Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Latinoamericana de Metalurgia y Materiales
Print version ISSN 0255-6952
Rev. LatinAm. Metal. Mater. vol.34 no.2 Caracas June 2014
BAMBOO PULP TREATED WITH NaOH AND BENZOPHENONE TETRACARBOXYLIC DIANHYDRIDE AS REINFORCEMENT OF NEW POLYMERICS MATERIALS
Mario Guimarães Junior1*, Kátia Monteiro Novack2, Vagner Roberto Botaro3
1: Doctoral Candidate in Materials Engineering for REDEMAT/UFOP/CETEC, Department of Electronic, Federal Center of Technological Education of Minas Gerais â CEFET -MG, 55 34 3661 4746, Rua Mario Campos 520, Brazil.
2: Instituto de Ciências Exatas e Biológicas / Departamento de QuÃmica, UFOP-MG (Universidade Federal de Ouro Preto), REDEMAT, Ouro Preto -Minas Gerais, Brazil. 3: Department of Post-graduate Studies in Materials Science, Federal University of São Carlos, UFScar-SP, P.O. Box 3031, Sorocaba âSP, Brazil, 18052-780, REDEMAT, Brazil. *e-mail: mgjunior@araxa.cefetmg.br
ABSTRACT
Bamboo admittedly and engineering material widely used for buildings in several countries. However, lack of good interfacial adhesion, low melting point, and poor resistance towards moisture make the use of natural fiber reinforced composites less attractive. The aim of this work was esterify the refined pulp of bamboo came from kraft industrial pulping, previously treated with sodium hydroxid and organic solvents for the production of less hydrophilic materials. In order to verify the occurrence of reaction, assays of spectroscopy in the region of IV, thermogravimetry, elementary analysis and water absorption were performed. After the reaction, the infrared spectra presented bands related to the ester and carboxylic acid groups, evidencing the exchange of OH groups for ester groups. This test also shows a decrease in the absorbance ratio due to the presence of ester group causing a reduction in the crystallinity index of the sample. These results were proven by the gain in the value of carbon content after the elementary analysis. In the water absorption test, the pulp modified with dianhydride showed the lowest level as compared to other samples. The thermogravimetric analysis also showed a decrease in initial temperature of pulp degradation, as well as decrease in the moisture, facts that proved the efficiency in the modification using dianhidrido BTDA.
Keywords: Polymeric matrices, bamboo pulp, chemical treatment, polymeric composites
PULPA DE BAMBÃ TRATADA CON NaOH Y DIANHÃDRIDO BENZOFENONA TETRACARBOXÃLICO COMO REFUERZO DE NUEVOS MATERIALES POLIMÃRICOS
RESUMEN
El objetivo de este trabajo fue esterificar pulpa refinada procedente de bambú industrial kraft, pre-tratados con hidróxido de sodio y disolventes orgánicos para producir materiales menos hidrofÃlicos. Con el fin de verificar la aparición de reacción de esterificación, espectroscopia infrarroja, análisis termogravimétrica, análisis elemental y absorción de agua fueron realizadas. Después de la reacción, el espectro infrarrojo mostró bandas relacionadas con los grupos éster y grupos de ácido carboxÃlico, que muestra el intercambio de grupos OH por grupos éster. Esta pruebla también mostró una proporción reducida de la absorbancia debido a la presencia del grupo éster causando una reducción en el Ãndice de cristalinidad de la muestra. Estos resultados se corroboraron por un aumento en la cantidad de contenido de carbono después del análisis elemental. En la prueba de absorción de agua, la pulpa modificada con dianhÃdrido mostró el nivel más bajo en comparación con otras muestras. El análisis termogravimétrica mostró también una reducción en la temperatura de inicio de la degradación de pulpa refinada, asà como una disminución en el contenido de humedad, los eventos que demostraron la eficacia de la modificación utilizando BTDA dianhÃdrido.
Palabras clave: Matrices poliméricas, pulpa de bambú, tratamientos quÃmicos, materiales compuestos poliméricos.
Recibido: 18-04-2013 ; Revisado: 29-05-2013
Aceptado: 08-06-2013 ; Publicado: 11-07-2013
1. INTRODUCTION
The use of natural plant fibers in composites exists since ancient times [1, 2]. However, the use of lignocellulosic fibers, as are known the natural plant fibers in the engineering area, has decreased over time, mainly due to the emergence of new materials. In the last decades, along the increase of pressure from the society and the appearance of new environmental legislations for a more rational use of natural resources, the vegetable fibers returned to be an alternative for various applications, mainly for the plastic industry for they are renewable natural resources and biodegradable [2, 3, 4].
Nowadays, several types of natural fibers have been investigated to be used in composites, including flax, hemp, ramie, sisal, jute, rice husk, rattan, kenaf, rye, piassava, among others [1, 2, 5, 6].
In this context, bamboo, in recent years, has been receiving considerable attention as a substitute for wood and other fibers, because its mechanical properties are very similar [7].
In contrast of the woods, that presents four layers of microfibrils arranged in different thicknesses and orientation angles in the formation of its cell wall, bamboo is made of several layers which alternate in longitudinal and transversal directions, oriented at angles that enhance their values toward the inside of the wall. The absence of anatomical elements in the radial directions and also the features mentioned above makes this grass a functional gradient material (FGM), in others words, a material in which its properties change gradually or continue along a specific direction. Because of anatomical characteristics differentiated, the bamboo does not suffer abrupt changes in their properties, quite the contrary; the properties vary from point to point according to the actual need [8, 9].
These fibers are considered natural polymers, composed, basically of cellulose, hemicelluloses and lignin, which are constituents that contribute differently amongst each other to the mechanical resistance [2, 10, 11, 12].
The application of vegetable fibers as reinforcement allows the acquisition of composite products, which, besides presenting smaller apparent specific mass and higher porosity, present, still, satisfactory values of traction and impact resistance, bigger control of cracking, besides ductile behavior during break [13, 14, 15].
Cellulosic fibers, such as bagasse from sugar cane, sisal, banana, coconut, wood and jute have been incorporated into thermoplastic and thermoset matrices, as reinforcement or load [3, 16, 17, 18].
However, natural fibers present some disadvantages, for example, the low compatibility with the polymeric matrix, due to the presentation of hydrophilic character, while the polymeric matrix has hydrophobic character. This difference in affinity for water results in a low superficial adherence, which prevents the transfer of effective stress, reducing, consequently, the mechanical properties of the composite [19, 20].
To enhance the adherence of the fiber/matrix interface, the natural fibers have to be modified superficially, so that they can interact with the polymeric matrix [21, 22]. By studying different modification methods of sisal fibers, Singha & Thakur [23]; Kumar et al., [24] e Taherzadeh & Karimi [25], argue that the fibers can be modified effectively by chemical substances, such as isocyanates and silanes, and physical treatments, such as plasma and corona discharge.
The alkaline treatment of fibers is one of the low cost possibilities [26]. The effects of mercerization as a treatment to improve the mechanical properties, especially traction resistance to lignocellulosic fibers, have been extensively studied [27].
In this context, the chemical modification of lignocellulosic fibers by esterification reaction with dianhydrides, after mercerization, presented itself as an attractive method for increasing the compatibility of natural fibers with polymeric matrices of high hydrophobic character.
The objective of this work was to modify chemically refined bamboo pulp with dianhydride benzofenonatetracarboxÃlic, after mercerization and extraction in organic solvents, aiming your use as strengthening agent in polymeric matrices of hydrophobic nature. The final structure of the sample after esterification is show in figure 1.
The changes in the chemical structure of the surface of pulp were evaluated by means of spectroscopic and chemical analyses, while the thermal stability and the cristallinity were determined through thermogravimetric analysis and spectroscopic observations, respectively.
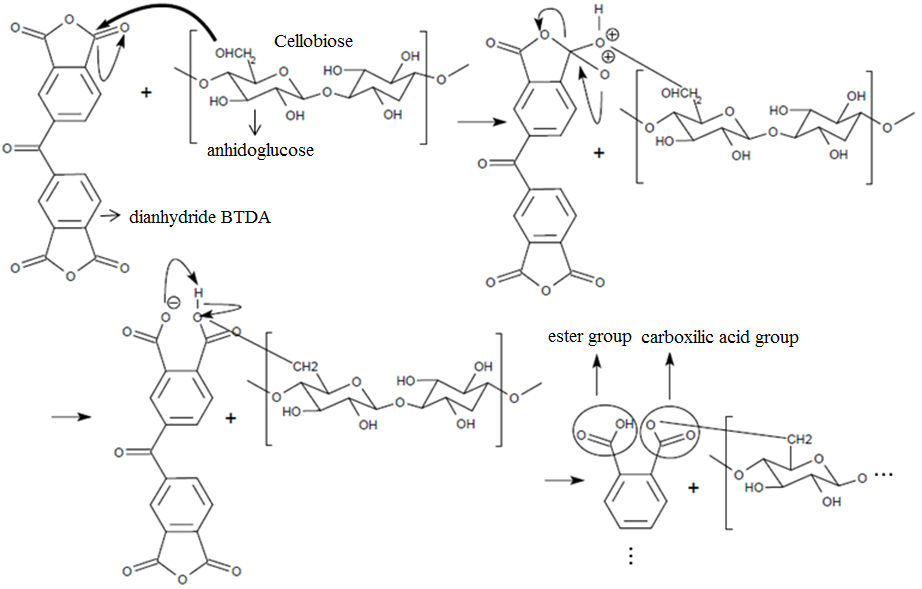
Figure 1. Esterification reaction between the hydroxyl group of the C6 atom of an anhydroglucose unit in cellobiose and BTDA.
2. EXPERIMENTAL PART
2.1 Refined Pulp
The refined samples of bamboo pulp from the species Bambusa vulgaris Schrad were provided by CEPASA â Celulose e Papel de Pernambuco S/A, located in the city of Jaboatão dos Guararapes. The refined pulps underwent the industrial pulping process known as KRAFT, with screened yield around 46% and KAPPA number between 45 e 55. At the wash exit, the pH varied from 9 to 10, while the ºSR suffered alterations between 60º and 70º after refining and 25 and 30º before the refining, in a disc refiner. The active alkali varied between 16% and 18%.
The bamboo pulp was washed with distilled water and dried in a climatized room for 15 days, at 23 ºC and 65% of relative humidity, aiming at its equilibrium moisture, according to the TAPPI T 257 cm-00 [28] Standard. Following this, the samples were ground in rotor mills and granulometrically classified in a series of Tyler sieves, following the recommendations of the WS Tyler Standard and ASTM 1921 [29].
The mercerization, performed after the extraction process with solvents, was carried out with 2% of NaOH, at room temperature, for a period of 2 hours, washed in distilled water until the complete elimination of alkali. The samples were, then, dried in an oven at 100 ± 5 ºC. After the mercerization, the refined pulp has undergone the process of extraction of organic solvents, followed by its placement in a round-bottomed flask with benzophenone tetracarboxylic dianhydride, used for the esterification reaction, and triethylamin, as a catalyst, plus acetone, as a solvent, for 48 hours, at 55 °C, which was used until suspension the fibrous blanket. The proportion between the reagents was of 1.0 g of pulp to 2.5 g of BTDA and 1.5 mL of triethylamin. After the reaction was concluded, the pulp was washed in acetone in abundance until the removal of unreacted solids adhered and dried in an oven at 100 ± 5 ºC, to constant mass.
The thermogravimetric, elementar and spectroscopic analysis were performed with samples retained in the 60 and 270 mesh sieves, respectively.
2.2 Characterization
The Spectroscopy in the infrared region (4000-400 cm -1^) was performed in a Perkin Elmer GX spectrophotometer, operating with the resolution of 16 cm-1 and 32 scannings, using KBr pellets, compressed at 10-12 Kgf.cm-2 .
The thermal analyses were performed in a SDT 2960 Simultaneous DSC-TGA device, from the TA Instruments. The tests were carried out in triplicate and the analysis carried out at temperature of 10 ºC to 630 ºC for the samples treated with sodium hydroxide and submitted to extraction in organic solvents and 10 ºC to 790 ºC for the samples refined and unrefined, with heating rate of 20 ºC.m-1 . The natural and esterified samples were analyzed at 10 ºC to 600 ºC with heating rates of 10 ºC.m-1 . All tests were carried out under an N2 atmosphere with a flow of 100 mL.m-1 .
Elementar analysis was done in an elementar analyzer EAS VARIO MICRO CHNS-O, using helium as transporter gas at 1020 °C.
The determination of the water absorption of the fibers and pulps, submitted to the process kraft pulping was conducted in accordance to the ASTM D570. Because of the various types of testing described by the standard, chose to determine the water absorption by immersion long up to saturation.
The values of the crystallinity indexes were obtained through the technique of spectroscopy in the infrared region (FTIR), according to methodologies adopted by Nelson & OâConnor [30] and Akerholm et al. [31].
Although has not been subject for discussion in this article, the unrefined and refined pulps were characterized according to their main dimensions and its anatomy, following the standards of IAWA committee [32] and Milanez and Foelkel [33]. After refined the pulp had her size and diameter reduced
(2.299 mm x 13.93 µm to 2.098 mm x 11.72 µm), flattened, defibrillated, low degree of collapse and small lumen. There was a decrease in the indices that measure its stiffness, but not enough to make her flexible.
3. RESULTS AND DISCUSSION
3.1 Spectroscopy Analysis in the Infrared Region â FTIR
The decrease of lignin levels, hemicellulose and extractives, resulting in the increase of alpha-cellulose level, was also confirmed after the FTIR test, which also showed a decrease in the absorption of the sample.
Figure 2a present the curve obtained for dianhydride BTDA, while Figure 2b shows the curves obtained for each type of treatment performed. These curves are the results of the vibrations that correspond to the bands of these chemical elements in the range from 4000 cm-1 to 400 cm-1 .

Figure 2. a) Absorption spectrum in the IR region, from 4100 to 400 cm-1, of the BTDA [34]. b) Absorption spectrum in the IR region, from 4100 to 400 cm-1, of the natural fiber samples (FNAT), industrial unrefined kraft pulp (POKR), industrial refined kraft pulp (PKRR), industrial refined kraft pulp, chemically modified with sodium hydroxide (PHIS), industrial refined kraft pulp, chemically modified with sodium hydroxide + ethanol/toluene + ethanol (PETE) and industrial refined kraft pulp, chemically modified with sodium hydroxide + ethanol/toluene + ethanol + dianhydride (NETEB).
The peak of 1734 cm-1, related to the acetyl group extraction with organic solvents, confirming the and carboxyl group of the xylanes disappeared with extraction of lignin and extractives from the sample, the treatments of pulping, refining, mercerization, but reappeared during the esterification treatment, indicating the incorporation of ester groups in the pulp.
The fall in the values of lignin, hemicelluloses and extractives was also confirmed by the decrease of the 1636 cm-1 bands, which represents vibrations in the aromatic skeleton plus stretching (C=O, carbonyl stretching) indicating a decrease in the level of poliosis; 1252 cm-1 , which represents asymmetric axial deformation of (=C-O-C-, asymmetric axial deformation ether), common in an aqueous medium where (=C-O-), is present, as in ester and ether; 1376 cm-1 , regarding the deformation vibration of the C-H group, due to the removal of polysaccharide of low molecular mass and 1512 and 1431 cm-1, related to the vibration of aromatic groups of lignin, specific of the guaiacyltype rings.
The decrease of the 3422 cm-1 band indicates the success of the esterification reaction in the sample and also in the humidity decrease. The widening of the bands of 897 and 1057 cm-1 point to an increase in the values of alpha-cellulose levels, while the increase of the 1162 cm-1 band indicates the incorporation of the ester group to the pulp. The little decrease in the 1320 cm-1 band (guaiacylsyringyl lignin ring) occurred, mainly, due to the treatment of mercerization. The 2916 cm-1 band, attributed to the symmetric and asymmetric vibrational stretching of the CH2 e CH3 from the aliphatic groups, became smaller, due to the removal of hemicellulose.
3.2 Spectroscopy Analysis in the Infrared Region for Crystallinity Index
The results of the absorbance ratios, representing the indexes found in each type of sample, treated and free of treatment ones, are presented in Figure 3, according to the methodologies adopted by Nelson & OâConnor [30] and Akerholm et al., [31]. The ratio between the heights of the bands at 1372 and 2900 cmâ1 proposed by Nelson and OâConnor as total crystalline index TCI was used to evaluate the infrared (IR) crystallinity ratio. The band 2900 cmâ 1 is the internal standard correction in the sample and does not depend of changes in crystallinity. The 1372 cmâ1 measures the intensity according to the change in crystallinity. The band at 1429 cm-1 associated with the amount of crystalline structure of cellulose, while the band at 897 cmâ1 is assigned with the amorphous region in cellulose. The ratio between the areas of the bands at 1429 and 897 cm-1 are used as a lateral order index (LOI). Considering the chain mobility and bond distance, the hydrogen bond intensity (HBI) of cellulose is closely related to the crystal system and the degree of intermolecular regularity, that is, crystallinity [35, 36, 37]
In this sense, it is implied that the crystalline region of the native cellulose presented stage β, to the detriment of stage α, when submitted to the processes of industrial pulping and refining and, also, mercerized with sodium hydroxide, as there were gradual raises in the crystallinity indexes of the samples according to removal of parts amorphous without the consequent degradation of the same. These characteristics inherent to cellulose I type β may have possibly occurred in the stage of industrial pulping, as reported by Tserki et al., [38] e Hult et al., [39], which might have happened due to electrostatic interactions amongst cellobiose units that provoked a more favorable alignment to the formation of hydrogen bonds that stabilized the charges of the molecule.
With regard to the decrease in the values of the refined samplesâ crystallinity indexes, treated with organic solvents, it is believed that has occurred, according to the utilized methodology a penetration of the solvents in the samples and the entry of new functional groups on the polymer chains, in the case of modification with dianhydride, provoking the rearrangement of the packing of native cellulose chains, aligned in parallel, for cellulose II, where the chains are antiparallel. The material had its chains of polysaccharide expanded and rearranged, which culminated in a greater amount of less organized material, as well as the consequent fall in the pulpâs crystallinity [40]. Siqueira [41] commented that the esterification increases the amorphous part of the sample, reducing therefore, the crystallinity of the same.
In a way, the lignin that acted as a kind of glue that unites the beams was removed with the treatments, easing the penetration of reagents and their access to the hydroxyl groups. Oliveira and Frollini [42] found similar behavior when modifying sisal fibers with ultrasound.
Tserki et al., [38] and Karnitz Junior et al., [43] obtained the same results by treating the surface of other lignocellulosic fibers, using other types of dianhydride.
The increase in value of crystallinity index after the refining process may have occurred for several situations, among them: a) the insufficient amount of hemicellulose, which, according to Cláudio-da Silva [44], potentiates the external fibrillation in detriment of internal, since the presence of hemicellulose in the cell wall, which is hydrophilic, is essential for the occurrence of internal fibrillation and reduction in crystallization. These factors contribute to the occurrence of the decrease in the crystallinity index. Somehow industrial process of refining and pulping altered the critical value of the hemicellulose content, prioritizing the external fibrillation, b) The fibers with thick wall, such as case, have resisted to external fibrillation [45], c) The decrease in the value of diameter of the lumen and in the value of the cell wall thickness, after refining, as well as the reduction in number of pores formed in the amorphous regions, identified after anatomical analizes, and recommended by Lindstron and Carlsson [46], may have contributed e d) the drying occurred during the tests may have contributed to the growth of crystallites becoming the samples more crystalline, as stated by Nazhad and Paszener [47].
Since the crystallinity index relates to water absorption of lignocellulosic samples, it may be affirmed that occured a decrease in hydrophilicity of pulp chemically modified with the dianhydride used, since there has been an increase in the crystallinity index the same. Microfibrils with the highest crystallynity indexes have strong hydrogen bonds or cellulose-cellulose between the same, which prevent or hamper its swelling.
3.3 Thermogravimetry
The thermogravimetric analysis curves (TGA), in nitrogen atmosphere, of the samples submitted to the industrial process of kraft pulping of the refined pulp, the refined pulp modified with sodium hydroxide and the refined pulp modified with sodium hydroxide and organic solvents (ethanol/toluene) are found in figure 4(a), while figure 4(b) presents the results of the analysis for the natural fiber and refined pulp modified with sodium hydroxide and organic solvents (ethanol/toluene) + BTDA dianhydride. By virtue of more rigorous and detailed monitoring promoted by modification of pulp with dianhydride, the heating rate of 10 °C.min-1 for natural fiber (FNAT) and modified pulp (BTDA) was used.
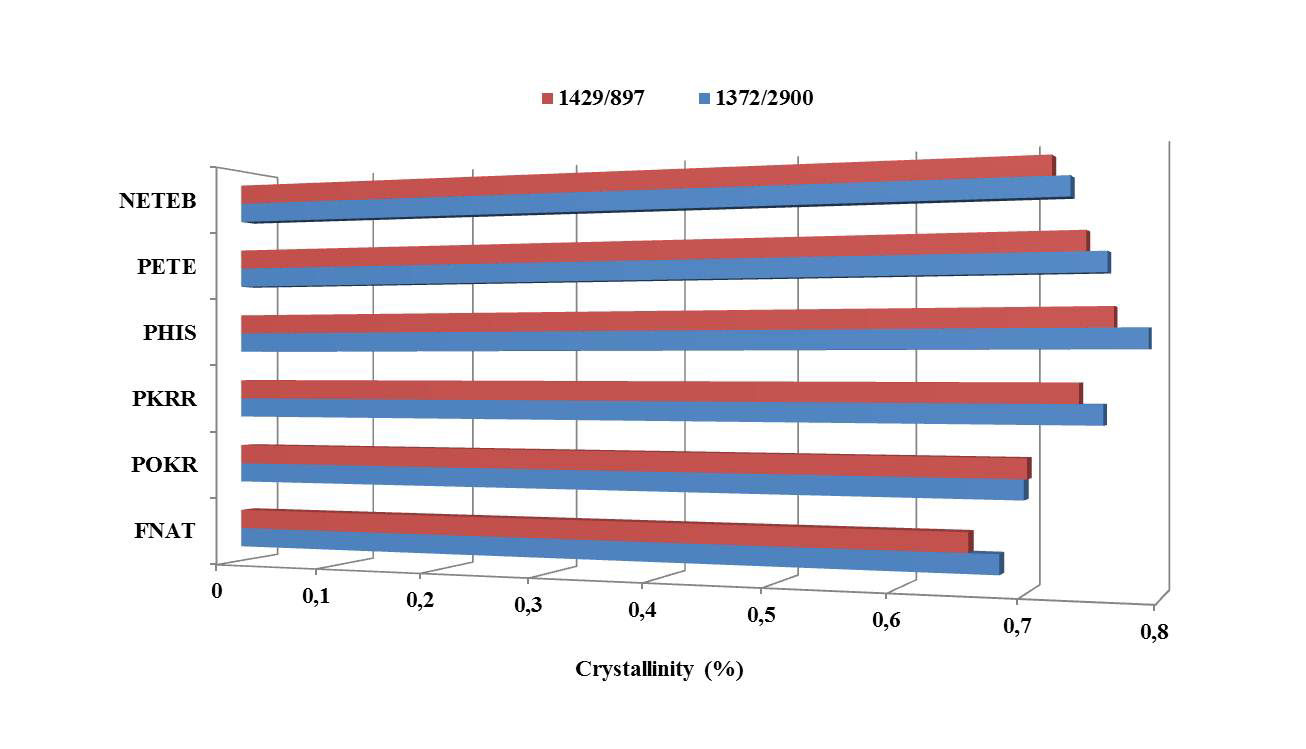
Figure 3. Crystallinity index of refined and unrefined fibers and pulps of Bambusa vulgaris schrad, with and without chemical modifications. Results obtained indirectly through analysis of absorption spectroscopy in the infrared region, according to Nelson & OâConnor45 and Akerholm et al.46 FNAT: natural fiber; POKR: unrefined pulp; PKRR: refined pulp; PHIS: refined pulp treated with NaOH at 2%; PETE: refined pulp treated with NaOH at 2% and submitted to extraction with organic solvents; NETEB: refined pulp treated with NaOH at 2% + extraction with organic solvents + esterification with dianhydride.

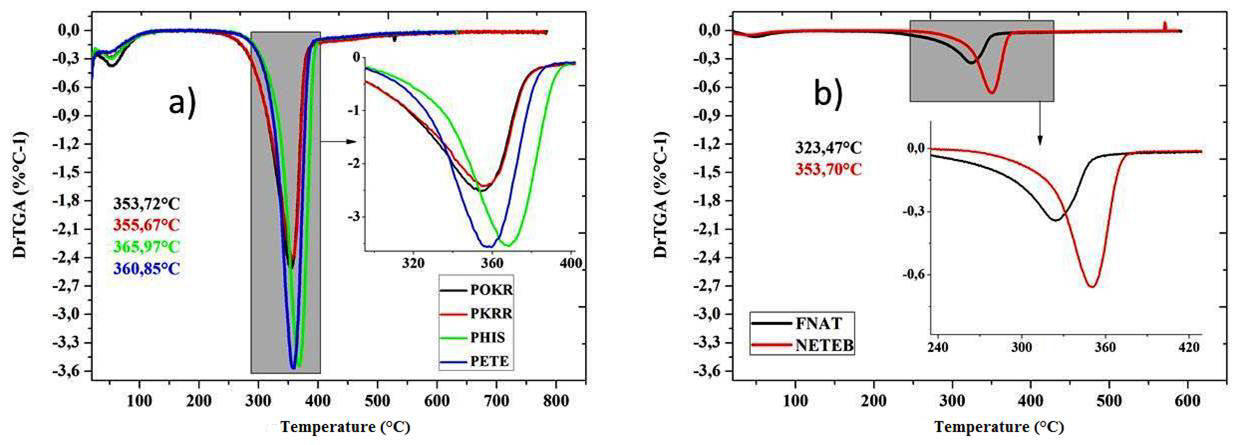
Figure 4. TGA Curves of modified and not-modified Bambusa vulgaris Schrad samples. 3(a) â Heating rate of 20 °C.min-1 (POKR, PKRR, PHIS e PETE); 3(b)-Heating rate of 10 °C.min-1 (FNAT e NETEB). All samples tested in N2 atmosphere under a flow of 100mLmin-1 .
In general, the fibers presented a similar thermal behavior. The TGA curves displayed in Figure 4 showed an average decrease in mass of approximately 9% at 100 °C, for all the samples of modified and not-modified Bambusa vulgaris schrad. This loss of mass corresponds to the water evaporation of the samples. Between the temperatures of 105 °C and 275 °C, the samples presented little loss of mass, indicating thermal stability up to 275 °C.
It has been also observed a difference with respect to the mass loss of the modified samples and the natural sample from around 330 to 415 °C, and all samples of Bambusa vulgaris Schrad presented greater loss than the natural samples. The difference in percentage of the products derived from the pyrolysis, as the atmosphere used in the test was N2, was attributed to the difference of the natural fiberâs composition, regarding the remaining samples.
Oliveira & Frollini [38] researching the modifications in fibers in order to use them in composites, found, for sisal fibers, a decomposition start temperature at 250 °C. In this study, by chemically treating refined pulps of bamboo, it was found, after a treatment with sodium hydroxide at 2%, for 2 hours, a decomposition start temperature of 275 °C, a value that is higher than the one found in literature for several types of fibers.
in derivative thermogravimetry, the first derivative of the mass change with respect to temperature is recorded as a function of time or temperature. The resulting curve consists of a series of peaks instead of a stepwise curve, if the mass change takes place in more than one step. The maxima of the DTG curve represent the inflection points, where the rate of mass change is maximum. The data are displayed in a more visually accessible manner. Besides, the DTG curve provides ready information about the temperature at which the rate of mass change is maximum. In a TG curve, if the subsequent changes are very close to each other, the different stages cannot be easily distinguished. But, the DTG curve provides sharp maxima corresponding to different stages and hence they can be easily differentiated. It indicates precisely the temperature corresponding to the beginning and the end of a mass change.
The DTG curves confirmed the data found in the TGA analysis, suggesting two degradation stages for all the samples analyzed. In all cases, an initial peak between 28 and 90 °C occurred, which corresponds to the elimination of water in the samples. The lowest percentage of humidity loss in the esterified pulp, when compared to other pulps and also the natural fiber, may be attributed to the greater hydrophobic character, acquired by the fibers modified with BTDA, due to the reaction of esterification. There was a difference of approximately 20%, on average, in the indexes water absorption between the modified pulp (BTDA) and natural fibers (FNAT).
The natural fiber presented a second decomposition peak with maximum at 323.47 °C, attributed to the depolymerization, deacetylation and dehydration of hemicelluloses and rupture of α and lignin β aryl-alkyl-ether bonds, with stabilization at 560 °C and loss of mass of 65.53%. The fiber samples after industrial kraft pulping and refining processes presented main decomposition peaks with maximum at 353.7 ºC and 355.6 °C and stabilization at 589.1 and 598.9 °C, respectively, with mass losses of 74.7 and 72.9%, in that order. The main peaks of mass loss are presented with maximum at 365.9 °C, stabilization at 610.3°C and 81.8% of mass loss for the sample treated and modified with NaOH at 2%, and at 360.8 °C, with stabilization at 586.7 °C and 80.7% of mass loss for the samples treated with organic solvents. The sample modified with dianhydride, through the process of esterification presented a second decomposition peak with maximum at 353.7 °C, stabilization at 569.6 °C and 79.2% of mass loss. Figure 5 shows the results found for the DTG tests.

Figure 5. DTG Curves of modified and unmodified Bambusa vulgaris schrad samples. 4(a)-Heating rate of 20 °C.min-1 (POKR, PKRR, PHIS e PETE); 4(b)-Heating rate of 10 °C.min-1 (FNAT e NETEB). All samples tested in N2 atmosphere under a flow of 100mL.min-1 .
Despite exhibiting more hydrophobia, the samples modified with organic solvents and dianhydride presented themselves as less thermally stable, due to the changes in their chemical structures, which made them more prone to degradations at low temperatures, fact that is confirmed by the introduction of ethanol/toluene and the ester group. It is believed that the reduction in the crystallinity indexes of the samples, provoked by the reagents, may have enhanced this reduction in thermal stability.
3.4 Elementary analysis â CHNS-O
Table 1 shows the result of elementar analysis made with bamboo samples. It is possible to observe a clear increase on carbon and hydrogen contents of the refined pulp modified with BTDA dianhydride, thus proving the efficiency in the modification of the pulp surface, becoming less hydrophilic. The modification caused an increase of the molecular mass of the fiber walls, which compounds the pulp in function of the esterification reaction, which was caused by the increase of carbon content, becoming more compatible with polymeric matrices of hydrophobic character. In the esterification reaction of cellulose the replacement of hydroxyl groups by ester groups causes an increase in carbon content [48, 49]. The increase in carbon content during the esterification reaction is according with the FTIR analysis, which indicated carbonyl stretching at 1734 cm-1 due to the formation of the ester group.
In this sense, Gatenholm & Gellerstedt [50] comment that besides reacting with hydroxils (OH) of celluloses chains, it is also suggested that esterification reactions of lignocellulosic fibers may occur with hydroxyls of hemicelluloses and lignin molecules, present in the fibers that compound bamboo pulp, forming esters derivatives. It also was observed that OH groups of these molecules (lignin and hemicellulose) also were available to take part in the esterification. It is estimated that the increase on nitrogen content is related to triethylamine, which despite being used only as catalyst in the process, eventually took part in the reaction due to the basic character of amine and hydrogen acid in the final product.
Table 1. Elementary analysis of the fiber, refined and without refining pulps of Bambusa vulgaris Schrad chemically
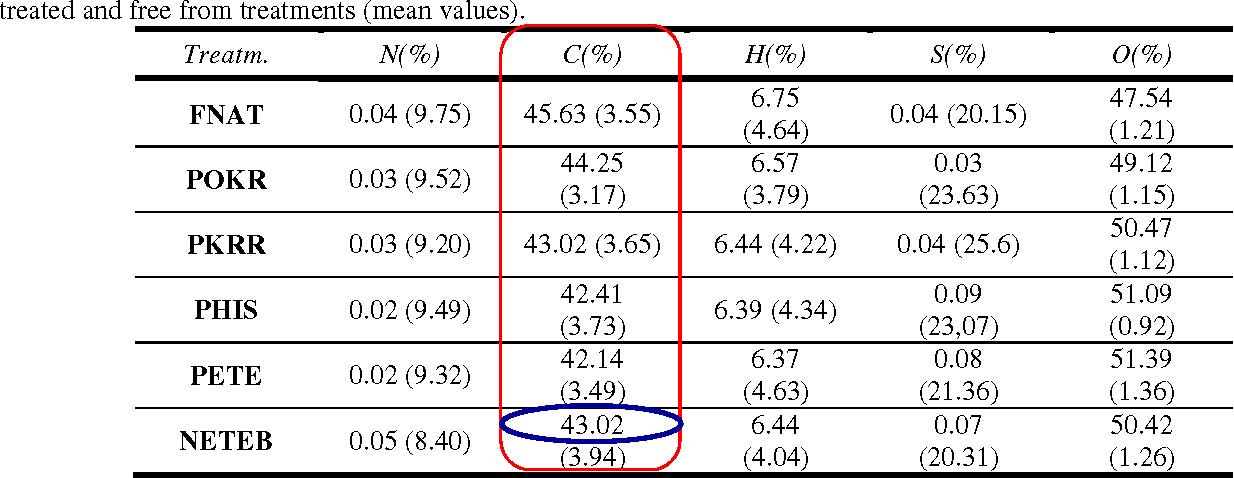
N(%): Nitrogen content; C(%): Carbon content; H(%): Hydrogen content; S(%): Sulfur content; O(%): Oxygen content; NOTE: Values in brackets refer to variation coefficients VC (%). FNAT: natural fiber; POKR: pulp without refining; PKRR: refined pulp; PHIS: refined pulp treated with NaOH 2%; PETE: refined pulp treated with NaOH 2% and submitted to extractions with organic solvents; NETEB: refined pulp treated with NaOH 2% + extraction with organic solvents + esterification with dianhydride.
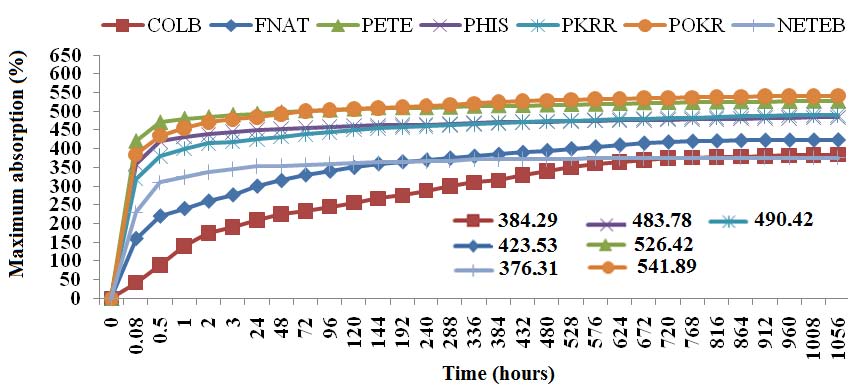
Figure 6. Comparative graphic of maximum absorption of water from the culm, culm fiber and unrefined pulps and refined unmodified and chemically modified Bambusa vulgaris Schrad. COLB: culm of bamboo; FNAT: natural fiber; POKR: pulp without refining; PKRR: refined pulp; PHIS: refined pulp treated with NaOH 2%; PETE: refined pulp treated with NaOH 2% and submitted to extractions with organic solvents; NETEB: refined pulp treated with NaOH 2% + extraction with organic solvents + esterification with dianhydride.
It is also possible to observe an increase in the carbon percentage for the pulp modified with dianhydride in relation to the pulp treated with organic solvents. This result confirms the improvement of carbon percentage in the esterified pulp. Another important observation is related to the hydrogen content of refined pulp treated with NaOH, equal to 42.41%. It is observed that in relation to the previous treatment, there was a reduction of about 1.5%. it is estimated that the treatment have broken the extensive hydrogen bonds of the lignocellulosic structure, making the hydroxilic groups more accessible for esterification reactions.
3.5 Analysis of the maximum water absorption
Figure 6 shows the percentage of water absorption of samples of Bambusa vulgaris Schrad in function of time in accordance with the type of process they were subjected. The water absorption is an important feature of composites systens reinforced by vegetables fibers, since it evaluates the potential of these composites for specific final applications. Generally, the water absorption occurs due to the hygroscopic and hydrophilic nature of the natural fibers.
Water absorption tests are useful to evaluate different methods of surface modification in these fibers. The high water absorption by the natural fibers may cause a decrease of mechanical properties, favoring the biodegradability and changes in fiber dimensions. Were observed high values of maximum absorption refering to unrefined fiber (POKR) and modified pulp with solvents (PETE) regarding natural fiber (FNAT). The pulp esterified (NETEB) submitted a absorption index lower than that of natural fiber. The treatment of the pulp with dianhydride was the cause for this decrease, since the ester groups formed after the modification are highly hydrophobic.
Another important conclusion is that the refining operation has been performed in aqueous solution with a pH between 9 and 10 since the literature suggests moderately alkaline pH, more precisely between 7 and 8, as Ferreira [51] and Uesaka [52]. This difference may contribute in reducing the absorption index and porosity of the pulp, since the surface tension should always be greater than the amount of cations present in the pulp so that there is swelling of the samples [53]. Ferreira [51] and Clark [54] contend that the increased crystallinity index reflects in a larger inaccessibility of the hydroxyl groups harming the swelling, and consequently the absorption of water.
This information is according to the results obtained after the refining process and treatment with sodium hydroxide, which have achieved elevations in their crystallinity index. The graphs concerning refined and unrefined pulps exhibit the same behavior, ie, high values of absorption at first with few variations in absorption over time. Opposite behavior was observed by the fiber and the stem Talisca. The samples presented a characteristic gradual absorption throughout most of the time with stabilizing trend more delayed in their absorption. All samples exhibit a logarithmic behavior to their respective absorption curves with R2 values around 0.97 for fibers and slivers and 0.63 for pulp. This difference is just in the initial stage where the pulps exhibit a more linear behavior in relation to the natural fiber.
4. CONCLUSIONS
There was a difference of approximately 20%, on average, in the indexes water absorption between the modified pulp (BTDA) and natural fibers (FNAT). The start decomposition temperature also decreased due to the incorporation of the ester group, which caused an improvement of carbon content in the final structure of the lignocellulosic material. Infrared spectra showed bands relative to the ester and carboxylic acid groups, evidencing the exchange of OH by ester groups in the cell wall of the material. Results showed the efficiency of the use of benzophenone tetracarboxylic dianhydride as crosslinker agent in the modification of superficial chemical composition of the bamboo refined pulp, becoming less hydrophilic and more adequate to be used as reinforcement agent in polymers of apolar nature, as has already been done with acetic anhydride in the process known as acetylation. The test of maximum water absorption performed in the samples, confirms definitively that modification with dianhydride was a success. 376.31% of maximum water absorption was the lowest value found among all samples tested.
5. REFERENCES
[1]. Chawla, K. K. âComposite Materials: Science and Engineeringâ. 1st Ed. New York, Springer-Verlag, 1987, 292p. [ Links ]
[2]. Taj, S.; Munamar, A. M.; Khan. S. âNatural Fiber-Reinforced Polymer Compositesâ. Proceedings of the Pakistan Academy of Science, 2007; 44: p. 129-144.
[3]. Chattopadhyay, S. K.; Khandal, R. K.; Uppaluri, R.; Ghoshal, A. K. âBamboo Fiber Reinforced Polypropylene Composites and Their Mechanical, Thermal, and Morphological: Propertiesâ. Journal of Applied Polymer Science, 2011, 119: p.16191626.
[4]. Lopez, L. F.; Correal, J. F. âExploratory study on the glued laminated bamboo Guadua angustifolia kunt as a structural materialâ. Maderas: Ciencia y Tecnologia, 2009, 11: p.171-182.
[5]. Mengeloglu, F.; Karakus, K. âThermal Degradation, Mechanical Properties and Morphology of Wheat Straw Flour Filled Recycled Thermoplastic Compositesâ. Sensors, 2008, 8: p.500-519.
[6]. Dhakal, H.; Zhang, Z.; Richardson, M. âEffect of Water Absorption on the Mechanical Properties of Hemp Fibre Reinforced Unsaturated Polyester Compositesâ. Composites Science and Technololy, 2007, 67: p.1674-1683.
[7]. Kushwaha, P. K.; Kumar, R. âInfluence of Chemical Treatments on the Mechanical and Water Absorption Properties of Bamboo Fiber compositesâ. Journal of Reinforced Plastics and Composites, 2011, 30: p.73-85.
[8]. Bruck, A.; Evans, J. J.; Peterson, M. L. âThe role of mechanics in biological and biologically inspired materialsâ. Experimental mechanics, 2002, 42: p.361-371.
[9]. Amada, S.; Ichikawa, Y.; Munekata, T.; Nagase, Y.; Shimizu, H. âFiber texture and mechanical structure of bambooâ. Composites part B, 1997,28: p.13-20.
[10]. Zou, L.; Jin, H.; Lu, W.; Li X. âNanoscale structural and mechanical characterization of the cell wall of bamboo fibersâ. Materials Science and Engineering C, 2009, 29: p.1375-1379.
[11]. Rao, K. M. M.; Rao, K. M. âExtraction and Tensile Properties of Natural Fibers: Vakka, Date and Bambooâ. Composite Structure, 2007, 77: p.288-295.
[12]. Bledzki, A. K.; Gassan, J. âComposites reinforced with cellulose based fibersâ. Progress Polymer Science, 1999, 24; p.221-274.
[13]. Kumar, V.; Kushwaha, P. K.; Kumar, R. âImpedance-Spectroscopy Analysis of Oriented and Mercerized Bamboo Fiber-Reinforced Epoxy Compositeâ. J Mater Sci, 2011, 46: p.3445-3451.
[14]. Sen, T.; Reddy, H. N. J. âApplications of Sisal, Bamboo, Coir and Jute and Natural Composites in Structural Up Gradation. International Journal of Inovationâ. Management and Technology, 2011, 2: p.186-191.
[15]. Chandramohan, D; Marimuthu, K. A. âReview on Natural Fibersâ. International Journal of Research and Reviews in Applied Sciences, 2011, 8: p.194 206.
[16]. Prasad, A. V. R.; Rao, K. M.. âMechanical Properties of Natural Fibre Reinforced Polyester Composite: Jowar, Sisal e Bambooâ. Materials and Design, 2011, 32: p.4658-4663.
[17]. Islam, M. N., Rahman, M. R.; Haque, M. M.; Huque, M. M. âPhysico-mechanical properties of chemically treated coir reinforced polypropylene compositesâ. Composites Part A, 2010, 41: p.192â198.
[18]. Bertoti, A. R.; Luporini, S.; Esperidião, M. C. A. âEffects of acetylation in vapor phase and mercerization on the properties of sugarcane fibersâ. Carbohydrate Polymers, 2009, 77: p.20 24.
[19]. Habibi, Y.; El-Zawaway, W. K.; Ibrahim, M. M.; Dufresne, A. âProcessing and characterization of reinforced polyethylene composites made with lignocellulosic fibers from Egyptian agroindustrial residuesâ. Escole Française de Papeterie et des Industries Graphiques, Institut National Polytechnique de Grenoble, 2007.
[20]. Troedec, M. L.; Sedan; D.; Peyratout, C.; Bonnet, J. P.; Smith, A.; Guinebretiere, R.; Gloaguen, V.; Krausz, P. âInfluence of Various Chemical Treatments on the Composition and Structure of Hemp Fiberâ. Composites: Parte A, 2008, 39: p.514-522.
[21]. Liu, L.; Wang, Q.; Xia, Z.; Yu, J.; Cheng, L. âMechanical Modification of Degummed Jute Fibre for High Value Textile and Usesâ. Ind. Crop. Prod, 2010, 31: p.43-47.
[22]. Weyenberg, V.; Truong, T.; Vangrimde, C. B.; Verpoest, I. âImproving the Properties of UD Flax Fibre Reinforced Composites by Applying an Alkaline Fibre Treatmentâ. Composites Part A Applied Science and Manufacturing, 2006, 37: p.1368-1376.
[23]. Singha, A. S.; Thakur, V. K. âMorphological, Thermal, and Physicochemical Characterization of Surface Modiï¬ed Pinus Fibersâ. International Journal of Polymer Anal. Charact, 2009, 14: p.271â289.
[24]. Kumar, P.; Barret, D. M.; Delwiche, P.; Stroeve,
M. J. âMethods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Productionâ. Ind. Eng. Chem. Res, 2009, 48: p.3713-3729.
[25]. Taherzadeh, M. J.; Karimi, K. âPretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Reviewâ. Int. J. Mol. Sci, 2008, 9: p.1621-1651.
[26]. Razera, I. A. T.; Frollini, E. âComposite based on jute fibers and phenolics matrices: properties of fibers and compositesâ. Journal of Applied Polymer Science, 2004, 9: p.1077-1085.
[27]. Sanchez, S. E. M.; Cavani, C. S.; Leal, C. V.; Sanchez, C. G. âCompósito de resina de poliéster insaturado com bagaço de cana-de-açúcar: influência do tratamento das fibras nas propriedadesâ. PolÃmeros. 2010, 20: p.194-200.
[28]. TAPPI â Testing Procedures of Technical Association of the Pulp and Paper Industr. In: TAPPI Standard Method. Atlanta, USA. Cd-Rom. 2001.
[29]. ASTM D 1921 Standards Test Methods for Particle Size (Sieve Analylis) of Plastic Materials, Philadelphia (EUA): Americam Society For Testing And Material Standards. 2001.
[30]. Nelson, M. L.; O'connor, R. T. âRelation on certain infrared bands to cellulose crystallinity and crystal lattice type. Part I: Spectra of lattice types I, II, III and of amorphous celluloseâ. Journal of Applied Polymer Science, 1964, New Orleans, 8: p.1311-1324.
[31]. Akerholm, M.; Histerstoisser, B.; Salmen, T. âCharacterization of the crystalline structure of cellulose using static and dynamic FTIR spectroscopyâ. Carbohydrate Research, 2004, 339: p.569-578.
[32]. International Association of Wood Anatomy â IAWA -List of microscopic features for wood identification. IAWA Bulletin, Leiden, 1989, 10: p.226-332.
[33]. Milanez, A. C.; Foelkel, C. E. B. âProcesso de deslignificação com oxigênio para a produção de celulose de eucaliptoâ. In: CONGRESSO ANUAL DA ABCP, 14. 1981, São Paulo. Anais... São Paulo: ABCTP 9 p.37-110. 1981.
[34]. Oliveira, V. A. âSÃntese e caracterização de géis de acetato de celullose reticulados com dianidrido piromelÃtico e dianidrido do ácido 3,3â, 4,4â benzofenona tetracarboxÃlicoâ. 2008. Dissertação (Mestrado em Engenharia e Materiais) -Universidade Federal de Ouro Preto, Ouro Preto, MG. 2008.
[35]. Carrilo, F.; Colom, X.; Suñol, J. J.; Saurina, J. âStructural FTIR analysis and termal characterization of lyocell and viscose-type fibresâ. European Polymer Journal, 2004, 40: p.2229-2234.
[36]. Akerholm, M.; Hinterstoisser, B.; Salmén, L. âCharacterization of the crystalline structure of cellulose using static and dynamic FTIR spectroscopyâ. Carbohydrate Research, 2004, 339: p.569-578.
[37]. Oh, S.Y.; Yoo, D. I.; Shin, Y.; Seo, G. âFTIR analysis of cellulose treated with sodium hydroxide and carbón dioxideâ. Carbohydrate Research, 2005, 340: p.417-428.
[38]. Tserki, V.; Zafeiropoulos, F. S.; Panayiotu, C. A. âStudy of the effect of acetylation and propionylation surface treatments on natural fibersâ. Composites Part A â Applied Science and manufacturing, 2005, 36: p.1110-1118.
[39]. Hult, E.; Larsson, P. T.; Iversen T. âCellulose fibril aggregation â an inherent property of kraft pulpsâ. Polymer, 2001, 42: p.3309-3314.
[40]. Sousa, K. S.; Airoldi, C. TermoquÃmica da Interação de cobre em quitosana quimicamente modificada com anidrido succÃnicoe etilenodiamina. In: IV SIMPÃSIO IBEROAMERICANO DE QUITINA. Natal, Brasil. 2007.
[41]. Siqueira, G. A. F.. âProdução e caracterização de compósitos fenólicos com fibras de sisal modificadasâ. 2006. Dissertação (Mestrado em Engenharia de Materiais) â Universidade Federal de Ouro Preto, Ouro Preto, MG. 2006.
[42]. Oliveira, F.; Frollini, E. âSisal fibers modified with sodium lignosulphonate: improvement of adhesion between fibers and phenolic type matricesâ. In: 26th Polymer Processing Society, Banff. 26th Polymer processing society. 2010.
[43]. Karnitz Junior, O.; Gurgel, L. V. A.; Freitas, R. P.; Gil, L. F. âAdsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by mercerized cellulose and mercerized sugarcane bagasse chemically modified with EDTA diahydride (EDTAD)â. Carbohidrate Polymers, 2009, 77: p.643-650.
[44]. Cláudio-da-Silva jr, E. âChemical pulp beating related to fiber structureâ. Thesis (Doctor of Philosophy), State University of New York, Syracuse, New York. 1981.
[45]. Paavilainen, L. âImportance of cross-dimensional fibre properties and coarseness for the characterization of softwood sulphate pulp, Paperi ja Puuâ. Paper and Timber, 1993, 75: p.343-351.
[46]. Lindstrom, T.; Carlsson, G. âThe effect os chemical environmental on fiber swellingâ. Svensk Papperstidining, 1982, 3: p.4-20.
[47]. Nazhad, M.; Paszner, L. âFundamentals of strenght loss in recycled paperâ. Tappi Journal, 1994, 77: p.171-179.
[48]. Brum, S. S.; Oliveira, L. C. A.; Bianchi, M. L.; Guerreiro, M. C.; Oliveira, L. K.; Carvalho, K. T. G. âSÃntese de acetato de celulose a partir da palha de feijão utilizando N-bromossuccinimida (NBS) como catalisadorâ. PolÃmeros, 2012, 22: p.447 452.
[49]. Abreu, A. L. âModificação quÃmica de ResÃduo lignocelulósico para preparação de compósitoâ. 2011. Dissertação (Mestrado em Engenharia de Materiais) -Universidade Federal de Ouro Preto, Ouro Preto, MG. 2011.
[50]. Gatenholm , P.; Gellerstedt, F. âSurface properties of lignocellulosic fibers bearing carboxylic groupsâ. Cellulose, 1999, 6: p.103â121.
[51]. Ferreira, P. J. T. âEstudo de pastas kraft de Eucalyptus globulus: CaracterÃsticas estruturais e aptidão papeleiraâ. 2000. Tese (Doutorado em Engenharia QuÃmica) -Universidade de Coimbra, Coimbra, Portugal.
[52]. Uesaka, T. Determination of fiber-fiber bond properties, Mark, R. E. (Ed.), âHandbook of physical and mechanical testing of paper and paperboardâ. Marcel Dekker, New York: Basel, 2: 379-402 (1984).
[53]. Guimarães Junior, M.; Novack, K. M..; Botaro, V. R.; Protásio, T. P.; Couto, A. M. âcaracterização fÃsico-quÃmica de fibra e polpas de bambusa vulgaris schrad para utilização em compósitos poliméricosâ. Revista Latinoamericana de Metalurgia y Materiales, 2013, 33: p.33-42.
[54]. Clark, J. D. A. âPulp technology and treatment for paperâ. 2ª ed. San Francisco: Miller Freeman Publications Inc. San Francisco, 752p. 1985.