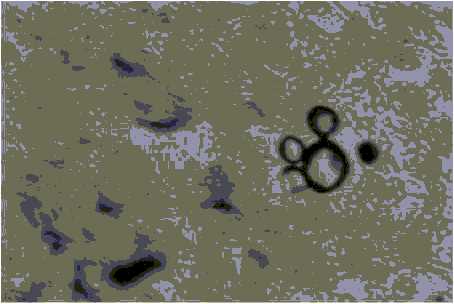
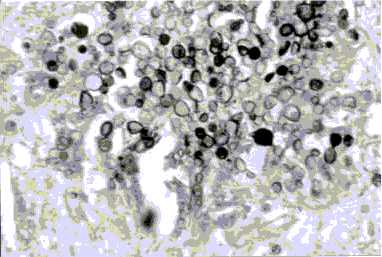
Fig. 4. Histology section of skin showing characteristic multibudding forms of P. brasiliensis (Grocott; X 40).
The cerebrospinal fluid (CSF) showed a glucose 146 mg/dL, protein 35 mg/dL, LDH 23 IU/L. The fungus was seen in direct examination. The serum complement fixation test with the paracoccidioidin antigen was positive. Based on the sputum results, treatment with amphotericin B was begun, reaching a total accumulated dose of 2.5 g. He also received amphotericin intrathecally 0.5 mg 3 days per week. After that treatment he received itraconazole 100 mg/day as well as parenteral nutrition. The patient had an irregular evolution and suddenly presented a dense left hemiparesis, loss of consciousness and severe dispnea requiring mechanical ventilation. He suffered a nosocomial pneumonia caused by Pseudomonas aeruginosa as a complication and finally died.
Autopsy revealed an intraparenchymatous necrotic nodule in the left cerebral hemisphere of 8.0 × 5.0 cm, cerebral edema, bilateral pneumonia with multiple nodules suprarrenal glans abcesses and traqueal adenopathy. The histopathology of suprarrenal, lungs and brain showed multiple paracoccidioidal granulomas in CNS (Fig. 5).
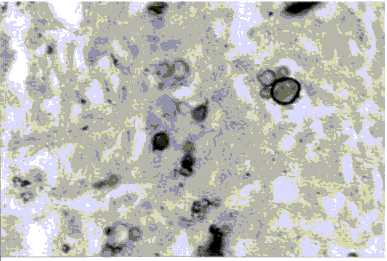
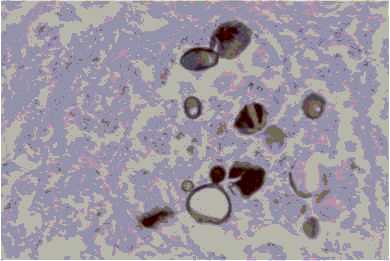
Fig. 5. Multibudding forms of P. brasiliensis in A: brain; B: suprarenal; C: lung (Grocott; X 40).
DISCUSSION
Involvement of the nervous system by paracoccidioidomycosis has been previously reported as rare. The infrequency of the diagnosis in this specific area is generally owing to its asymptomatic presentation, cursory neurological examination, lack of specialized tests such as cerebral CT-Scan or MRI and low frequency of CNS studies in autopsies (1, 13).
With the new diagnostic methods in the last decades, the frequency of cases reported in the literature have increased. Until 1962 there were reported 28 cases of neuroparaccocidioidomycosis. In our English and Latin America search through Medline and Lilacs data bases we found 58 reported neuroparaccocidioidomycosis cases (Table I). Thirty one cases of paracoccidioidomycosis have been reported in the Domingo Luciani Hospital from 1987 to April 2002 (Table II), one in 1987, two in 1988, four in 1989, five in 1990, four in 1991, eight 1992, three in 1993, three in 1994, and one in 1995. Nine cases involved the lungs, four oropharyngeal mucosa, three larinx, one lymph node and two were cutaneus. Nine patients had more than one organ involved simultaneously, two of whom had gastrointestinal system involvement. Only in three patients was the CNS affected, two of whom had pulmonary involvement and only one reported the fungus in the CFS direct exam similar to the clinical case presented.
TABLE I
NEUROPARACOCCIDIOIDOMYCOSIS: CASES REPORTED
| Authors | Gender | Age | CNS | Other | XR | CSF | TT | Results |
| Farage et al | M | 42 | C7 | lung | ampho. B, TMP-SMX, surgery | No change | ||
| Moura et al | M | 57 | T3-T11 | libs, lung | fluconazole | good | ||
| Pacheco et al | F | 45 | T11 | libs, lung | TMP-SMX, surgery | no change | ||
| Plá et al | F | 48 | left frontal parietal lobe right cerebelar hemisphere | lung | abnormal | N | ampho. B, TMP-SMX | death |
| M | 62 | vermix | lung | abnormal | N | dexametasone, cloranphenicol, penicillin G, acetazolamide | death | |
| M | 55 | mesencephalo thalamus, hypo. pons pedun. left cerebelllum | lung | abnormal | DE | ampho. B., TMP-SMX | death | |
| ? | ? | occipital lobe | N | N | sulfonamide | cure | ||
| ? | ? | left frontoparietal | N | N | sulfonamide | |||
| ? | ? | right frontal lobe | N | N | sulfonamide | |||
| ? | ? | right parietal lobe | N | N | sulfonamide | |||
| ? | ? | temporal lobe | N | N | sulfonamide | cure | ||
| M | 48 | left occipito parietal right cerebellar hemisphere | abnormal | pleocytosis | ampho. B, TMP-SMX | death | ||
| M | 62 | vermix | abnormal | ampho. B, surgery | death | |||
| M | 55 | mesencephalo dyencephalo | abnormal | N | ampho. B, TMP-SMX | death | ||
| M | 51 | spinal | N | ampho. B, surgery | death |
| Authors | Gender | Age | CNS | Other | XR | CSF | TT | Results |
| Plá et al | M | 50 | C4-T1, right frontal lobe | abnormal | pleocytosis ↓ glucose ↑ protein | sulfonamide | worse | |
| Marchiori et al | M | 51 | C4-C5 | lung, skin | abnormal | N | ampho. B | death |
| Braga et al | M | 45 | T11-T12 | lung, lymph node |
| ampho. B | improve | |
| Morato et al | M | 50 | C4-C6 | lung, tongue libs | abnormal | N | ampho. B, sulfonamide | improve |
| Pereira et al | M | 52 | left fronto parietal | skin, spleen node, lung |
| N |
| death |
| M | 45 | leptomeningeal cortex, mesencephalo, thalamus | larynx, trachea, esophagus |
| death | |||
| M | 56 | left fronto parietal | sulfadiazina | death | ||||
| M | 44 | cerebellar right | lung, right adrenal | abnormal | ampho. B | death | ||
| M | 42 | left parietal | pharynx, lung, tonsils node |
| ampho. B | death | ||
| M | 49 | left cerebellar |
|
| DE positive | sulfadiazine, ampho. B | cure | |
| F | 57 | temporal | lung | abnormal | ampho. B | cure | ||
| M | 50 | temporoparietal left | lung | abnormal | ampho.B | cure | ||
| M | 39 | vermix | lung | abnormal | TMP-SMX, surgery | cure | ||
| M | 39 | left fronto parietal and cerebellar | lung |
|
| death | ||
| Pedro et al | M | 34 | T5-T6 | larynx, lung | abnormal | ampho. B | no change | |
| M | 47 | right cerebral hemisphere |
|
| ampho. B, sulfadiazine | improve | ||
| Pereira et al | ? | meningeal | skin, tonsils, mouth, tongue larynx, lung node, adrenal | abnormal |
| death | ||
| ? | meningeal spinal |
| normal |
| death | |||
| ? | meningeal encephalo | trachea, esopha. larynx | normal |
| death |
| Authors | Gender | Age | CNS | Other | XR | CSF | TT | Results |
| Pereira et al | ? | left parietal | skin, lung spleen node | abnormal | death | |||
| ? | bulb | pharynx, larynx lung node adrenal | abnormal | death | ||||
| ? | pons | lung node | abnormal | death | ||||
| ? | right cerebellum | lung adrenal | abnormal | death | ||||
| ? | right parietal | mouth, tonsils lung, pharynx, node | abnormal | death | ||||
| ? | right frontotemporal left parietoccip. thalamus | larynx, esophagus trachea | normal | death | ||||
| ? | hemispheres cerebral | skin, lung, adrenal | abnormal | death | ||||
| ? | left fronto parietal |
| normal | death | ||||
| ? | left fronto parietal, cerebellum | lung | abnormal | death | ||||
| M | 46 | cerebral hemispheres | legs ulcers lung, node | abnormal | ampho. B | cure | ||
| Guerreiro et al | M | 59 | right fronto parietal | lung | abnormal | TMP-SMX | cure | |
| Colli et al | M | 55 | T3-T4 | lung |
| sulfadiazine, surgery | cure | |
| Valle et al. | M | 57 | C4-C6 | lung |
|
| death | |
| M | 37 | T2 | lung | abnormal | fluconazole, TMP-SMX, surgery | cure | ||
| Duarte et al | M | 37 | encephalo | lung, mouth, | abnormal | ampho. B, TMP-SMX, itraconazol | death | |
| Villa et al | M | 55 | right thalamus, mesencephalon | lung, skin | abnormal | itraconazol | cure | |
| Silva et al | F | 34 | cerebellum |
| abnormal | TMP-SMX, surgery | cure | |
| Lambertucci et al | M | 46 | occipital | lung | abnormal | TMP-SMS, surgery | cure |
CNS= central nervous system. CSF=spinal fluid. DE= direct examination. N= no data. TMP-SMX=trimethoprin sulfamethoxazole. TT= treatment. XR=Chest film.
TABLE II
PARACOCCIDIOIDOMYCOSIS:
CASES REPORTED AT DOMINGO LUCIANI HOSPITAL (1987-2002)
| Location | Cases (n) |
| Lungs | 9 |
| Oropharyngeal | 4 |
| Larinx | 3 |
| Lymph node | 1 |
| Cutaneous | 2 |
| Gastrointestinal | 2 |
| CNS | 3 |
| Multiple | 9 |
CNS: central nervous system.
The most characteristic clinical picture includes symptoms of endocraneal hypertension, seizures, hemiparesis and changes in consciousness or personality. Ocasionally there is spinal cord involvement simulating tumorous lesions. The differential diagnosis include cerebral abcesses, gliomas, metastasis and neurocysticercosis (13, 14). CT scan and MRI lesions are presented as single or multiple rounded lesions with low attenuation values in the center and contrast enhancement in a ring like pattern. There is little perifocal edema and mass effect, unless lesions are localized in the posterior fossa. No bone destruction or neoformation are seen (15-18).
Cerebrospinal fluid is usually normal or with slight pleocytosis, proteins maybe normal or raised, reaching values of up to 200mg/dL, glucose values are normal or reduced. Only four reported cases had abnormal CSF. Direct examination rarely shows the fungi. In our search we reported 3 patients; (one in 1965, another in 1994) and the one we are describing in this paper (6, 8).
Histopathologically we may see lesions with a central zone of necrosis tisular with the fungus surrounded by a cellular infiltrate that includes epithelioid cells, giant cells, lymphocytes and plasma cells (13).
Pulmonary involvement is frequent, thorax roentgenogram findings vary from bilateral symmetrical infiltrates in the middle and lower lung fields to unilateral apical densities or a solitary mass (4, 7, 19, 20). The diagnosis is established by direct examination of sputum, bronchoalveolar lavage with microscopic visualization of the fungi or by biopsy with special tinctures (8).
Twenty four patients of the published cases had pulmonary involvement; twenty of them had more than one affected organ (suprarenal glands, skins, oropharinx, liver, bones, gastrointestinal system and lymphatic nodes). Therefore involvement of two or more sites is not infrequent. So it is important not to overlook any clue to the diagnosis specially abnormal thorax x-rays, accesible lesions or a previous history of paraccocidioidomycosis.
Treatment of neuroparacoccidioidomycosis is based predominantly on the use of amphotericin intravenously and intrathecally with optional combination of sulfonamides (8, 21, 22). Ketoconazol has been used in some cases with discouraging results. Others use it for infections in patients in good clinical condition because it is less expensive than other options. Treatment for 6 to 12 months substantially improves or cures chronic pulmonary and chronic disseminated paraccocidioidomycosis in 88 to 95% of patients (16, 22, 23).
Fluconazole 200-400 mg/day- for at least 2 months (median duration of 5 months) was an effective treatment for paracoccidioidomycosis in 27 of 29 patients in one study (12, 16, 17). Even though it is the only azole to cross the blood brain barrier it has been used infrequently in neuroparaccocidiodomycosis, with a report of administration in an intramedullary lesion with partial result (23). Available data supports itraconazole as the drug of choice for paraccocidioidomycosis. Almost all patients treated with itraconazole 50-100 nmg/day- for aproximately 6 months demonstrated considerable improvement or cure. Most patients in this study had chronic disseminated disease (23). Villa y col. reported a Colombian patient with neuroparacoccidioidomycosis whose symptoms and neurological signs improved or resolved during therapy with itraconazole (24). All patients who are severely ill (inmunocompromised) and those with CNS involvement should be treated with amphotericin B and sulfadiazine (21). Amphotericin B, either intravenously or intrathecally, has been regarded as the best drug for the treatment of neuroparaccocidioidomycosis (10, 11). The prognosis is not good; even with adequate treatment the mortality reaches up to 20% in disseminated forms. In our case the delay in the diagnosis and treatment and the nutritional status of the patient probably contributed to his death.
REFERENCES
1. Moura LP, Raffin NC, Del Negro GM, Ferreira SM. Paracoccidioidomicose evidenciado comprometimento medular tratada com sucesso por fluconazol. Arq Neuropsiquiatr 1994; 52: 82-86. [ Links ]
2. Pacheco AB, Arruda OW, Hunhevicz CN, Tsubouchi HM, Torres BL. Thoracic intraspinal paracoccidioidomycosis. Arq Neuropsiquiatr 1996; 54: 474-478. [ Links ]
3. Saravia J and Toro G. Paracoccidioidomicosis del SNC. In: Saravia J and Toro G. Enfermedades infecciosas y Parasitarias. Colombia; 1985, p238-243. [ Links ]
4. Marquez SA, Conterno LO, Sgarbi LP, Villagra AM, Sabongi VP, Bagatin E, Goncalves VL. Paracoccidioidomycosis associated with acquired immunodeficiency syndrome. Report of seven cases. Rev Inst Med Trop S Paulo 1995; 37: 261-265. [ Links ]
5. Pereira W, Raphael A, Sallum J. Lesoes neurológicas na blastimicose sul-americana. Estudio anatomopatológico de 14 casos. Arq Neuropsiquiatr (Sao Paulo) 1965; 23: 95-112. [ Links ]
6. Pereira W, Tenuto RA, Raphael A, Sallum J. Localizacao encefálica da blastomicose sul-americana. Consideracóes a propósito de 9 casos. Arq Neuropsiquiatr (Sao Paulo) 1965; 23: 113-125. [ Links ]
7. Machiori E, Freitas ML, Lima MR. Paracoccidioidomicose medular. Relato de um caso. Arq Neuropsiquiatr ( Sao Paulo) 1989; 47: 224-229. [ Links ]
8. Plá MP, Hartung C, Mendoza P, Stukanoff A, Moreno MJ. Neuroparacoccidioidomycosis: Case reports and review. Mycopathologia 1994; 127: 139-144. [ Links ]
9. Nobrega JPS, Spina-França Netto A. Neuroparacoccidioidomycosis. In: Franco, M, Lacaz, CS, Restrepo, A & Del Negro, G. Paracoccidioidomycosis. Boca Ratón, CRC Press; 1994. p 321-330. [ Links ]
10. Pedro RJ, Branchini ML, Lucca RS, Silveira ML, Facure NO, Neto VA. Paracoccidioidomicose de sistema nervoso central. A propósito de dois casos. Rev Inst Med Trop S. Paulo 1980; 22: 269-274. [ Links ]
11. Guerreiro C, Chuluc S, Branchini M. A new treatment for large cerebral paracoccidioidomycosis. Arq Neuropsiquiatr (Sao Paulo) 1987; 45: 419-423. [ Links ]
12. Barraviera B, Mendes RP, Machado JM, Pereira PC, de Souza MJ, Meira DA. Evaluation of treatment of paracoccidioidomycosis with clotrimazine (combination of sulfadiazine and trimetoprim). Preliminary report. Rev Inst Med Trop S Paulo 1989; 31: 53-55. [ Links ]
13. Minguetti G, Madalosso L. Paracoccidioidal granulomatosis of the brain. Arch Neurol 1983; 40: 100-102. [ Links ]
14. Teive HAG, Ramina R, Torres BL, Arruda WO, Meneses MS, Filho FQ. Paracoccidioidomycosis granuloma simulating posterior fossa tumour. J Royal Soc Med 1991; 84:562-563. [ Links ]
15. Restrepo A, Robledo M, Giraldo R, Hernández H, Sierra F, Gutierres F, Londono F, Lopez R, Calle G. The gamut of paracoccidioidomycosis. Am J Med 1976; 61: 33-42. [ Links ]
16. Morato-Fernandez MR, Beraldo PS, Masini M, Costa PH. Paracoccidioidomicose de localizacao intramedula e cerebral. Arq Neuropsiquiatr (Sao Paulo) 1991; 49: 192-197. [ Links ]
17. Magalhaes AC, Caramelli P, Silva ED, Bacheschi LA, Lo L, Menezes JR, Shikanai-Yasuda MA, Magalhaes A, Polachin JR. Magnetic resonance imaging in intracranial paracoccidioidomycosis. J Neuroimaging 1993; 3: 216-219. [ Links ]
18. Rodacki MA, Toni Gde, Borba LA, Oliveira GG. Paracoccidioidomycosis of the central nervous system: CT finding. Neuroradiology 1995; 37: 636-641. [ Links ]
19. Dos Santos JW, Andrade CF, Lopes AT, Londero AT. Pseudotumoral presentation of chronic pulmonary paracoccidioidomycosis: Report of a case. Mycopathologia 1996; 134: 135-136. [ Links ]
20. Silletti RP, Glezerov V, Schwartz IS. Pulmonary paracoccidiodomycosis misdiagnosed as Pneumocytis pneumonia in an immunocompromised host. J Clin Microbiol 1996; 34: 2328-2330. [ Links ]
21. Marques SA, Dillon NL, Franco MF, Habermann MC, Lastoria JC, Stolf HO, Marcondes J, Grizzo W, Silva NC, Cavariani MR, Curi PR. Paracoccidioidomycosis: a comparative study of the evolutionary serologic, clinical and radiologic results for patients treated with ketoconazole or amphotericin B plus sulfonamides. Mycopathologia 1985; 89: 19-23. [ Links ]
22. Hamill R. Infectious Diseases: Mycoitic. In: Tierney L, McPhee S, Papadakis M. Current Medical Diagnosis & Treatment. Appleton & Lange. Connecticut; 1998, p1427. [ Links ]
23. Gomes MC. Tratamiento de paracoccidioidomicose com ketoconazol. Rev Inst Med Trop S Paulo 1983; 25: 127-132. [ Links ]
24. Villa LA, Tobón A, Restrepo A, Calle D, Rosero D, Gómez BL, Restrepo A. Central Nervous System paracoccidioidomycosis. Report of a case successfully treated with itraconazol. Rev Inst Med Trop S Paulo 2000; 42: 231-234. [ Links ]
25. Colli BO, Assirati JA, Machado HR, Figueiredo JF, Chimelli L, Salvarani CP, Dos Santos F. Intramedullary spinal cord paracoccidioidomycosis. Report of two cases. Arq Neuropsiquiatr 1996; 54: 466-473. [ Links ]
26. Valle AC, Skacel M, Costa RL, Ribeiro CT, Montagna NA, Cruz LC. A case report of intraspinal paracoccidioidomycosis. Rev Inst Med Trop S Paulo 1998; 40: 466-473. [ Links ]
27. Duarte PA, Baruffa G, Terra GC, Renck VD, Moura D, Petrucci C. Paracoccidioidomycose sistemica com envolvimento do sistema nervoso central. Rev Soc Bras Med Trop 1999; 31: 439-442. [ Links ]
28. Silva CE, Cordeiro FA, Gollner MA, Cupolilo NS, Filgueiras QM, Curzio FM. Paracoccidoidomicose do sistema nervoso central. Relato de caso. Arq Neuropsiquiatr 2000; 58: 741-747. [ Links ]
29. Lambertucci JR, Lana-Peixoto MA, Pitella JH. Paracoccidioidomicose do sistema nervoso central. Rev Soc Bras Med Trop 2001; 34: 395-396. [ Links ]












 uBio
uBio