Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Acta Botánica Venezuelica
Print version ISSN 0084-5906
Acta Bot. Venez. vol.28 no.2 Caracas 2005
VEGETATIVE ANATOMY OF ARATITIYOPEA LOPEZII (XYRIDACEAE)
Anatomía vegetativa de Aratitiyopea lopezii (Xyridaceae)
Lisa M. CAMPBELL and Dennis W. STEVENSON
The New York Botanical Garden, Bronx, NY 10458, U.S.A. lcampbell@nybg.org, dws@nybg.org
ABSTRACT
The center of generic diversity of the monocot family Xyridaceae is northern South America, where four of the five genera are endemic. Many species of these four small genera have restricted distributions and have not been critically studied, including the monospecific genus Aratitiyopea. Populations of this unusual Xyridaceae occur in mid-elevation, forested montane sites. Most Xyridaceae occur in exposed habitats, and although they are at least seasonally wet, many species exhibit xeromorphic adaptations. In contrast, Aratitiyopea presents a morphology and internal anatomy of a mesophyte (trailing stems, leaves with a relatively broad lamina, little mechanical tissue, and lacking a hypodermis, the latter two features present in other Xyridaceae with bifacial leaves) as well as effects of seasonal drying (cell death in the root and thickened endodermal cell walls). Structural details of the vegetative body of Aratitiyopea are presented here, and compared to that known for other Xyridaceae. Although the "gestalt" of Aratitiyopea is aberrant in Xyridaceae, it is structurally consistent with the family, and unusual features are interpreted as adaptations to its habitat, which few other Xyridaceae occupy.
Key words: Xyridaceae, Aratitiyopea, Guiana Shield, Guayana, Mesophyte,Vegetative anatomy, Xerophyte
RESUMEN
El centro de mayor diversidad genérica de la familia Xyridaceae se encuentra al norte de América del Sur, donde se han detectado cuatro géneros endémicos de los cinco reconocidos en la familia. Muchos taxones representativos de estos géneros endémicos tienen una distribución geográfica restringida y no se han estudiado críticamente, incluyendo la única especie del género Aratitiyopea. Poblaciones de esta Xyridaceae extraordinaria se encuentran a elevaciones medias en bosques montañosos. La mayoría de las Xyridaceae se encuentran en ambientes expuestos, oligotróficos y que a pesar de ser al menos estacionalmente húmedos, muchas especies muestran adaptaciones xeromórficas. En contraste, Aratitiyopea presenta características morfológicas y de anatomía interna particulares de un mesófito (tallos rastreros, hojas con una lámina relativamente ancha, poco tejido mecánico y la falta de una hipodermis presente en otras Xyridaceae con hojas bifaciales) así como efectos de resecamiento estacional (muerte de células en la raíz y engrosamiento de las paredes celulares endodermales). En este trabajo se presentan estudios morfológicos y anatómicos de las partes vegetativas de Aratitiyopea y se comparan con estudios previos de otros miembros de las Xyridaceae. Aunque el aspecto general de Aratitiyopea es aberrante en Xyridaceae, el género es estructuralmente consistente con la familia, y los caracteres poco usuales son interpretados como adaptaciones a su ambiente típico que comparte con muy pocas otras Xyridaceae.
Palabras clave: Xyridaceae, Anatomía vegetativa, Aratitiyopea, Escudo de Guayana, Guayana, Mesófito, Xerófito
INTRODUCTION
Xyridaceae are a medium-sized family of petaloid monocots currently included in Poales s.l. (Chase et al. 2000; Soltis et al. 2000; APG II 2003). As presently understood, the family comprises about 385 species in five genera (Campbell 2004a), with a mostly pantropical distribution and with the greatest species diversity in South America (Wanderley 1992; Kral 1998; Campbell 2004b). About ninety percent of the species are in the wide spread genus Xyris; the other four genera, Abolboda (ca. 22 spp.), Achlyphila (1 sp.), Aratitiyopea (1 sp.), and Orectanthe (2 spp.) are known only from northern South America, where many species are endemic to the Guiana Shield, especially the tepuis of the
Roraima formation. These latter species are among the most enigmatic xyrids, due to the inaccessibility of the remote mesas on which they occur. Relationships amongst Xyridaceae, and of Xyridaceae to other Poales are not yet well established. Although formal systems of classification recognize a single family (Cronquist 1981; Takhtajan 1997; Thorne 2000; APG II 2003), it has long been recognized as a morphologically diverse assemblage (Maguire & Wurdack 1960; Dahlgren et al. 1985), and some cladistic analyses do not resolve the family as monophyletic (Michelangeli et al. 2003; Davis et al. 2004).
Xyridaceae are adapted to a variety of habitats that are oligotrophic, and at least seasonally wet, such as sand savannas, bogs, and rocky outcrops. Even species that occur in constantly wet sites have probably acquired strategies to overcome restricted water availability due to rapidly draining soils (e.g. sandy soils), or physiological constraints (e.g. high acidity; Seddon 1974). Basic anatomical features of the family include strengthening tissue common in all vegetative organs; roots with a persistent exodermis, endodermal cell walls with conspicuous U-shaped thickenings (Van Fleet 1961); stems with amphivasal vascular bundles and leaf traces invested in a sheath of sclerenchyma; and leaves unifacial or bifacial, the mesophyll without or with only a weakly developed palisade layer and air spaces, vascular bundles with a bilayered sheath, the inner strengthening (Carlquist 1960; Tomlinson 1969; Wanderley 1992).
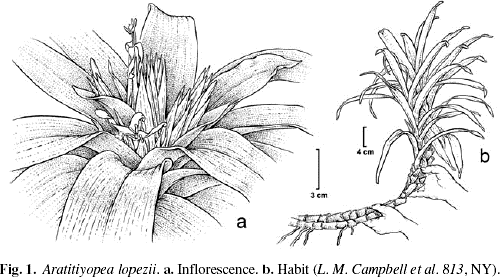
The genus Aratitiyopea was relatively recently described for the transference of a single species of Bromeliaceae, Navia lopezii L.B. Sm. (Steyermark 1984). Although Bromeliaceae are not generally considered closely related to Xyridaceae (Thorne 2000; Davis et al. 2004), the "gestalt" of Aratitiyopea lopezii (L.B. Sm.) Steyerm. & P.E. Berry is strikingly that of Navia (Kral 1992; Campbell 2004b), a moderate-sized genus of Bromeliaceae endemic to the Guayana region (Holst & Luther 2004). Plants of Aratitiyopea lopezii are herbaceous perennials with long, decumbent stems that terminate in a congested inflorescence (Fig. 1). Most other Xyridaceae are characterized by a stout caudex, ranked or spiraled leaves with short internodes, and a long naked, or occasionally bracteate, peduncle that bears the inflorescence. Isolated populations of Aratitiyopea occur in semideciduous forests in areas of near highest annual precipitation for the region (2.800–4.000 mm; Campbell 2004b), that are located on ancient sandstone and granite of the Guiana Shield and also on the geologically similar, although younger (Foster & Beltran 1997), Cordillera del Cóndor in northern Peru. The habit and nidular inflorescence, with large flowers and colorful bracts, is strikingly different from species in the more familiar genera Xyris and Abolboda (Campbell & Stevenson, in press). Although Aratitiyopea was known to share with some Xyridaceae a uniseriate androecium, appendages on the gynoecium, and inaperturate, spinose pollen (Steyermark 1984), the species remained poorly characterized (Rudall & Sajo 1999) until recent collections allowed more detailed investigations (Campbell 2004b; Campbell & Stevenson, in press). Here we present aspects of the vegetative structure of Aratitiyopea and compare it to that known for other Xyridaceae.
MATERIALS AND METHODS
Observations are based on field studies and specimens that were fixed in formalin-propionic acid-alcohol (1:1:18 v/v, 50% ethanol), and later transferred to 70% ethanol (L. M. Campbell et al. 734, 766, 813 [NY, TFAV, VEN]). Additionally, specimens of Aratitiyopea from the following herbaria have been studied: COL, F, GH, K, MO, NY, SEL, US, and VEN. Some stems were sectioned using a sliding microtome, and the presence of lignin was detected with saturated phloroglucinol acidified with HCl (Jensen 1962). Other sectioned material (including some stems) was embedded in Paraplast ® Plus (Oxford Labware, St. Louis, Missouri, USA) using standard procedures, sectioned with a rotary microtome, and stained with safranin and astra blue. Leaves were cleared with 5% NaOH, followed by 10% commercial bleach, and stained in safranin (Ruzin 1999). Terminology of epidermal analysis follows Dilcher (1974).
For scanning electron microscopy, specimens were dissected in 70% ethanol, dehydrated in a series to 100% acetone and critical point dried. Samples were coated with gold palladium in a Hummer 6.2 sputtering system and viewed with a JEOL JSM-5410LV scanning electron microscope.
RESULTS AND DISCUSSION
Plant
Perennial, densely rooting proximally; rhizome long-trailing, decumbent, leaves dorsiventral, spirally inserted, cauline, internodes shorter distally, where the plants are rosulate and bromeliad-like, rarely sympodially branched distally from the terminal inflorescence (Fig. 1).
Root
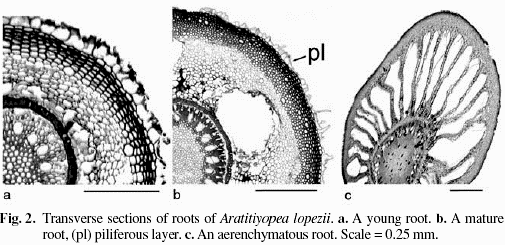
Epidermis thin walled, persistent, cells of the same length. Roots densely covered by uniseriate, unicellular hairs, hairs from the center of the cell (Type 1 development, without specialized cells [Leavitt 1904]). Exodermis many cell layers thick, peripherally the cells in radial files, compact, of a similar size, thick-walled and heavily lignified, staining red with safranin (Fig. 2). Cortical cells thinner-walled, and increasing in size near the endodermis (Fig. 2), some areas of cell collapse (Fig. 2b). Aerenchyma present in some roots (Fig. 2c). Endodermis very conspicuous of thick-walled cells, staining bright orange with safranin, the small lumen near the exterior (Fig. 2a, b). Central cylinder containing a continuous pericycle, polyarch, the vessels forming a single ring with no central vessels present, and little or no phloem. Toward the endodermis vessels invested in very thick-walled fibers with minute lumina, the cells grading to sclerenchyma, that of the wide pith weakly lignified (Fig. 2).
Dense root hairs and a multilayered exodermis are present in many Xyridaceae (Carlquist 1960; Tomlinson 1969; Campbell 2004b). The roots of Aratitiyopea present a relatively wide cortex and pith, typical of mesophytes (Fahn & Cutler 1992). Within the family, variation in the cortex includes the presence in some species of stellate cells, or short cells in transverse rows (Xyris and Abolboda; Carlquist 1960; Tomlinson 1969) and the degree to which intercellular spaces are developed. The patches of cell death between the exo- and endodermis appear to be the result of seasonal drought (Dickison 2000) that most Xyridaceae experience, whereas well-developed aerenchyma found in some roots of Aratitiyopea probably form as a response to periods of saturation. Thickened endodermal cell walls are common among monocots, as well as other vascular plants growing in adverse conditions (Van Fleet 1961), and characterize roots of Xyridaceae (Malme 1924; Carlquist 1960; Tomlinson 1969). The histochemical distinction between endodermal and other lignified cells detected in Aratitiyopea was also observed in Achlyphila (Carlquist 1960), and may represent an adaptation to conditions of high elevation habitats. Vessel are confined to the periphery of the central cylinder in most of the family, but are distributed uniformly throughout the stele in Orectanthe (L.M. Campbell, unpubl. data) and some Abolboda species (Carlquist 1960).
Rhizome
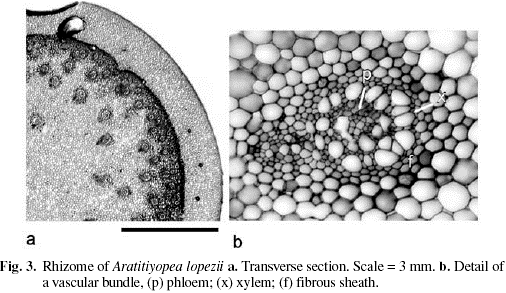
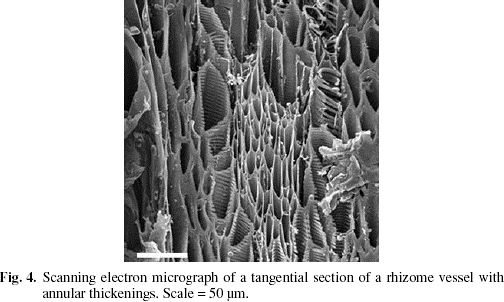
Epidermis with a thin cuticle. Epidermal cells square in shape in transverse section, the outer periclinal walls slightly sunken, the anticlinal walls slightly protruding, without suberin. Cortex narrow, composed of dense, starchless, isodiametric parenchyma cells, and delimited by a multi-layered sheath of small, thick-walled sclereids (Fig. 3). Internal to this sheath, the majority of the vascular bundles are embedded in lignified ground tissue, the cells of which become progressively larger and more parenchymatous centripetally. A few vascular bundles also occurring near the center of the stem. The bundles amphivasal and surrounded by a sheath of lignified cells. Vessels with annular thickenings (Fig. 4). The central pith broad, composed of dense, unlignified, starchless parenchyma.
Most Xyridaceae have a short vertical stem, with short internodes and the leaves usually forming a congested rosette. Species occurring in some edaphic conditions (e.g. sand savannas) have a high below ground to above ground biomass ratio (Campbell 2004b). Longer stems and internodes are most pronounced in taxa that occur in high elevation scrub vegetation: Aratitiyopea, Orectanthe ptaritepuiana (Steyerm.) Maguire, Achlyphila disticha Maguire & Wurdack, and some Xyris spp. (e.g. Xyris ptariana Steyerm.).
A strengthening schlerenchymatous sheath demarcating the central cylinder, and surrounding the leaf traces found in Aratitiyopea characterize stems of most Xyridaceae (Carlquist 1960; Tomlinson 1969). Within the family, variation occurs in the relative width of cortex, whether peripheral cells are suberized, the presence of spongy ground tissue in some Xyris and the small species of Abolboda, and presence of a sclerenchymatous central pith in Orectanthe sceptrum (Oliv. ex Thurn) Maguire (Carlquist 1960).
Leaf
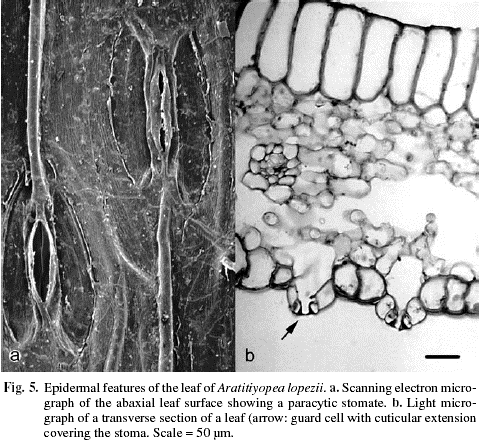
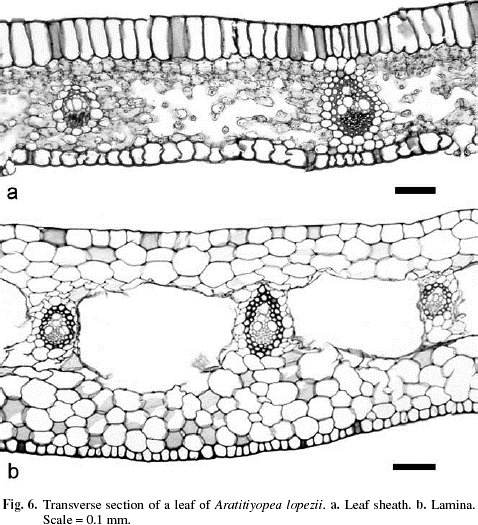
Leaf sheath open, short and broad; lamina linear-lanceolate and falcate, distally shortly cymbiform, the apex terete, margins entire. Surface area of a median leaf 49.4 cm2. Epidermis with a thin cuticle, without epicuticular wax (Fig. 5), glabrous. In transverse section, epidermal cell walls evenly thickened, epidermal cells colorless and of two types. Those of the adaxial epidermis elongate (Fig. 6a), approximately twice the height of intercostal abaxial epidermal cells. In surface view rectangular, the anticlinal wall undulate. Cells of the abaxial epidermis more or less square in transverse section, the anticlinal walls irregular in shape, cells over the costae smaller. In surface view rectangular to hexagonal, the end walls mostly truncate, sometimes oblique. Stomata abaxial (hypostomatic), randomly arranged, frequency of 12 per mm2, more or less flush with the epidermis, the stomatal complex about twice as long as broad, paracytic (Fig. 5a), without the subsidiary cells completely enclosing the guard cells (brachyparacytic), subsidiary cells with numerous chloroplasts, the outer periclinal walls of the guard cells thickened, with cuticular extensions forming an outer stomatal ledge over the aperture (Fig. 5b), apertures average 5.49 x 51.97 µm. Along the leaf margins a narrow band of fibers forms mechanical tissue.
Mesophyll not differentiated into an adaxial palisade parenchyma, with numerous intercellular spaces (Fig. 6a), including substomatal chambers (Fig. 5b). Parenchyma adjacent to the dermal layers more or less isodiametric, those of the spongy central tissue irregular in shape and periclinally elongate. Vascular bundles equidistant from each surface, surrounded by a sclerenchymatous sheath of 1- or 2-cell layers, which is surrounded by a single layer of large, colorless parenchyma. Together with mesophyll cells, the sheath forms a girder between the ad- and abaxial surfaces. Vascular bundles smaller in diameter alternate with ones larger in diameter. A few compound vascular bundles, surrounded by a common sclerenchymatous sheath occur. In surface view, cross anastomoses infrequent, diverging at acute angles. In the terete tip, three large vascular bundles consisting mostly of xylem, are invested in dense chlorenchyma.
The leaf sheath is broad and without a distinct articulation at the lamina. In transverse section, the cells of abaxial epidermis have somewhat thickened outer periclinal walls, and are shorter in length than those of the adaxial epidermis. The mesophyll is composed of tightly arranged, isodiametric, colorless parenchyma with regularly spaced intercostal lacunae (Fig. 6b). No starch was observed.
The genera of Xyridaceae are often considered in two groups, which correspond to leaf morphology and phyllotaxy (Nakai 1943; Rudall & Sajo 1999; Thorne 2000). Xyris and Achlyphila have unifacial leaves that are ranked, and Aratitiyopea, Abolboda, and Orectanthe have bifacial leaves that are spirally inserted. The latter two genera also share the presence of a multi-layered, colorless, adaxial hypodermis in the lamina; an abaxial hypodermis is also present in Orectanthe sceptrum (Carlquist 1960).
Aratitiyopea occurs in a moister, shadier habitat than most Xyridaceae, and has a relatively thin lamina that contains less supportive tissue. Despite this, there is no distinct palisade parenchyma typical of mesophytes (Esau 1965). The internally elongate adaxial epidermal cells in Aratitiyopea comprise a substantial portion of the leaf and may function in water storage (cf. Eriocaulaceae, Tomlinson 1969; Roth 1990), as does the hypodermis in other Xyridaceae. Aratitiyopea shares with representatives of all the genera except Achlyphila the presence of irregularly shaped mesophyll chlorenchyma (Carlquist 1960; Tomlinson 1969), and has a mesophyll similar to that of Abolboda and Orectanthe: spongy in the lamina and dense and lacunate in the sheath.
Most Xyridaceae have xeromorphic adaptations, such as sclerophylly, in response to the intense insolation and lack of continually available optimal water levels they incur. Sclerophylly is also associated to deficiency of some minerals (Specht 1979; Medina et al. 1990), and the habitats Xyridaceae occupy are typically nutrient poor (e.g. acidic peat soils and sand savannas). Thickened cell walls and fibrous bundle sheath extensions present in the leaves of many Xyridaceae (Tomlinson 1969) provide mechanical support when turgor pressure is low. Epidermal cells of many Xyridaceae have either uniformly thickened walls, or thickened periclinal walls, which are rugose or papillate in Achlyphila and some Xyris species (Carlquist 1960; Tomlinson 1969).
Cell inclusions
No tannins, calcium oxalate crystals, or starch were detected in the material observed in Aratitiyopea.
Epidermal cells of some Xyridaceae include tannins, and silica sand is found in some species, either in the rhizome (Abolboda) or leaf (Xyris, Tomlinson 1969). The fact that starch was depleted in the material of Aratitiyopea examined could be because the specimens collected were flowering and/or fruiting. Some Xyris species accumulate abundant starch in the leaf base (Tomlinson 1969; L.M. Campbell, unpubl. data). Mucilage may function in water storage (Roth & Lindorf 1991; Fahn & Cutler 1992), and amongst Xyridaceae is known only to be present in the genus Xyris (Campbell 2004b) where it is associated with leaf axil hairs (Tomlinson 1969).
CONCLUSIONS
Aratitiyopea lopezii is an unusual Xyridaceae that exhibits numerous adaptations to the moist, montane forest sites it occupies. Although populations occur in areas that are moist compared to sites in which most other Xyridaceae occur (Campbell 2004b), plants of Aratitiyopea experience periods of relative dryness, as well as periods of saturation. Thus, the vegetative anatomy presented by Aratitiyopea is interpreted as a combination of features found in mesophytes, such as broad, thin laminas, as well as adaptations to prevent water loss, for example a water-storing leaf epidermis. In contrast, most other Xyridaceae occur in exposed habitats with high insolation, and only seasonal water availability, and exhibit xeromorphic adaptations, including structural rigidity, reduction in stem and leaf size, and a foliar hypodermis.
Although Aratitiyopea had been considered aberrant in Xyridaceae (M. Wanderley, pers. com.), the increasing body of data (Campbell & Stevenson, in press; Campbell 2004b) indicates that Aratitiyopea exhibits ecological adaptations within the continuum of structural variation found in Xyridaceae. In gross vegetative morphology, plants of Aratitiyopea are most similar to those of the more robust genus Orectanthe, particularly the longer-stemmed species, O. ptaritepuiana (Steyermark 1984; Campbell 2004b), which scrambles over tepuian vegetation (Huber 1992; O. Huber, pers. com.). The genera of Xyridaceae with bifacial leaves share numerous vegetative as well floral features (Carlquist 1960; Steyermark 1984; Campbell 2004b; Campbell & Stevenson, in press). However, anatomical (Carlquist 1960) and morphological (Takhtajan 1997; Campbell 2004b) data do not support division of the family into groups characterized by leaf morphology, and the relationship of Achlyphila to other Xyridaceae needs further evaluation.
ACKNOWLEDGEMENTS
We are grateful for logistic support in Venezuela that was provided by: the Instituto Nacional de Parques (INPARQUES); Oficina de Asuntos Indígenas del Ministerio de Educación; the Ministerio del Ambiente y de los Recursos Naturales Renovables; the Gobernación del Estado Amazonas; Grupo Aéreo de las Fuerzas Armadas de Cooperación; G.A. Romero-González (HUH); Carlos Gómez (Puerto Ayacucho); Luis Alvarez (TFAV); and the community of Raudal de Danto, Amazonas state, Venezuela. Fieldwork was conducted with funds from The Explorers' Club, New York, NY; The Fund for Neotropical Plant Research, Institute of Systematic Botany, The New York Botanical Garden; The International Association for Plant Taxonomy; Ph.D. Alumni Association Dissertation Support Fund, City University of New York. We thank the curators and staff of the herbaria cited for use of their collections. Bobbi Angell skillfully prepared the illustration in Figure 1. Ivonne Sánchez del Pino (NY, MEXU) kindly translated the abstract into Spanish. An anonymous reviewer provide suggestions that allowed great improvement to the manuscript.
BIBLIOGRAPHY
1. APG II. 2003. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. J. Linn. Soc., Bot. 121: 399–436. [ Links ]
2. Campbell, L.M. 2004a. Xyridaceae. In: Flowering Plants of the Neotropics (Smith, N., S.A. Mori, A. Henderson, D.W. Stevenson & S.V. Heald, eds.), pp. 492–493. Princeton University Press, Princeton, NJ. [ Links ]
3. Campbell, L.M. 2004b. Anatomy and systematics of Xyridaceae with special reference to Aratitiyopea. Ph.D. Dissertation. City University of New York. [ Links ]
4. Campbell, L.M. & D.W. Stevenson. In press. Inflorescence architecture and floral morphology of Aratitiyopea lopezii (Xyridaceae). Proceedings of the Third International Conference on the Comparative Biology of the Monocotyledons. [ Links ]
5. Carlquist, S. 1960. Anatomy of Guayana Xyridaceae: Abolboda, Orectanthe, and Achlyphila. Mem. New York Bot. Gard. 10(2): 65–117. [ Links ]
6. Chase, M.W., D.E. Soltis, P.S. Soltis, P.J. Rudall, M.F. Fay,W.H. Hahn, S. Sullivan, J. Joseph, M. Molvray, P.J. Kores, T.J. Givnish, K.J. Sytsma & J.C. Pires. 2000. Higher-level systematics of the monocotyledons: an assessment of current knowledge and a new classification. In: Monocots: Systematics and Evolution (Wilson, K.L. & D.A. Morrison, eds.), pp. 3–16. CSIRO Publishing, Collingwood. [ Links ]
7. Cronquist, A. 1981. An integrated system of classification of flowering plants. Columbia University Press, New York. [ Links ]
8. Dahlgren, R.M.T., H.T. Clifford & P.F. Yeo. 1985. The families of the Monocotyledons. Springer-Verlag, New York. [ Links ]
9. Davis, J.I., D.W. Stevenson, G. Petersen, O. Seberg, L.M. Campbell, J.V. Freudenstein, D.H. Goldman, C.R. Hardy, F.A. Michelangeli, M.P. Simmons, C.D. Specht, F.Vergara-Silva & M.A. Gandolfo. 2004. A phylogeny of the monocots, as inferred from rbcL and atpA sequence variation, and a comparison of methods for calculating jackknife and bootstrap values. Syst. Bot. 29: 467–510. [ Links ]
10. Dickison,W.C. 2000. Integrative plant anatomy. Academic Press, San Diego, ca. [ Links ]
11. Dilcher, D.L. 1974. Approaches to the identification of angiosperm leaf remains. Bot. Rev. 40: 1–157. [ Links ]
12. Esau, K. 1965. Plant Anatomy. Second Edition. John Wiley & Sons, Inc., New York. [ Links ]
13. Fahn A. & D.F. Cutler. 1992. Xerophytes. Gebrüder Borntraeger, Berlin. [ Links ]
14. Foster, R.B. & H. Beltran. 1997. Vegetation and flora of the eastern slopes of the Cordillera del Cóndor. In: (Schulenberg, T.S. K. Awbery & G. Fabregas, eds.) pp. 44–63. The Cordillera del Cóndor region of Ecuador and Peru: A biological Assessment. Conservation International, Washington, DC. [ Links ]
15. Holst, B.K. & H.E. Luther. 2004. Bromeliaceae. In: Flowering plants of the Neotropics (Smith, N., S.A. Mori, A. Henderson, D.W. Stevenson & S.V. Heald, eds.), pp. 418–421. Princeton University Press, Princeton, NJ. [ Links ]
16. Huber, O. 1992. El Macizo del Chimantá. Oscar Todtmann Editores, Caracas. [ Links ]
17. Jensen,W.A. 1962. Botanical Histochemistry. W. H. Freeman & Co., San Francisco. [ Links ]
18. Kral, R. 1992. A treatment of American Xyridaceae exclusive of Xyris. Ann. Missouri Bot. Gard. 79: 819–885. [ Links ]
19. Kral, R. 1998. Xyridaceae. In: (K. Kubitzki, ed.) pp. 461–469. The Families and genera of vascular plants. Vol. IV. Alismatanae and Commelinanae (except Gramineae). Springer, New York. [ Links ]
20. Leavitt, R. G. 1904. Trichomes of the root in vascular cryptogams and angiosperms. Proc. Boston Soc. Nat. Hist. 31: 273–313. [ Links ]
21. Maguire, B. & J.J. Wurdack. 1960. Xyridaceae. In: (Maguire, B., J.J. Wurdack & Collaborators, eds.), pp. 1–19. The botany of the Guayana HighlandPart IV. Mem. New York Bot. Gard. 10(2): 1–37. [ Links ]
22. Malme, A.N. 1924. Xyridologische beiträge. Ark. Bot. 19(13): 1–8. [ Links ]
23. Medina, E., V. García & E. Cuevas. 1990. Sclerophylly and oligotrophic environments: relationships between leaf structure, mineral nutrient content, and drought resistance in tropical rain forests of the upper Río Negro region. Biotropica 22: 51–64. [ Links ]
24. Michelangeli, F.A., J.I. Davis & D.W. Stevenson. 2003. Phylogenetic relationships among Poaceae and related families as inferred from morphology, inversions in the plastid genome, and sequence data from mitochondrial and plastid genomes. Amer. J. Bot. 90: 93–106. [ Links ]
25. Nakai, T. 1943. Ordines, familiae, tribi, genera, sectiones, species, varietates, formae et combinationes novae a Prof. Nakai-Takenosin adhuc ut novis edita. Appendix. Imperial University, Tokyo. [ Links ]
26. Roth, I. 1990. Leaf structure of a Venezuelan cloud forest. Handbuch der Pflanzenanatomie Bd. 14, 1. Borntraeger, Berlin. [ Links ]
27. Roth, I. & H. Lindorf. 1991. Leaf structure of two species of Pachira indigenous of Venezuela from different habitats. Bot. Jahrb. Syst. 113: 203–219. [ Links ]
28. Rudall, P.J. & M.G. Sajo. 1999. Systematic position of Xyris: flower and seed anatomy. Int. J. Plant Sci. 160: 795–808. [ Links ]
29. Ruzin, S.E. 1999. Plant Microtechinque and Microscopy. Oxford University Press, New York. [ Links ]
30. Seddon, G. 1974. Xerophytes, xeromorphs, and sclerophylls: the history of some concepts in ecology. Biol. J. Linn. Soc. 6: 65–87. [ Links ]
31. Soltis, D.E., P.S. Soltis, M.W. Chase, M.E. Mort, D.C. Albach, M. Zanis, V. Savolainen, W.H. Hahn, S.B. Hoot, M.F. Fay, M. Axtell, S.M. Swensen, L.M. Prince, W.J. Kress, K.C. Nixon & J.S. Farris. 2000. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. J. Linn. Soc. Bot. 133: 381–461. [ Links ]
32. Specht, R.L. 1979. Heathlands and related shrublands of the world. In: Ecosystems of the World (Specht, R.L., ed.), pp. 1–18. Vol. 9A. Elsevier Publ. Co., Amsterdam. [ Links ]
33. Steyermark, J.A. 1984. Flora of the Venezuelan GuayanaI. Ann. Missouri Bot. Gard. 71: 297–340. [ Links ]
34. Takhtajan, A. 1997. Diversity and Classification of Flowering Plants. Columbia University Press, New York. [ Links ]
35. Thorne, R.T. 2000. The classification and geography of the monocotyledon subclasses Alismatidae, Liliidae, and Commelinidae. In: Plant Systematics for the 21st Century (Nordenstam, B., G. El-Ghazaly, M. Kassas & T.C. Laurent, eds.), pp. 75–124. Portland Press, London. [ Links ]
36. Tomlinson, P.B. 1969. Anatomy of the Monocotyledons. Vol. III. Commelinales–Zingiberales. Clarendon Press, Oxford. [ Links ]
37. Van Fleet, D.S. 1961. Histochemistry and function of the endodermis. Bot. Rev. 27: 165–220. [ Links ]
38. Wanderley, M.G.L. 1992. Estudos Taxonômicos no Gênero Xyris L. (Xyridaceae) da Serra do Cipó, Minas Gerais, Brasil. Ph.D. Dissertation. Instituto de Biociências, São Paulo. [ Links ]