Acta Botánica Venezuelica
versión impresa ISSN 0084-5906
Acta Bot. Venez. v.33 n.1 Caracas jun. 2010
Morphology and anatomy of flowers of Dalechampia stipulacea Müll.Arg. (Euphorbiaceae)
Morfología y anatomía de flores de Dalechampia stipulacea Müll.Arg. (Euphorbiaceae)
Luiz Antonio de Souza, Aparecido Caetano da Silva e Ismar Sebastião Moscheta
Universidade Estadual de Maringá, Departamento de Biologia, Avenida Colombo, 5790, Maringá, Paraná, Brasil lasouza@uem.br
RESUMEN
Las flores de Dalechampia han sido reportadas como modelo para los estudios de evolución floral. Sin embargo, la literatura registra escasos estudios sobre la anatomía floral de estas plantas. El análisis estructural de las inflorescencias y flores de Dalechampia stipulacea es el objetivo del trabajo. El pseudanto consiste en inflorescencias masculinas y femeninas con dos brácteas grandes y flores monoclamídeas. En la inflorescencia masculina hay una glándula resinosa. En las brácteas y flores se encuentran tricomas no glandulares, tricomas glandulares y glándulas. Las brácteas y el perianto no presentan parénquima en empalizada. La antera es tetrasporangiada con la pared formada por epidermis, endotecio, capa media y tapete secretor. En el ovario se observaron dos meristemas, el adaxial con origen epidérmico y otro intermedio proveniente del mesófilo. El óvulo es anátropo, bitegumentado y crasinucelar. El estilo tiene el tejido transmisor central con una hendidura reducida. Los resultados anatómicos obtenidos son comparados con las flores e inflorescencias de otras especies del género y discutidos en relación con la ecología de la polinización.
Palabras clave: Bráctea, Dalechampia, Euphorbiaceae, flor femenina, flor masculina, perigonio, pseudanto
ABSTRACT
The flowers of Dalechampia species have been reported as a model for the study of floral evolution. However, the literature does not record floral anatomical studies of these plants. The structural analyses of inflorescences and flowers of Dalechampia stipulacea was the objective of this paper. The pseudanthium consists of male and female inflorescences with two large bracts and monochlamydeous flowers. In the male inflorescence there is a resinous gland. In the bracts and flowers occurred nonglandular, glandular trichomes and glandular emergencies. The bracts and perianth without palisade parenchyma. The anther is tetrasporangiate with wall formed by epidermis, endothecium, middle layer and secretory tapetum. In the ovary wall meristematic regions were observed in the epidermis and mesophyll regions. The ovule is anatropous, bitegmic and crassinucellate. The style has central transmitting tissue with a reduced rift. The obtained anatomical results are compared with flowers of other species of the genus and discussed in relation to pollination ecology.
Key words: Bract, Dalechampia, Euphorbiaceae, female flower, male flower, perigone, pseudanthium
Recibido: 03/12/2008
Aceptado: 30/11/2009
INTRODUCTION
The neotropical species of Dalechampia Plum. ex L. (Euphorbiaceae) constitute a model system for the study of floral evolution. This system was chosen because it comprises a tractable number of closely related species exhibiting considerable variation in pollination ecology. The pseudanthia of most Dalechampia species secrete a triterpene resin that is collected by bees that use resin in nest construction. The resin-reward system apparently evolved by modification of preexisting resin-secretion system that defended the flowers from herbivores (Armbruster 1996).
Most of the Dalechampia species are also noteworthy for the presence of stinging trichomes in the flowers and other plant organs, for which they are commonly called pó-de-mico, cipó-urtiga, urtiga and urtiga-de-boi. The pseudanthium inflorescence that occurs in the genus is also considered unique among Euphorbiaceae (Maia et al. 2002).
The flower morphology of Dalechampia species has been presented in several investigations of taxonomic nature, in pollination studies or floral biology (Graner 1942; Capinpin & Bruce 1955; Webster & Webster 1972; Armbruster & Webster 1979; Armbruster 1996; Sazima et al. 1985; Armbruster et al. 1992; Freitas et al. 2001; Silva et al. 2001; Maia et al. 2002). Anatomical studies of flowers of Euphorbiaceae are scarce, but include notably studies of: Manihot utilissima Pohl (Manihot esculenta Crantz) microsporogenesis and megasporogenesis (Graner 1935); ontogenetic and structural analysis of the Manihot utilissima flowers (calyx, stamen and gynoecium) (Toledo 1963); ovule integuments, chalaza and vascularization of Crotonoideae, Euphorbioideae and Acalyphoideae (Tokuoka & Tobe 1998, 2002, 2003); and Croton sarcopetalus Müll.Arg. floral and extrafloral nectaries (Freitas et al. 2001). Therefore, the present investigation has as objective the morphological and anatomical analysis of the Dalechampia stipulacea Müll.Arg. inflorescence which is a liana that occurs at high frequency in forest remnants of the Parana, Brazil.
MATERIAL AND METHODS
Inflorescences of two Dalechampia stipulacea were collected at the forest remnant (Bosque dos Pioneiros), in Maringa, Brazil (state of Parana). Voucher materials were deposited at the UEM Herbarium, collection number: K.S. Mourão 11720 and L.A. Souza 11746.
Morphological analyses of the inflorescences, flowers and floral buds were conducted with fresh and fixed material. The terminology used here follows that of Sazima et al. (1985), Weberling (1992), Armbruster (1996) and Maia et al. (2002).
The Fosters (1950) clearing technique was used for study of venation pattern in the bracts. The terminology of venation pattern was based on Hickey (1979).
Anatomical preparations of inflorescences were made from fixed material in FAA (formalin, acetic acid, ethanol), dehydrated through alcohol series, embedded in hydroxymethacrylate (Gerrits 1991), sectioned via rotary microtome (cross- and longitudinal sections), and stained following the toluidine blue 0,05% in phosphate buffer pH 4,7 technique (OBrien et al. 1964).
Photomicrographs were prepared using an Olympus BX50 optical microscope and Nova Optical Systems stereoscope fitted with a digital Canon Power Shot A95 camera and subsequently prepared using software Zoom Browser EX 4.6. RESULTS AND DISCUSSION
Inflorescence and flower morphology
The monoecious plant of D. stipulacea possesses a pseudanthium inflorescence with two white or yellow-greenish bracts which subtend the staminate and pistillate cymes. Bracts are ovate with round and slightly cordate bases, and 3, 4-lobed apices (Fig. 1a). The bracts have actinodromous venation with seven primary veins (five thick and two fine veins) diverging radially from a single point; the veins have basal position and marginal development (Fig. 1a). The bracts have trichomes and stalked glands.
In Dalechampia the inflorescence is a bilaterally symmetrical pseudanthium, a unique configuration within the Euphorbiaceae. The sequence of changes in color and position of the bracts during inflorescence development reflects successive adaptation to protection of flowers (before anthesis), to attraction of pollinators (at anthesis) and to protection of capsules (after anthesis) (Webster & Webster 1972).
The male inflorescence (Fig. 1b, d) was considered by Sazima et al. (1985) as a pleiochasium. It has six pedicellate, monochlamydeous flowers with green sepaloid perianth, an androphore and numerous stamens (Fig. 1c). The anthers are bithecate and rimose. The inflorescences presented two small, obovate and pilose bracts.
The male inflorescence includes a laminar gland (Fig. 1b) which exudes a highly sticky compound. Armbruster & Webster (1979) recorded that this gland complex in Dalechampia secretes resin as a reward for insect visits. Only female bees of Euglossa melanotricha, Apidae, Euglossini, are attracted to the D. stipulacea inflorescences, where they gather resin; these euglossine bee species are considered as the most effective pollinator in D. stipulacea (Sazima et al. 1985).
The female cyme inflorescence (Fig. 1b, d) consists of three flowers with two short, obovate, pilose and white bracts. The pistillate flowers are monochlamydeous with green perianth, trilobate and pilose ovary, long pilose style, and clavate stigma with a central cavity. The perianth possesses trichomes and stalked glands.
Inflorescence and flower anatomy
The pseudanthium presents nonglandular and glandular trichomes, and glands in the bracts and flowers (Fig. 2). Nonglandular trichomes are unicellular, thick-walled and pointed (Fig. 2a). Unicellular glandular trichomes (Fig. 2c) occur sparsely on the perianth; these trichomes have wide bases and pointed extremities; the literature does not record unicellular glandular trichomes for Dalechampia. Pluricellular glandular trichomes (Fig. 2b) present a central cell armed with a sharp-pointed crystal, surrounded by several epidermal jacket-cells.
Pluricellular glandular trichomes of D. stipulacea are similar to the stinging hairs of Tragia (Thurston 1976) and Dalechampia scandens L. hairs (Webster & Webster 1972). The trichomes of these species can be irritating to the skin. Maia et al. (2002) made note of these stinging trichomes, considering them to be an important character in the recognition of most of the Dalechampia species.
The glands (Fig. 2d) consist of a long stalk with uniseriate epidermis that presents nonglandular trichomes and stomata, parenchyma with chloroplasts, and a collateral vascular bundle. The head of each gland (Fig. 2d, e) consists of a palisade-like secretory epidermis subtended by parenchyma.
The D. stipulacea stalked glands have structural similarity to special outgrowths (nectaries) on the teeth of leaf margins (Prunus and Ailanthus) or on different parts of the floral organs (Fahn 1990). Studies of floral biology of D. stipulacea (Sazima et al. 1985) and of other Dalechampia species (Armbruster & Webster 1979, 1981) make no reference to the nectaries in pseudantha. On the contrary, in these investigations the emergencies are denominated improperly as glandular hairs that occur in the female flower perianth. It is possible that the D. stipulacea glands have an important role in the pollination, but that were not considered by workers of floral biology. The perianth and bract glands of D. stipulacea could be considered as perigonal nectaries and extrafloral ones, respectively according to Fahn (1990) classification. Croton sarcopetalus outer nectary of female flower (Freitas et al. 2001) is similar to the D. stipulacea one, consisting of a secretory epidermis (column-shaped cells) subtended by secretory parenchyma and ground parenchyma.
The two pseudanthium bracts (Fig. 2f) present uniseriate epidermis with trichomes, glands and stomata. The ordinary epidermal cells possess sinuous anticlinal walls and thick outer periclinal walls. Mesophyll possesses uniform photosynthetic parenchyma.
Pseudanthium bracts of D. stipulacea have external structure similar to dicotyledonous foliage leaves, but they do not have differentiated palisade and spongy parenchyma. The striking structural features of D. stipulacea bracts, such as the uniform mesophyll with chloroplasts and stinging trichomes, should be related to the photosynthetic function and the anti-herbivore mechanism.
The female flower bracts (Fig. 1d) present uniseriate epidermis with stomata on both surfaces. In bracts nonglandular trichomes, stinging trichomes and glands were also observed. Mesophyll consists of uniform parenchyma with few chloroplasts. The number of mesophyll layers is higher in the bract base, while towards the apex this number decreases gradually. In the bract tip the volume of intercellular spaces is greater than at the base. The vascular bundles are collateral and occur in the mesophyll proper.
The anatomical studies of bracts remain relatively poor in the literature. It is probable that the most of species present bracts with uniform mesophyll, as observed in D. stipulacea inflorescences. Bracts with dorsiventral mesophyll occur in Piperaceae (Iwazaki et al. 2006).
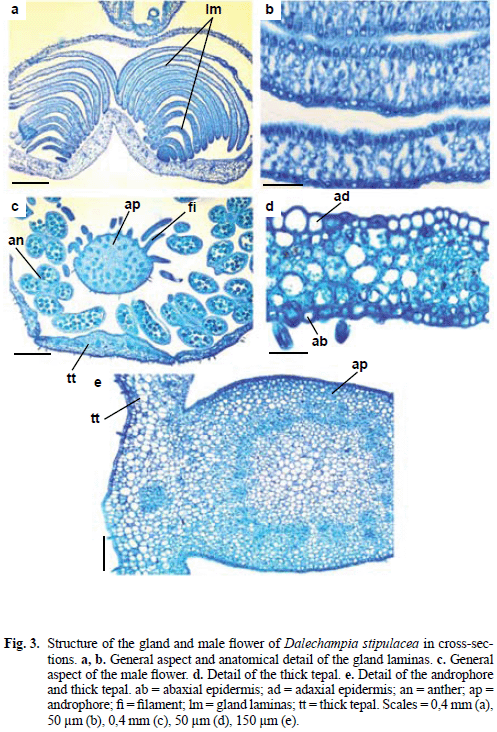
Glands of the male inflorescence of D. stipulacea (Fig. 1b) consist of two tightly appressed groups of laminas with variable dimensions (Fig. 3a). In D. scandens there is a similar gland which was interpreted by Webster & Webster (1972) as modified bractlets with secretory epithelium. Each lamina (Fig. 3a, b) is formed by parenchyma and glabrous uniseriate epidermis. The laminas close to male flowers are larger and show epidermal cells with thick-walls and spongy parenchyma; successive laminas have thinner epidermal cells and fewer intercellular spaces among the parenchyma cells. The lamina parenchyma sometimes contains druses.
The male flower perianth (Fig. 3c, d) consists of four tepals; one of these is thicker (top to bottom) and narrower (side to side). This thicker tepal consists of uniseriate epidermis with stomata on abaxial surface only and uniform parenchyma with chloroplasts and druse idioblasts.
In angiospermous, coloured perianth elements usually have poorly developed vascular system, veins without sclerenchyma, and mesophyll with spongy parenchyma in which the cells may have chloroplasts or pigments in the cell sap, or both (Fahn 1990). The male flower perianth of Dalechampia stipulacea lacks pigments in the cell sap but otherwise shows structure similar to that described by Fahn (1990).
The androphore is fused to the base of the thicker/narrower tepal (Fig. 3c, e). The androphore has pilose uniseriate epidermis, parenchyma, and a cylinder of collateral bundles which encloses a parenchymatous pith. Anthers are tetrasporangiate; and, when immature, anther walls consist of a uniseriate epidermis, an endothecium with thin-walled cells, one middle layer and secretory tapetum (Fig. 4a).
Davis (1966) defined four types of anther wall based on a survey of 80 dicotyledonous families: basic, dicotyledonous, monocotyledonous and reduced. Anthers of D. stipulacea should belong to the dicotyledonous type in which only cells of the outer secondary parietal layer divide, giving rise to the endothelium and the single middle layer, while the cells of the inner layer develop directly into tapetum. Alternatively, but less probable, the D. stipulacea anther could belong to the Monocotyledonous type that also has a single middle layer. Therefore, studies about details of anther wall formation and definition of the type of D. stipulacea are necessary.
In maturity, the anther wall (Fig. 4b) consists of discontinuous epidermis with tabular or lenticular cells, endothecium with thick-walled cells, and secretory tapetum with binucleate cells. In certain areas the tapetum is discontinuous and shows cell projections that penetrate between the developing pollen grains, imitating an amoeboid type of tapetum. However, Carniel (1963) reported that the secretory tapetum can be confused with amoeboid or periplasmodial tapetum in final stage of degeneration.
The female flower perianth (Fig. 4c, d) has a single epidermis with small and cube-shaped cells, nonglandular trichomes, and unicellular and pluricellular glandular trichomes. In the abaxial surface and leaf margin there are glands, notably numerous. Stomata are absent. In the mesophyll occur two layers of photosynthetic parenchymas: the abaxial surface parenchyma is formed by large and isodiametric cells, and the adaxial surface parenchyma shows tangentially elongated cells. Vascularization is made by collateral bundles immersed in the mesophyll. The female flower perianth is different from that of the male flower, not only in shape, but also for exhibiting unicellular glandular trichomes, glands and heterogeneous parenchymatous mesophyll.
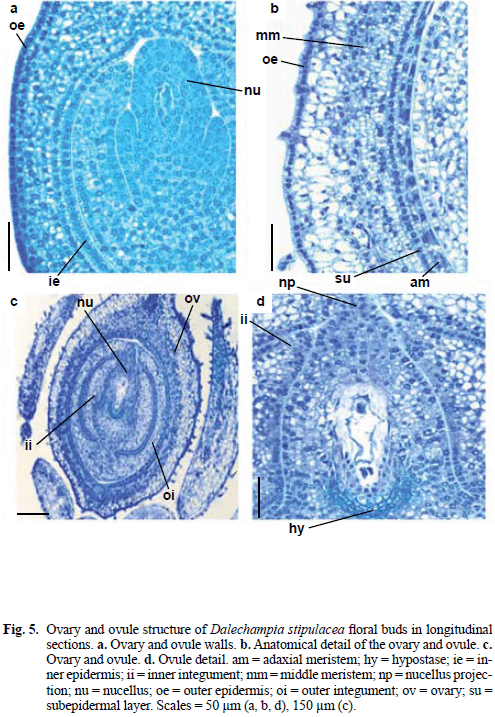
Prior to anthesis, the ovary wall surface (Fig. 5a) consists of outer epidermis, with small and cuboidal cells containing dense cytoplasm. The mesophyll is parenchymatous and consists of four or more cell layers. The subepidermal mesophyll layer and inner epidermis are constituted by shortly elongated cells.
Still prior to anthesis (Fig. 5b), the inner epidermis and middle cell layers divide periclinally forming two meristematic regions in the ovary wall, the adaxial meristem and the middle meristem. Between the meristematic regions a layer of radially elongated cells can be discerned. In this stage the mesophyll cells became vacuolized; trichomes began the differentiation in the inner epidermis.
The presence of meristems in the ovary wall (preanthesis or postanthesis stage) is frequent in plants with follicles, legumes or capsules (Roth 1977; Souza 2006). Toledo (1963) reported parenchyma in the Manihot utilissima (Manihot esculenta) ovary in which cell layers close to locule differentiate in endocarp. Mahihot caerulescens Pohl. and Manihot tripartita Müll.Arg. form adaxial meristem, not in the flower, but in the developing fruit (Oliveira 2007). Dalechampia stipulacea did not just develop a meristem, which usually is verified in ovaries and/or fruits, but two meristems, an adaxial meristem and other middle one.
The ovary mesophyll usually has uniform parenchyma. Differently of most of the studied species, D. stipulacea possess mesophyll in preanthesis with different tissues, mainly for the meristem differentiation. Other Euphorbiaceae species, as Manihot utilissima (Manihot esculenta) (Toledo 1963), Manihot caerulescens and Manihot tripartita (Oliveira 2007) have heterogeneous mesophyll. Besides, Erythroxylaceae species belonging to Malpighiales presented subadaxial and subabaxial meristems, and parenchyma in the mesophyll (Nakamura 2003).
Dalechampia stipulacea ovules (Fig. 5c, d) are anatropous, bitegmic and crassinucellate. The integuments are multi-layered, with vascular tissue just in the outer integument, and micropyle occluded by the nucellar beak; there is a hypostase characterized by thick-walled cells. The hypostase is a cup-shaped region of thick-walled cells occurring at the chalazal end of the ovule, abutting directly with embryo sac and partially surrounding its chalazal end (Tiwari 1983). The hypostase probably has an integral function in the translocation of nutrients into the megagametophyte and, after fertilization, into the embryo sac (Tilton 1980); as reported in Euphorbiaceae (Bouman 1984). Ovule structure has systematic implications in Euphorbiaceae (Tokuoka & Tobe 2003); these authors published a series of papers that deal with Euphorbiaceae ovule and considered all 20 tribes of Acalyphoideae examined with bitegmic and non-vascularized inner integument ovules. The inner integument of the ovule of D. stipulacea is non-vascularized, but Silva & Souza (2009) registered in the seed of this species that the basal part of the tegmen develops by intercalary growth, and few vascular bundles extend from the chalaza by postchalazal branching.
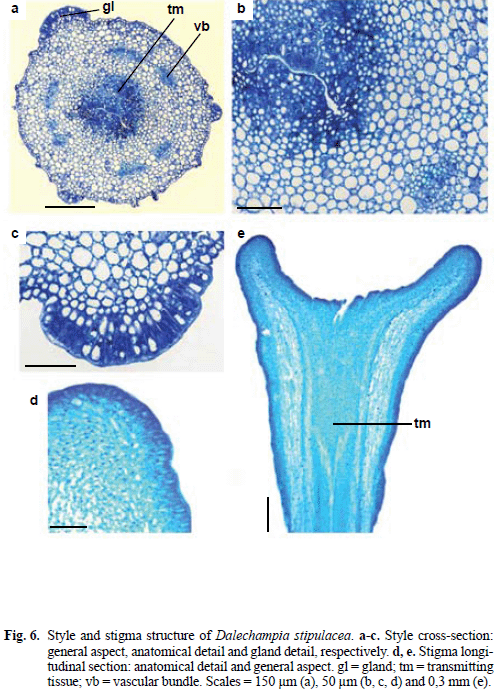
The style (Fig. 1b, d, 6a, b) has uniseriate epidermis presenting nonglandular trichomes and stomata, multi-layered parenchyma, six collateral vascular bundles and central transmitting tissue. In the surface of the style there is a gland with radially elongated cells disposed as a palisade similar to the epidermal cells in the head of stalked glands. The style glands could have the nectary function, although this was not mentioned by Armbruster & Webster (1979, 1981) nor Sazima et al. (1985) in floral biology studies of D. stipulacea.
Styles of D. stipulacea are intermediate between the commonly encountered states of hollow or solid (Fahn 1990). It presents central rifts (Fig. 6a, b) that makes possible to identify the transmitting tissue of each carpel.
The enlarged stigma consists of a central cavity of circular contour which is covered by secretory epidermis with non-papillose elongated cells (Fig. 6d, e). Armbruster (1996) described different types of stigmas of Dalechampia, without mentioning D. stipulacea. The author considered that the expanded stigma appears to be associated with fragrance secretion throughout the genus, which suggests that the large stigmatic surface evolved to promote fragrance production for attraction of pollinators.
Some striking structural features of the inflorescence and flower of D. stipulacea, such as pseudanthium with a unique configuration within the Euphorbiaceae, laminar gland in the male inflorescence, stalked glands in the bracts and male and female inflorescences, stinging trichomes, adaxial and middle meristems formed in the ovary wall, and micropyle occluded by the nucellar beak, are important for taxonomic and pollination ecology studies of the genus Dalechampia. On the other hand, the Dalechampia literature makes no reference to the encountered features in D. stipulacea, such as unicellular glandular trichomes and the style glands.
ACKNOWLEDGMENTS
We thank Dr. Olavo A. Guimarães of the Universidade Federal do Paraná for the identification of studied species. We thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil) for funding this research.
BIBLIOGRAPHY
1. Armbruster, W.S. 1996. Evolution of floral morphology and function: an integrative approach to adaptation, constraint, and compromise in Dalechampia (Euphorbiaceae). In: Lloyd, D.G. & S.C.H. Barrett (eds.). Floral biology studies on floral evolution in animal-pollinated plants, pp. 241-272. Chapman & Hall, New York. [ Links ]
2. Armbruster, W.S., A.L. Herzig & T.P. Clausen. 1992. Pollination of two sympatric species of Dalechampia (Euphorbiaceae) in Suriname by male euglossine bees. Amer. J. Bot. 79: 1374-1381. [ Links ]
3. Armbruster, W.S. & G.L. Webster. 1979. Pollination of two species of Dalechampia (Euphorbiaceae) in Mexico by Euglossine bees. Biotropica 11:278-283. [ Links ]
4. Armbruster, W.S. & G.L. Webster. 1981. Sistemas de polinização de duas espé cies simpátricas de Dalechampia (Euphorbiaceae) no Amazonas, Brasil. Acta Amazon. 11: 13-17. [ Links ]
5. Bouman, F. 1984. The ovule. In: Johri, B.M. (ed.). Embryology of angiosperms, pp. 123-157. Springer-Verlag, New York. [ Links ]
6. Capinpin, J.M. & V.C. Bruce. 1955. Floral biology and cytology of Manihot utilissima. Philip. Agric. 39: 306-316. [ Links ]
7. Carniel, K. 1963. Das Antherentapetum. Österr. Bot. Z. 110: 45-176. [ Links ]
8. Davis, G.L. 1966. Systematic embryology of the angiosperms. John Wiley & Sons, New York. [ Links ]
9. Fahn, A. 1990. Plant anatomy. Pergamon Press, Oxford. [ Links ]
10. Foster, A.S. 1950. Techniques for the study of venation patterns in the leaves of angiosperms. Proceedings 7th International Botanical Congress Stockholm. [ Links ]
11. Freitas, L., G. Bernardello, L. Galetto & A.A.S. Paoli. 2001. Nectaries and reproductive biology of Croton sarcopetalus (Euphorbiaceae). Bot. J. Linn. Soc. 136: 267-277. [ Links ]
12. Gerrits, P.O. 1991. The application of glycol methacrylate in histotechnology (some fundamental principles). Leica Gmbh., Germany. [ Links ]
13. Graner, E.A. 1935. Contribuição para o estudo citológico da mandioca. Public. Esc. Sup. Agric. Luiz de Queiroz 1-28. [ Links ]
14. Graner, E.A. 1942. Notas sobre florescimento e frutificação da mandioca. Bragantia 2: 1-12. [ Links ]
15. Hickey, L.J. 1979. A revised classification of the architecture of dicotyledonous leaves. In: Metcalfe, C.R. & L. Chalk (eds.). Anatomy of the dicotyledons, pp. 25-39. Clarendon Press, Oxford. [ Links ]
16. Iwazaki, M.C., L.A. Souza & J.H.G. Oliveira. 2006. Morfo-anatomia comparativa da flor de Peperomia dahlstedtii C. DC., Ottonia martiana Miq. e Piper gaudichaudianum Kunth (Piperaceae). Hoehnea 33: 545-558. [ Links ]
17. Maia, V.C.R., M. Emmerich & L.S. Valle. 2002. Dalechampia Plum. ex L. (Euphorbiaceae) - Taxonomia das espécies ocorrentes nas restingas do Estado do Rio de Janeiro, Brasil. Bol. Mus. Nac. (Bot.) 119: 1-29. [ Links ]
18. Nakamura, A.T. 2003. Morfologia e anatomia dos frutos e sementes de três espécies de Erythroxylum P.Browne (Erythroxylaceae) de cerrado do Estado de São Paulo. Dissertação de Mestrado. Universidade Estadual Paulista. Botucatu (Brasil). [ Links ]
19. OBrien, T.P., N. Feder & M.E. McCully. 1964. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59: 368-373. [ Links ]
20. Oliveira, J.H.G. 2007. Morfologia, anatomia e desenvolvimento do fruto e semente de Manihot caerulescens Pohl. e M. tripartita Müll.Arg. (Euphorbiaceae). Tese de Doutorado. Universidade Estadual Paulista. Botucatu (Brasil). [ Links ]
21. Roth, I. 1977. Fruits of angiosperms. In: Linsbauer, K. (ed.). Encyclopedia of plant anatomy, pp. 21-26. Gebrüder Borntraeger, Berlin. [ Links ]
22. Sazima, M., I. Sazima & R.M. Carvalho-Okano. 1985. Biologia floral de Dalechampia stipulacea (Euphorbiaceae) e sua polinização por Euglossa melanotricha (Apidae). Revista Brasil. Biol. 45: 85-93. [ Links ]
23. Silva, A.C. & L.A. Souza. 2009. Morphology and anatomy of the developing fruit and seed of Dalechampia stipulacea Müll.Arg. (Euphorbiaceae). Acta Scient. Biol. Sci. 31: 425-432. [ Links ]
24. Silva, R.M., G. Bandel, M.I.F. Faraldo & P.S. Martins. 2001. Biologia reprodutiva de etnovariedades de mandioca. Sci. Agric. 58: 101-107. [ Links ]
25. Souza, L.A. 2006. Fruto. In: Souza, L.A. (ed.). Anatomia do fruto e da semente, pp. 10-123. Editora Universidade Estadual de Ponta Grossa, Ponta Grossa. [ Links ]
26. Thurston, E.L. 1976. Morphology, fine structure and ontogeny of the stinging emergence of Tragia ramosa and T. saxicola (Euphorbiaceae). Amer. J. Bot. 63: 710-718. [ Links ]
27. Tilton, V.R. 1980. Hypostase development in Ornithogalum caudatum (Liliaceae) and notes on the other types of modifications in chalaza de angiosperm ovules. Canad. J. Bot. 58: 2059-2066. [ Links ]
28. Tiwari, S.C. 1983. The hypostase in Torenia fournieri Lind.: a histochemical study of the cell walls. Ann. Bot. 51: 17-26. [ Links ]
29. Tokuoka, T. & H. Tobe. 1998. Ovules and seeds in Crotonoideae (Euphorbiaceae): structure and systematic implications. Bot. Jahrb. Syst. 120: 165-186. [ Links ]
30. Tokuoka, T. & H. Tobe. 2002. Ovules and seeds in Euphorbioideae (Euphorbiaceae): structure and systematic implications. J. Plant Res. 115: 361-374. [ Links ]
31. Tokuoka, T. & H. Tobe. 2003. Ovules and seeds in Acalyphoideae (Euphorbiaceae): structure and systematic implications. J. Plant Res. 116: 355-380. [ Links ]
32. Toledo, A.P. 1963. Anatomia e desenvolvimento ontogenético da flor de mandioca. Bragantia 22: 465-476. [ Links ]
33. Weberling, F. 1992. Morphology of flowers and inflorescences. Cambridge University Press, Cambridge. [ Links ]
34. Webster, G.L. & B.D. Webster. 1972. The morphology and relationships of Dalechampia scandens (Euphorbiaceae). Amer. J. Bot. 59: 573-586. [ Links ]












 uBio
uBio