INTRODUCTION
Neuropathic pain usually has a chronic progression and affects a significant number of individuals globally, leading to physical, emotional, and economic burdens 1. It arises due to a dysfunction in the nervous system, causing abnormal signalling and processing of pain signals 2. Patients with neuropathic pain experience various symptoms such as shooting or burning pain, numbness, tingling, and hypersensitivity to stimuli 3. Various factors can cause neuropathic pain, including injury, infection, diabetes, or chemotherapy. This condition causes a substantial burden to the patients, and the current treatments for neuropathic pain are often limited in efficacy and associated with adverse effects 4. Hence, the development of alternative treatment agents for the clinical therapy of neuropathic pain is urgently needed.
Toll-like receptor 4 (TLR4) belongs to the TLR family that initiates the innate immune response 5. TLR4 has been found in various cells, including immune cells and neurons. It was reported that TLR4 could be activated by lipopolysaccharides (LPS) and damage-associated molecular patterns (DAMPs) 6. Activation of TLR4 leads to the upregulation of myeloid differentiation factor 88 (MyD88). Moreover, the activated TLR4 could promote the nuclear factor-κB (NF-κB) pathway, resulting in the upregulation of various inflammatory factors and chemokine production 7. TLR4 participates in the occurrence and progression of neuropathic pain. Publications have shown that TLR4 increases in the spinal cord in animal models of neuropathic pain 8, and TLR4- deficient mice exhibit reduced pain behavior in various neuropathic pain models 9. Therefore, implementing TLR4 antagonists or inhibitors might have analgesic effects in neuropathic pain treatment.
Phillygenin (PHI) is a bioactive compound found in Forsythia suspensa, traditionally used for treating inflammatory and infectious diseases 10. PHI exhibits a wide range of bioactive capabilities, including improving the inflammatory status, preventing cancer progression, and protecting against neuron injury 11. The modulation of different cellular processes, such as NF-κB-related inflammation and PI3K/Akt-mediated tumor cell proliferation, accounts for the diverse properties of the substance 12. Recent research indicates that PHI could suppress TLR4 activation, further inhibiting the NF- κB signalling pathway downstream, suggesting its potential as a treatment target for the therapy of inflammatory and neuropathic conditions 13. However, the mechanism of action underlying the potential use of PHI in treating neuropathic pain remains unclear.
This study explored the potential effects of PHI in neuropathic pain progression and its underlying mechanism. We implemented rat chronic constriction injury (CCI) models for in vivo studies on neuropathic pain. We hypothesized that treatment with PHI could significantly inhibit TLR4 expression and suppress MyD88/NF-κB signalling, reducing the neuropathic behavior in CCI rats. Our study aimed to provide new insights into the potential use of PHI for the clinical therapy of neuropathic pain.
METHODS
Animal models
Male Sprague-Dawley rats (210-250 g) were purchased from the Hunan Sileck Jingda Experimental Animal Co., Ltd. All rats were kept in individual cages with a 12 h light/dark cycle, constant temperature (22 ± 2°C) and humidity (50 ± 10%), and food and water were freely available. Both male and female rats were included in the sample. The final allocation of 40 rats comprised an equal representation of both sexes to ensure a comprehensive assessment of the experimental outcomes. The acclimatization of animals (at least seven days) was performed before they underwent various treatments and experiments. The Animal Care and Use Committee of the Wuhan Fourth Hospital reviewed and authorized all animal experiments.
The CCI model was implemented as per a previous study 14. During surgery, the rats received continuous anesthesia with 2% isoflurane in oxygen, and the exposition of the left sciatic nerve was made at the mid-thigh level. Subsequently, 4-0 chromic gut was used as loose ligatures for making ties around the nerve with a 1-mm interval. The tightened ligatures were further made to produce a mild nerve constriction, which induced the CCI condition. Layers of 4-0 silk sutures were used to close the wound, and all rats were then kept in a warm environment for recovery. Rats that exhibited motor dysfunction or showed signs of infection were excluded from the study. A total of 40 rats were then randomly allocated into four groups (10 rats in each, according to previous studies)15,16:
(1) Sham group: rats underwent the same surgical process as the CCI group, but without performing the nerve ligation; (2) CCI group, where CCI surgery was implemented; (3) CCI+PHI group, where CCI surgery was implemented and rats received daily intragastric administration of PHI (20 mg/kg, using DMSO as vehicle, accordingly to previous studies ) 11,17 for 14 consecutive days. PHI was obtained from a commercial supplier, specifically the Shanghai Yuanye Biotechnology Co., Ltd., which provided a high-purity grade of PHI suitable for our experimental needs; (4) CCI+NC group, where CCI surgery was implemented and rats were treated with the same volume of normal saline. Once the CCI model was established and the rats had recovered from the anesthesia, they received daily intragastric administration of PHI (20 mg/kg) or the same volume of normal saline in the CCI+NC group for 14 consecutive days. Researchers performing data analysis and histological assessments were blinded to the treatment groups. After model establishment, the rats were euthanized, and their spinal cords were collected for subsequent experiments. Researchers performing data analysis and histological assessments were blinded to the treatment groups.
Von Frey Test
Von Frey assay (Ugo Basile, Italy) was used to assess mechanical allodynia before surgery and on postoperative days 0, 2, 4, 6, 8 10, 12 and 14. All rats were maintained in boxes that contained a wire mesh bottom and acclimatized for 30 minutes. After acclimation, the von Frey test was conducted on the mid-plantar surface of the ipsilateral and contralateral hind paw. The cutoff was set at 26 g, and the data were collected automatically. Pain-like responses, such as an abrupt withdrawal of the paw, licking, or vigorously shaking, were noted. The 50% paw withdrawal threshold (PWT) was determined through the up-down approach as described in a previous study 18.
Hargreaves Test
The Hargreaves test was performed on rats before surgery and on postoperative days 0, 2, 4, 6, 8, 10, 12, and 14 to assess thermal hyperalgesia. The Plantar Test Apparatus (Ugo Basile, Italy) was obtained for this purpose. A total of 30 minutes of acclimatization were first made. A radiant heat source treated the mid-plantar surface of the right hind paw, and the paw withdrawal latency (PWL) was recorded. A 20-second cut-off threshold was set for the heat stimulation test to prevent injury. A decrease in PWL was considered a sign of heat hyperalgesia. The behavioral tests were accomplished by experimenters blinded to the group assignments 18.
Nitric oxide (NO) assay
The expression of NO was detected using a 2,3-diaminonaphthalene (DAN) assay kit. First, the tissue samples were first isolated and homogenized in PBS at 4°C and then centrifuged at 4°C for 15 minutes (12,000 rpm). Next, the supernatant was obtained, and 50 μL of the sample and 50 μL of DAN solution (5 mM in 0.62 M HCl) were added and maintained for 15 minutes at room temperature. Afterward, NaOH (100 μL, 2 M) was used to terminate the reaction. The intensity was measured using a fluorescence spectrophotometer (excitation wavelength: 365 nm, emission wavelength: 450 nm).
ELISA assay
Following the manufacturer’s protocol, TNF-α, IL -1β, and IL -6 expressions were measured using a commercial ELISA kit (R&D Systems, USA). Briefly, the spinal cord tissue samples were obtained as described above. Then, 200 μL of detection reagent was added and maintained for 2 h, after which 200 μL of substrate solution was added and maintained for 1 h in the dark. A 50 μL stop solution was added to terminate the reaction, and the absorbance was monitored through a microplate reader (450 nm).
RT-qPCR
TRIzol reagent (Invitrogen, USA) was obtained to isolate total RNA from the spinal cord tissues, following the manufacturer´s protocol. Then, total RNA was reverse transcribed into cDNA using a PrimeScript RT Reagent Kit (Takara Bio, Japan). The expression levels of mRNAs were quantified using an SYBR Premix kit (Takara Bio, Japan) and a StepOnePlus Real-Time PCR system (Applied Biosystems, USA). The mRNA levels were normalized to that of GAPDH, and the calculation was performed as per the 2-ΔΔCt method. The primers used are listed as follows:
TLR4-F: 5’- GAATGCTAAGGTTGGCACTCTC -3’
TLR4-R: 5’- CTCAGGCAGGAAAGGAA-CAATG -3’
MyD88-F: 5’- GCTGAGAGGAAGAGTTC-TAC -3’
MyD88-R: 5’- CAGTGATAACCCTGGAC-TAC -3’
NF-κB: 5’- AGACCTGGAGCAAGCCATTAG -3’
NF-κB: 5’- CGGACCGCATTCAAGTCATAG -3’
GAPDH: 5’- TTCAACGGCACAGTCAAGG -3’
GAPDH: 5’- GTCTTCTGAGTGGCAGT-GATG -3’
Western blotting
For Western blot analysis, spinal cord tissue samples underwent homogenization in RIPA buffer (Roche, Switzerland), which was added to the protease inhibitor cocktail. Then, quantification was performed via BCA Protein Assay kit (Thermo Scientific, USA). After separation on 10% SDS-PAGE, proteins were transferred to PVDF membranes (Millipore, USA). Then, 5% non-fat milk was obtained for blocking. Subsequently, the membranes were incubated with primary antibodies against TLR4 (ab22048, Abcam, 1:1000), MyD88 (ab219413, Abcam, 1:1000), p-NF-κB (#3039, CST, 1:1000), NF-κB (#6956, CST, 1:1000), and β-actin (ab8226, Abcam, 1:1000) overnight at 4°C. The membranes were further incubated with secondary antibodies (1:1000) for 2 hours at room temperature. The protein levels of target genes were quantified by visualizing protein bands using enhanced chemiluminescence reagents (Millipore, USA) and analyzed through ImageJ (National Institutes of Health, USA). Normalization to β-actin expression levels was performed.
Statistical analysis
All data were presented as means ± standard deviation (SD) and analyzed using GraphPad Prism 8. One-way analysis of variance (ANOVA) followed by Dunnett’s post-hoc test was used to determine the statistical significance of differences among multiple groups. P<0.05 was considered statistically significant.
RESULTS
The data illustrated in the behavioral tests revealed that rats subjected to the CCI model had significantly lower 50% PWT and PWL (Figs 1A and B) compared to the Sham group. However, when compared with CCI rats, the additional administration of PHI dramatically enhanced 50% of PWT and PWL. Moreover, the implementation of NO and ELISA assays revealed, respectively, that the levels of NO and the inflammatory cytokines TNF-α, IL-1β, and IL-6 significantly increased after establishing the CCI model (Fig 2). In contrast, administration of PHI significantly reduced these expression levels. Western blotting and RT-qPCR (Figs. 3A and 3B) results showed that the levels of TLR4, MyD88, and p-NF-κB were significantly increased by CCI induction, while treatment with PHI significantly reversed the change in these expression levels. And the results will be presented as “relative quantification (RQ) using the 2^(-ΔΔCt) method,” indicating the fold change in expression levels compared to the control group.
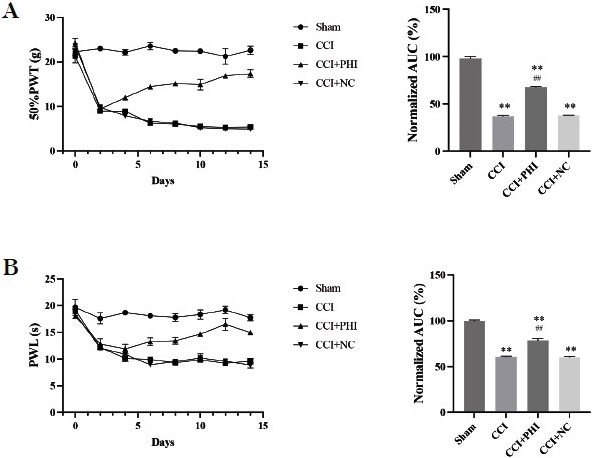
Fig. 1 PHI treatment significantly attenuated mechanical allodynia and thermal hyperalgesia. (A) The time course effect of PHI treatment on mechanical allodynia was assessed using the 50% paw withdrawal threshold (PWT). (B) The time course effect of PHI treatment on thermal hyperalgesia was determined by measuring paw withdrawal latency (PWL). Data are presented as means ± SD. Statistical analyses were performed using a One-way variance analysis (ANOVA). Significant differences are indicated as **P<0.01 vs Sham group and ##P<0.01 vs CCI group (n=10). Abbreviations: CCI, chronic constriction injury; PWT, paw withdrawal threshold; PWL, paw withdrawal latency.
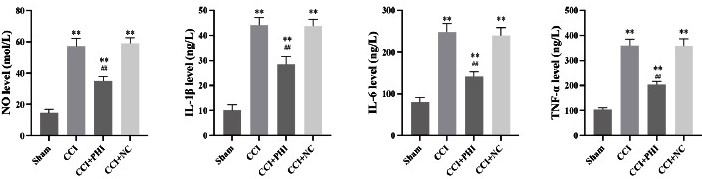
Fig 2 PHI treatment significantly reduced the inflammatory status in CCI rats, as indicated by the levels of nitric oxide (NO), tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL -1β), and interleukin-6 (IL -6) measured after treatment. Data are presented as means ± SD. Statistical analyses were conducted using a One-way analysis of variance (ANOVA). Significant differences are indicated as **P<0.01 vs Sham group and ##P<0.01 vs CCI group (n=10). Abbreviations: CCI, chronic constriction injury; NO, nitric oxide; TNF-α, tumor necrosis factor-alpha; IL -1β, interleukin-1 beta; IL -6, interleukin-6. The Y-axis of the graphs denotes the “Levels” of these inflammatory markers.
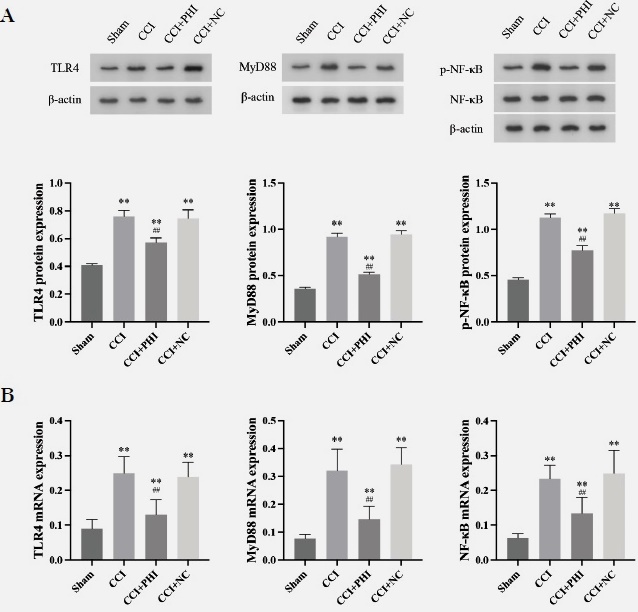
Fig. 3 PHI treatment inhibited CCI rats’ TLR4/MyD88/NF-κB signalling pathway. (A) Protein expression levels of TLR4, MyD88, and phosphorylated NF-κB (p-NF-κB) relative to total NF-κB were determined by western blotting. (B) mRNA expression levels of TLR4 and MyD88 were assessed using RT-qPCR. Data are presented as means ± SD. And the results will be presented as “relative quantification (RQ) using the 2^(-ΔΔCt) method,” indicating the fold change in expression levels compared to the control group. Statistical analyses were performed using a One-way analysis of variance (ANOVA). Significant differences are indicated as **P<0.01 vs Sham group and ##P<0.01 vs CCI group (n=10). Abbreviations: CCI, chronic constriction injury; TLR4, Toll-like receptor 4; MyD88, myeloid differentiation primary response 88; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; p-NF-κB, phosphorylated NF-κB.
DISCUSSION
In our study, treatment with Phillygenin (PHI) significantly enhanced the 50% paw withdrawal threshold (PWT) and paw withdrawal latency (PWL), indicating an effective analgesic effect. Notably, PHI treatment resulted in a marked decrease in nitric oxide (NO) levels, alongside a reduction in proinflammatory cytokines, including TNF-α, IL-1β, and IL-6. Furthermore, our findings demonstrated that PHI downregulated the expression of TLR4 and MyD88, key components in the inflammatory pathway, and inhibited the phosphorylation of NF-κB, a critical factor in inflammation and pain signalling.
These results suggest that PHI may alleviate pain by reducing inflammation and modulating key signalling pathways, highlighting its potential as a therapeutic agent in pain management.
In our study, we observed that treatment with Phillygenin (PHI) significantly enhanced the 50% paw withdrawal threshold (PWT) and paw withdrawal latency (PWL) in CCI rats, indicating its potential efficacy in alleviating neuropathic pain. This finding aligns with recent literature suggesting that neuropathic pain is closely associated with elevated inflammatory factors and increased TLR4 expression 18. Specifically, TLR4 signalling has been implicated in mediating neuropathic pain and exacerbating inflammatory responses, as demonstrated in studies indicating that partial sciatic nerve ligation leads to heightened TLR4 levels and subsequent pain sensations 19. Another study suggested that the increased TLR4 level was linked to partial sciatic nerve ligation-mediated neuropathic pain 20. Moreover, our findings support Zhang et al.’s observations that opioid receptor agonists can non-selectively actívate TLR4 and increase proinflammatory cytokines like TNF-α, IL-1β, and IL-6, contributing to neuroinflammation.
In contrast, our results demonstrate that PHI significantly decreased the levels of these inflammatory cytokines, suggesting a potential mechanism for its analgesic effects 21. Li et al. reported enhanced TLR4 and MyD88 levels in the dorsal root ganglion following paclitaxel treatment, which correlated with diminished PWT and PWL 18. Our study extends these findings by showing that PHI not only modulates TLR4 expression but also reduces inflammation, thereby improving pain thresholds in CCI rats. Overall, our results suggest that targeting TLR4 could be a viable strategy for alleviating neuropathic pain. The integration of our findings with existing literature underscores the importance of inflammatory pathways in neuropathic pain and positions PHI as a promising candidate for further investigation in pain management strategies.
PHI has been found to participate in various aspects of inflammatory status relief and in reducing TLR4 expression. For instance, in our study, we noted that administration of PHI inhibited LPS-induced inflammatory responses and apoptosis in BEAS-2B cells. This inhibition was linked to the subsequent activation of PPAR-γ signalling due to the downregulation of MMP-8. By reducing MMP-8 levels, PHI effectively alleviated acute lung injury 22. Additionally, a previous study found that dietary PHI supplementation reduced malondialdehyde and inflammatory mediator production, increased antioxidant enzyme contents and Bcl-2 levels, and ameliorated aflatoxin B1-induced liver damage 23. Xue et al. found that PHI reversed the expression of SOD and MDA and downregulated the levels of TNF-α, IL -1β, IL -6, and IL -10 in a model of colitis in mice, and reduced the proportion of tyrosine kinase Src activated by TLR4, suggesting that PHI might be a potential drug candidate that could effectively safeguard against colitis 13. Our study found that PHI administration might ameliorate neuropathic pain in CCI rats, potentially through mechanisms such as the downregulation of TLR4, inhibition of MyD88/NF-κB signalling, and reduction of proinflammatory cytokines. Similarly, our findings demonstrated that PHI played a beneficial role in reducing inflammation in CCI rats, suggesting it may serve as an alternative therapeutic strategy for neuropathic pain. In our study, we observed that treatment with Phillygenin (PHI) significantly enhanced the 50% paw withdrawal threshold (PWT) and paw withdrawal latency (PWL) in CCI rats, indicating its potential as an analgesic agent.
Our results suggest that PHI may alleviate neuropathic pain by targeting the TLR4 and MyD88/NF-κB signalling pathways. While these findings are promising, a more detailed mechanistic explanation is warranted to elucidate how PHI exerts its effects. One potential mechanism involves the downregulation of TLR4 expression by PHI, which could reduce the activation of downstream inflammatory pathways. By inhibiting TLR4, PHI may prevent the subsequent activation of MyD88 and the phosphorylation of NF-κB, leading to a decrease in proinflammatory cytokines such as TNF-α, IL-1β, and IL-6. This reduction in inflammatory mediators may alleviate pain and contribute to restoring normal neuronal function in the context of neuropathic pain. Additionally, PHI may modulate oxidative stress pathways. By decreasing nitric oxide (NO) levels, PHI could help mitigate oxidative damage that often accompanies neuropathic conditions, further supporting neuronal health and function. This dual action-reducing inflammation while protecting neurons-could provide a comprehensive approach to pain management. While our findings align with existing literature, further investigation is needed to explore these proposed mechanisms. Future studies should aim to clarify the specific interactions of PHI with TLR4 and MyD88/ NF-κB, potentially utilizing molecular and cellular assays to provide insight into the therapeutic implications of targeting these pathways in neuropathic pain. This enhanced mechanistic understanding would strengthen the rationale for PHI as a viable therapeutic strategy in clinical settings. We utilized a nitric oxide (NO) assay to assess NO levels, a key signalling molecule involved in various physiological and pathological processes, including inflammation and pain. Our results indicated that Phillygenin (PHI) treatment significantly reduced NO levels in CCI rats. This reduction is particularly noteworthy, as elevated NO levels are often associated with increased pain sensitivity and inflammatory responses in neuropathic pain models. The decrease in NO levels suggests that PHI may exert an analgesic effect by modulating the nitric oxide signalling pathway. Elevated NO contributes to pain by enhancing nociceptive signalling and promoting neuroinflammation. By lowering NO levels, PHI may help restore the balance of neuroinflammatory mediators, thereby alleviating pain sensations. Furthermore, the reduction of NO could also imply potential protective effects on neuronal health, as excessive NO can lead to oxidative stress and neuronal damage. This aspect is crucial, as it highlights PHI’s dual role in alleviating pain through anti-inflammatory effects and promoting neuronal survival in a neuropathic context.
Previous publications have investigated the role of the TLR4 and its downstream MyD88/NF-κB signalling in inflammation and neuropathic pain. Wang et al. indicated that the upregulation of the TLR4 and MyD88/NF-κB signalling participated in the development of inflammation and contributed to vascular dementia 24. MicroRNA-27a modulated the TLR4 and MyD88/NF-κB signalling, further decreasing proinflammatory cytokine levels and ameliorating acute lung injury 25. We referenced the findings of Liu et al., who suggested that activating the GABA receptor, specifically the GABAA receptor, might inhibit TLR4 and MyD88/NF-κB signalling pathways, thereby ameliorating the progression of diabetic neuropathic pain 26. Our results indicate that PHI influences the TLR4 expression and inhibits MyD88/NF-κB signalling, highlighting its crucial role in neuropathic pain.
In our study, we conducted behavioral tests to assess mechanical allodynia and thermal hyperalgesia in CCI rats following PHI treatment. A one-way ANOVA was initially applied to establish differences between the groups, and this analysis is represented in the bar graphs of Fig. 1. These graphs illustrate each group’s total magnitude of mechanical allodynia and thermal hyperalgesia. Additionally, the line graphs in the same figure display intriguing patterns of changes in nociceptive variables over time. Given the temporal nature of these data, we agree that a time-oriented analysis would enhance the depth of our results. Thus, we will perform a two-way ANOVA to analyze the time-dependent effects of PHI on these behavioral measures in our future studies. This approach will allow us to examine both the treatment and time factors, providing a more comprehensive understanding of the dynamics of pain response. The initial analysis using one-way ANOVA provided valuable insights into the overall effects of PHI on mechanical allodynia and thermal hyperalgesia. However, the rich temporal data displayed in the line graphs suggested that a more nuanced approach could be beneficial. A two-way ANOVA is more appropriate for analyzing changes across time points and treatment conditions.
By incorporating this analysis, we can better interpret the interaction effects of treatment and time on pain responses, enhancing our understanding of the pharmacodynamics of PHI. In acknowledging the potential limitations of our study, several factors warrant consideration. Although we used 40 rats divided into four groups, a larger sample size may have provided more robust data and enhanced the statistical power of our findings. Smaller sample sizes can lead to variability and may not fully capture the effects observed. Other factors, such as environmental stressors, the time of day, and individual animal differences, could influence pain responses. While we attempted to control for these variables, they may still introduce bias or variability in our results.
Our study demonstrated that PHI treatment could reduce TLR4 levels and attenuate the inflammatory status in CCI rats, indicating that targeting the TLR4 and its downstream MyD88/NF-κB signalling might be a viable therapeutic strategy for neuropathic pain. However, our study needed further investigation on other inflammatory factors and molecular pathways related to PHI and neuropathic pain to explore its underlying mechanism further.












 uBio
uBio 


