INTRODUCTION
Epilepsy is a neurological disorder characterized by sudden abnormal discharges of neurons in the brain, causing transient disruptions in brain function. It can lead to neuronal damage in regions such as the cerebral cortex and hippocampus, and it is one of the most common neurological disorders in children 1. Research shows that about 75% of epilepsy patients develop the disease during childhood 2. The primary treatment method for epilepsy is oral antiepileptic drugs. However, despite advances in the diagnosis and treatment of epilepsy, long-term use of antiepileptic drugs can lead to numerous adverse reactions. Furthermore, about 30% of children with epilepsy do not respond well to treatment, and severe cases can result into intractable epilepsy, which significantly affects the physical and mental health of patients 3. Therefore, exploring the pathogenesis of epilepsy and finding new therapeutic drugs and targets are crucial for the effective treatment of this disorder.
Research 4 indicates that during epileptic seizures, the NLRP3 (nodlike receptor protein 3) inflammasome is involved in cellular pyroptosis through the classical Caspase-1-dependent pathway, regulating the expression of IL -1β and IL -18. The P2X7R (purinergic ligand-gated ion channel 7 receptor), an ATP-gated cation non-selective channel receptor, is a crucial contributor to the formation and release of IL -1β and has been widely considered an essential target in brain tissue inflammatory responses 5. P2X7R is upregulated at different stages of seizure In various models of epilepsy, including the hippocampus, amygdala, piriform cortex, and neocortex, and its activation can induce and worsen inflammatory reactions 6. Multiple studies 7,8 have demonstrated that in innate myeloid cells such as monocytes, macrophages, and dendritic cells, the P2X7R is the most effective activator of the NLRP3 inflammasome during the inflammatory process. Current research 9 confirms that during epileptic seizures, the P2X7R/NLRP3/ Caspase-1 pathway is significantly upregulated, and downstream-specific inflammatory factors such as IL -1β and IL -18 significantly increase. These findings suggest that the P2X7R/NLRP3/Caspase-1 pathway plays a vital role in the development of epileptic seizures.
Gentiopicrin, the primary active ingredient of Gentiana macrophylla, a plant belonging to the Gentianaceae family, has demonstrated pharmacological properties such as anti-inflammatory, anti-oxidative stress, and anti-apoptotic effects, making it an effective natural medicine. Additionally, it has been found to have low toxicity, high safety, and multi-target synergistic effects 10. The study aims to induce status epilepticus (SE) in juvenile rats through intraperitoneal injection of lithium chloride-pilocarpine and examine whether gentiopicrin can impact the occurrence of epilepsy by regulating the P2X7R/NLRP3/Caspase-1 inflammatory pathway. The results of this study will provide a foundation for gentiopicrin to be considered as a potential candidate for the effective treatment of childhood epilepsy.
MATERIALS AND METHODS
Experimental Animals
Seventy-two specified pathogen-free male Sprague Dawley(SD) rats, aged 21 days and weighing 50-80g, were procured from Beijing Vital River Laboratory Animal Technology Co., Ltd. The production license number is SCXK (JING) 2021-0011. The rats were kept at the Central Animal Laboratory of our institute under optimal conditions, with a relative humidity of 70%, a temperature of 25°C, and a 12-hour light/dark cycle. We strictly follow the 3R principle in the experiment. The Animal Experiment Center of Tongling Vocational and Technical College Ethics Committee approved this study.
Reagents and Instruments
Main reagents: Tropisetron was purchased from Xi’an Yangsen Pharmaceutical Co., Ltd. Lithium chloride and pyrrolidine carboxamide were purchased from Fluka, USA. Gentiopicrin was purchased from Jingzhu Biotechnology Co., Ltd., and the Reverse transcription kit from Jiangsu Kangwei Century Biotechnology Co., Ltd. SYBR Premix Ex Taq II was purchased from Takara, Japan. BCA protein quantification kit and protein marker were purchased from Beijing Solarbio Science & Technology Co., Ltd. P2X7R, NLRP3, ASC, and Caspase-1 primary antibodies from Affinity, USA. Tunel staining solution was purchased from Shanghai Biyun Tian Biotechnology Co., Ltd.
Main instruments: A Micro 17R low-temperature high-speed centrifuge was purchased from Thermo, USA. CFX Connect real-time fluorescence quantitative PCR instrument was purchased from BIORAD, USA. EPS300 electrophoresis apparatus, EPS300 electrophoresis tank, and VE186 transfer apparatus were purchased from Zhejiang Tianneng Corporation. 610020-9Q chemiluminescence analyzer was purchased from Shanghai Qinxiong Scientific Instrument Co., Ltd. RM2016 pathological slicer was purchased from Shanghai Leica Instrument Co., Ltd. Nikon Eclipse C1 upright fluorescence microscope and Nikon DS-U3 imaging system were purchased from Nikon, Japan.
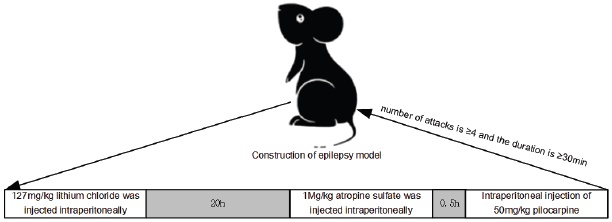
Animal grouping and model establishment A total of 72 SD rats were used in the study. Twelve rats were randomly assigned to the normal group, while the remaining 60 rats were used to establish an epilepsy model. The rats were first weighed and then injected with 127 mg/kg of lithium chloride into the abdominal cavity to induce epilepsy. After 20 hours, 1 mg/kg of atropine sulfate was also injected to reduce the peripheral cholinergic reaction of pilocarpine. Thirty minutes later, the rats were injected with 50mg/kg of pilocarpine. The success of the epilepsy model was determined by using the Racine scoring standard, which required a seizure frequency of ≥4 and a duration of ≥30 minutes 11. The model construction is shown in Fig. 1.
The rats that were successfully modeled were divided into groups randomly. The positive control group received 5.9 mg/kg of topiramate dissolved in 2 mL of normal saline via gavage. The low-dose, medium-dose, and high-dose groups were administered gentiopicrin solution via gavage, dissolved in 2 mL of normal saline, at doses of 1.28g/kg, 2.56g/kg, and 5.12g/kg, respectively. The model and normal groups were given the same volume of saline via gavage once a day for four weeks.
Behavioral assessment
Within two hours of administering pilocarpine, the rats were observed for any behavioral changes, and the onset latency and duration of the first seizure were recorded. The severity of SE in each group was graded according to the Racine scoring system, as follows: Stage 0, no seizures; Stage I, facial twitching and mouth movements; Stage II, frequent nodding or wet dog shakes; Stage III, localized clonic seizures in the forelimbs; Stage IV, generalized tonic-clonic seizures with forelimb clonus and rigid posture in the hindlimbs; and Stage V, generalized tonicclonic seizures with falling and rolling.
Tissue processing
After one, two, and seven days of modeling, rats from each group were intraperitoneally injected with a 5% chloral hydrate solution for anesthesia. Four rats from each group were then quickly decapitated to obtain brain tissue, rats were fixed on the operating table, and their chests were opened to expose the heart. The right auricle was incised, and 150 mL of saline was perfused to flush out the blood, followed by perfusion with 150 mL of 4% paraformaldehyde for fixation. The brain was quickly removed after fixation and placed in a 4% paraformaldehyde solution for an additional 48 hours of fixation before being subjected to Tunel staining. The rest of the brain tissue was stored at -80°C for subsequent analysis using ELISA kits, Western blotting, and qRT-PCR.
Tunel staining to observe pathological changes in hippocampal tissue
Brain tissue was fixed in 4% paraformaldehyde, and coronal brain sections containing the hippocampus, from 3 mm anterior to 3 mm posterior to the optic chiasm, were obtained. The sections were routinely dehydrated, transparentized, and embedded in paraffin before being pre-cooled for sectioning (thickness of 4 μm). The sections were then deparaffinized with xylene, rehydrated with gradient ethanol, and stained with Tunel staining solution for 1-10 minutes. After rinsing with distilled water twice for 15 seconds each, the sections were dehydrated with 95% ethanol for 5 minutes, transparentized with xylene for 5 minutes, and mounted with neutral gum. Under a microscope, the number of Tunel-positive stained cells in each high-power field was counted, and the average value was obtained.
ELISA detection of superoxide dismutase (SOD) activity and malondialdehyde (MDA) content in hippocampal tissue
Hippocampal tissue from rats obtained on the 7th day after modeling was added to pre-cooled PBS buffer, ground into a homogeneous slurry on ice, and centrifuged at 10,000 rpm for 10 minutes. The supernatant was collected and divided into Eppendorf (EP) tubes, then stored at -80°C. ① SOD activity detection: 20μL of the test sample was taken and sequentially added to the SOD detection buffer, WST-8 working solution, and reaction start working solution. The mixture was incubated at 37°C for 30 minutes, and the absorbance (A) value was measured at 450 nm wavelength. Using the formula inhibition percentage = (ΔA blank - ΔA test)/ΔA blank × 100%, the inhibition percentage was calculated. The SOD enzyme activity (U/ mg) of the test sample was determined from the standard curve. ② MDA content detection: 100μL of the test sample was taken and sequentially added to the working solution, distilled water, and test sample. The mixture was incubated at 100°C in a water bath for 60 minutes, cooled in an ice bath, and centrifuged at room temperature at 10,000g for 10 minutes. Next, 200 μL of the supernatant was transferred to a glass cuvette, and the absorbance (A) values at 450 nm, 532 nm, and 600 nm were measured. The ΔA450 = A450 test - A450 blank, ΔA532 = A532 test - A532 blank, and ΔA600 = A600 test - A600 blank were calculated. The MDA content (nmol/g) was calculated as MDA content = 5 × [12.9 × (ΔA532 - ΔA600) - 2.58 × ΔA450].
Western blot analysis of P2X7R, NLRP3, ASC, and Caspase-1 Protein expression in hippocampal tissue
Hippocampal tissue was collected and homogenized on ice on the seventh day after modeling. The supernatant was obtained after centrifugation, and protein concentration was measured using a BCA protein assay kit. Protein lysates were separated on a 15% SDS-PAGE gel with a 5% stacking gel, run at 80V for two hours, and transferred to a membrane at 60V for two hours. After blocking with 5% skim milk for two hours, the membrane was incubated overnight at 4°C with rabbit primary antibodies against P2X7R, NLRP3, ASC, and Caspase-1 (diluted 1:1000). The following day, the membrane was washed with TBST and incubated with a goat anti-rabbit secondary antibody (diluted 1:2000) for two hours at 37°C. After washing, the membrane was incubated with a chemiluminescence reagent, and the protein expression levels were quantified using ImageJ software with GAPDH as an internal reference protein. The relative protein expression levels were calculated as the protein gray value of the target protein divided by the protein gray value of the internal reference protein.
qRT-PCR detection of hippocampal P2X7R, NLRP3, ASC, and Caspase-1 mRNA expression
On the 7th day after modeling, hippocampal tissue was obtained and ground on ice. Total RNA was extracted using 1 mL of Trizol lysis buffer. The RevertAidTM First Strand cDNA Synthesis Kit synthesized cDNA as the fluorescent quantification template. Takara designed and synthesized Primers in Japan, and GAPDH was used as the internal reference for all samples. The reaction system included 0.5%μL dNTPs, 5μL 5×Buffer, 0.3 μL Taq enzyme, 21.5 μL MgCl, 2 μL cDNA template, and 1 µL of upstream and downstream primers. Deionized water was added to a total volume of 25µL. The reaction conditions were as follows: predenaturation at 95°C for 5 minutes, denaturation at 95°C for 30 seconds, annealing at 62°C for 30 seconds, extension at 72°C for 30 seconds, repeated for 40 cycles, and final extension at 72°C for 10 minutes. The reaction was terminated at 4°C for 5 minutes, and the experiment was repeated three times. The relative expression level of the target gene mRNA was calculated using the 2-△△CT method. Primer sequences are shown in Table 1.
Table 1 Primer Sequences.
| Gene | Sequence |
|---|---|
| P2X7R | Forward GGACGAGCTACCCTTCGGT |
| Reverse CTGTCTCACCCCCAGCATAG | |
| NLRP3 | Forward CACATCATGAAGGAGGAGG |
| Reverse GCTATCACACAGCCTGGGTC | |
| ASC | Forward ACCCTAATGCCTTGTTCCCA |
| Reverse TGGGGAAGTCTTCAGCAAC | |
| Caspase-1 | Forward GGCAGACCTATTTTGCACGA |
| Reverse TAGCTGTCGATGGATGCCTC | |
| U6 | Forward CTGATGAAATACGCCCAGGT |
| Reverse CTGTGGTTCTGGTTCGCTTT |
Statistical Analysis
The experimental data were analyzed using SPSS 26.0 software, and GraphPad 8.0 was utilized for plotting. Data that followed a normal distribution, such as pathological changes and protein expression level in each sample group, were presented as mean ± standard deviation (X̄±SD). One-way analysis of variance (ANOVA) was employed for comparisons among multiple groups, and t-tests were used for pairwise comparisons. A p of less than 0.05 was considered statistically significant.
RESULTS
Effects of Gentiopicrin on behavioral performance in juvenile rats with epilepsy
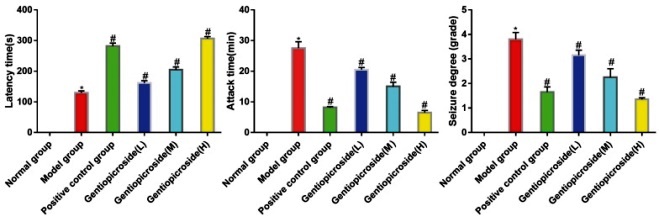
In the blank control group, the behavior of the rats was normal, and no epileptic seizures were observed, indicating a grade 0 level of epileptic seizures. In the positive control group and gentiopicrin low, medium, and high dose groups, the latency period was significantly increased compared to the model group. Moreover, the duration and severity of epileptic seizures were significantly reduced in the groups mentioned above compared to the model group (p<0.05) (Fig. 2).
Effects of Gentiopicrin on Tunel staining positive cell numbers in hippocampal tissue
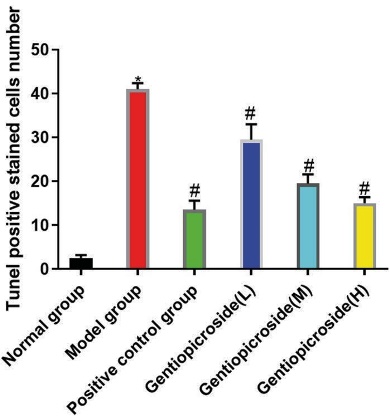
Tunel staining revealed that in the normal group, neurons in the CA3 region of rat hippocampus were evenly distributed across multiple layers, with pyramidal and granular cells showing normal morphology, uniform staining, tight arrangement, and round or oval cell bodies with distinct nucleoli. The cytoplasm of these cells had abundant Tunel bodies, and occasional nuclear condensation was observed. In contrast, compared to the normal group, the model group showed a reduction in the number and shape of neurons in the CA3 region, with decreased cell bodies, ruptures, nuclear condensation, and a significant decrease in Tunel bodies in the cytoplasm. Compared to the model group, the positive control group and gentiopicrin low, medium, and high dose groups showed a reduction in the degree of neuronal damage in the CA3 region of the rat hippocampus. The number of Tunel-stained positive cells in the model group was significantly higher than in the control group (p<0.05). However, the number of Tunel-stained positive cells in the positive control group and gentiopicrin low, medium, and high dose groups was significantly lower than that in the model group, and the differences were statistically significant (p<0.05)(Fig. 3).
Effects of Gentiopicrin on superoxide dismutase (SOD) activity and malonilaldehyde (MDA) content in hippocampal tissue
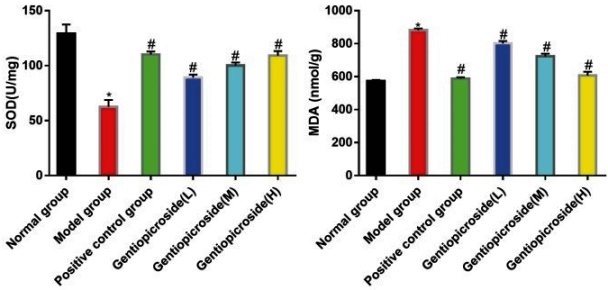
In the model group, the SOD activity was significantly lower, and the MDA content was significantly higher than that in the control group, with statistical significance (p<0.05). However, in the positive control group and gentiopicrin low, medium, and high dose groups, the SOD activity was significantly higher, while the MDA content was significantly lower than that in the model group, with statistical significance (p<0.05), (Fig. 4).
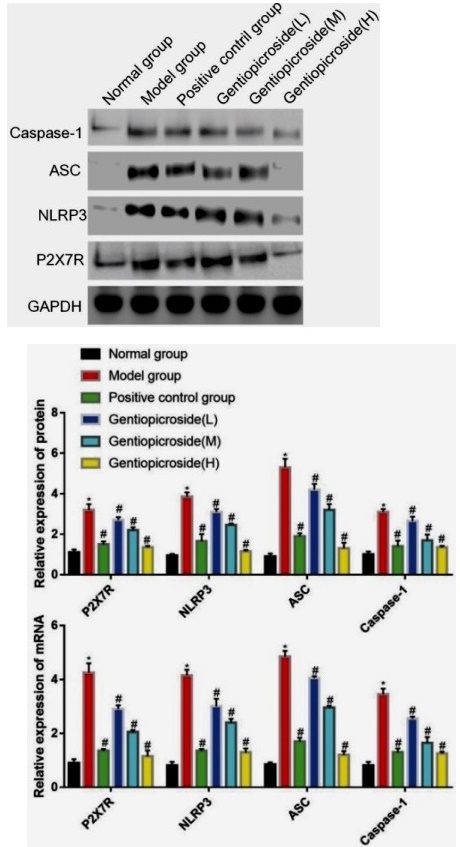
Effects of Gentiopicrin on the expression of P2X7R, NLRP3, ASC, Caspase-1 proteins, and mRNA in hippocampal tissue
The expression levels of P2X7R, NLRP3, ASC, Caspase-1 proteins, and mRNA in the model group were significantly higher than those in the control group, with statistical significance (p<0.05). However, in the positive control group and gentiopicrin low, medium, and high dose groups, the expression levels of P2X7R, NLRP3, ASC, Caspase-1 proteins, and mRNA were significantly lower than those in the model group, with statistical significance (p<0.05), (Fig. 5).
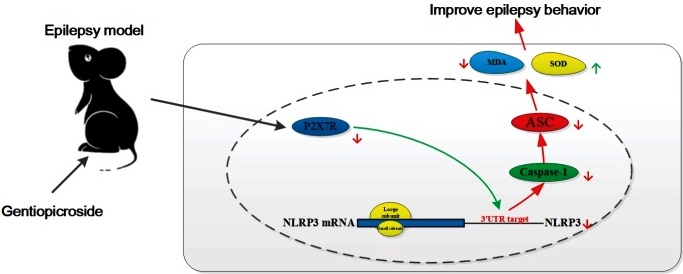
Gentiopicrin improves behavioral performance in juvenile rats with epilepsy by regulating the P2X7R/ NLRP3/Caspase-1 pathway to inhibit inflammatory factor expression.
Gentiopicrin significantly prolonged the latency period of epileptic rats, reduced the duration and severity of seizures, improved hippocampal tissue pathology, increased hippocampal tissue SOD activity, and decreased MDA levels. Furthermore, it down-regulated critical proteins in the P2X7R/ NLRP3/Caspase-1 pathway. Therefore, we speculate that gentiopicrin may improve behavioral performance in epileptic rats by regulating the P2X7R/NLRP3/Caspase-1 pathway to inhibit inflammatory factor expression. A schematic diagram of the mechanism is shown in Fig. 6.
DISCUSSION
Epilepsy is a common and severe neurological disorder that can cause recurrent, transient, and paroxysmal seizures, resulting in high mortality and disability rates, particularly among children. This condition significantly affects the quality of life of patients 12. At present, the primary clinical approach for treating epilepsy is through the use of antiepileptic drugs (AEDs). However, AEDs only provide partial and incomplete control of seizures in approximately 30% of epilepsy patients 13. Moreover, long-term use of AEDs can lead to significant adverse reactions, such as cognitive impairment, psychiatric side effects, teratogenic effects, and toxic effects on the liver. Therefore, it is of utmost importance to identify new drug targets and develop new antiepileptic drugs for children. Gentiopicrin is a natural bicyclic diterpenoid lactone compound with various biological activities, mainly extracted from Gentiana macrophylla, a characteristic medicinal plant in Ningxia, China 14. Previous studies 15 have shown that gentiopicrin can help alleviate anxiety and depression symptoms, memory impairment, and nerve damage.
The P2X7 receptor can affect the release of downstream inflammatory factors and the activation of immune cells by influencing various signaling pathways, such as the P-38MAPK, NF-ΚB, and NLRP3/Caspase-1 pathways 16. Recent studies have confirmed that the P2X7R/NLRP3/Caspase-1 pathway is significantly upregulated during seizures, leading to increased expression of specific inflammatory factors like IL -1β and IL -1817. This suggests that the P2X7R/NLRP3/Caspase-1 pathway is a critical mediator in seizure activity 18. In this study, we focused on the protective effect of gentiopicrin on neurons in a rat model of epilepsy by targeting the P2X7R/NLRP3/Caspase-1 pathway. We induced SE in rats using lithium chloridepilocarpine and used topiramate as the positive control drug. The results showed that the gentiopicrin intervention significantly prolonged the SE latent period, shortened seizure duration, and reduced SE in a dose-dependent manner, indicating its antiepileptic effects in SE rats. Seizures can lead to decreased brain nerve cells, neuronal damage, and cell apoptosis, directly affecting the patient’s prognosis and condition development 19. In this study, the protective effect of gentiopicrin on hippocampal damage in epileptic rats was observed through Tunel staining. Pathological section staining revealed that hippocampal neurons in the model group exhibited significant damage, with disordered arrangement, small or ruptured cell bodies, condensed nuclei, reduced Tunel bodies in the cytoplasm, and an increased number of damaged cells over time. Compared with the model group, the number of damaged neurons in the CA3 region of the hippocampus in the positive control and gentiopicrin low, medium, and high dose groups decreased, the degree of damage was reduced, and the number of damaged cells did not increase over time. These results suggest that gentiopicrin has an inhibitory effect on hippocampal neuronal damage caused by epilepsy. Oxidative stress-induced brain cell damage and weakened antioxidant capacity are essential mechanisms underlying the occurrence and persistence of epilepsy and other neurological diseases 20) SOD, an important antioxidant enzyme, reflects the body’s ability to remove free radicals, while MDA is related to the degree of tissue cell damage and is a lipid peroxide produced after tissue cells are damaged by oxygen free radicals 21. The findings of this study showed that epilepsy can increase the MDA content in hippocampal tissue and reduce SOD activity, whereas gentiopicrin can inhibit oxidative stress-induced damage caused by epilepsy, increase SOD activity, and reduce MDA production.
The NLRP3 inflammasome is a complex composed of three essential proteins: the NOD-like receptor NLRP3, the adapter protein ASC, and the effector protein Caspase-1 22. Once activated, NLRP3 releases Caspase-1, which in turn cleaves precursor forms of IL -1β and IL -18, generating mature and activated forms of these cytokines that promote the development of inflammation 23. The P2X7R receptor is a crucial factor in activating the NLRP3 inflammasome. When neural tissue is damaged, a large amount of ATP is released, which binds to the P2X7 receptor on microglia and promotes the formation of the NLRP3 inflammasome. This results in the acceleration of maturation and release of inflammatory factors such as IL - 1β and IL -18, initiating a cascade effect 24-26. The study results show that both the protein and mRNA levels of P2X7R, NLRP3, ASC, and Caspase-1 were significantly higher in the model group than in the control group. However, in the positive control group and the low, medium, and high dose groups of gentiopicrin, the protein and mRNA levels of P2X7R, NLRP3, ASC, and Caspase-1 were significantly lower than those in the model group. Yang Wenwei and other scholars have also found that gentiopicroside inactivates NLRP3 inflammatory corpuscles by inhibiting P2X7R expression, thus reducing the occurrence of epilepsy in young rats 27, which is similar to the results of this study.
In conclusion, the results of this study suggest that gentiopicrin may improve behavioral deficits in epileptic rats by regulating the expression of inflammatory factors through the P2X7R/NLRP3/Caspase-1 pathway.












 uBio
uBio