Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Ciencias Veterinarias
versión impresa ISSN 0258-6576
Rev. Fac. Cienc. Vet. v.48 n.2 Maracay dic. 2007
Renal Glycosuria Induced by Acute Thioacetamide Administration to Rats
Glucosuria Renal Producida por la Administración Aguda de Tioacetamida en Ratas
Freddy Gonzalez-Mujica*1, José D. Pradere** and Julio C. Gonzalez***
*Sección de Bioquímica Médica, Instituto de Medicina Experimental, Facultad de Medicina, Universidad Central de Venezuela. Apartado postal 50.587 Sabana Grande, Caracas. Venezuela.
**Departamento de Ciencias Biomédicas, Facultad de Ciencias Veterinarias, Universidad Central de Venezuela. Maracay, 2101, Venezuela.
***Escuela de Bioanálisis, Facultad de Ciencias de la Salud, Universidad de Carabobo. Valencia, Venezuela. Correo-E:gonzalef@cantv.net
Resumen
La administración de tioacetamida (TAA) en ratas (75 mg/kg de peso vía subcutánea) produjo un incremento estadísticamente significativo de la eliminación urinaria de glucosa, Na+ y fosfato. Adicionalmente, ocurrió una disminución de la presión arterial y de la velocidad de filtración glomerular, sin cambios en la excreción urinaria de K+, H+, aminoácidos ni en la glucemia. Se observó una disminución del 60% y 75% del Tmax y Tmin para la glucosa, respectivamente. La TAA alteró la captación de [14C]-glucosa por vesículas de membrana de borde apical renal (BBMV), disminuyendo en un 16% el pico de captación a los 30 s, en un 63% la Vmax y en aproximadamente un 82% el Km. También disminuyó en un 52% el Kd y cerca de un 59% el número de sitios de unión para [3H]-floricina en BBMV. Estos resultados sugieren fuertemente que la droga reduce la cantidad del co-transportador Na+/glucosa (SGLT1) presente en la membrana apical del túbulo contorneado proximal. La disminución de la temperatura de transición de la maltasa y de la fosfatasa alcalina de las BBMV así como el incremento de la cantidad de ácidos grasos insaturados presentes en los fosfolipidos de las BBMV sugieren un incrementoen la fluidez de dichas membranas. Estos resultados pudieran explicar el incremento en la afinidad del SGLT1 por la glucosa producida por la TAA.
(Palabras clave: Rata, tioacetamida, carcinógeno, glucosuria, SGLT1)
Abstract
Thioacetamide (TAA) administration to rats (75 mg/kg body weight by subcutaneous injection) produced a statistically significant increase in the urinary excretion of glucose, Na+ and phosphate. In addition, there was a decrease in the arterial pressure and in the glomerular filtration rate, without changes in the urinary excretion of K+, H+, amino acids or in the glycaemia. Decreases of 60% and 75% were observed in the Tmax and Tmin for glucose, respectively. TAA intoxication reduced in 16% the overshoot, by almost 63% the Vmax and by nearly 82% the Km of the brush border membrane vesicles (BBMV) [14C]-glucose uptake. It also decreased about 52% the Kd and by almost 59% the number of binding sites of BBMV [3H]-phlorizin. These results strongly suggest that TAA reduces the amount of Na+/glucose co-transporter (SGLT1) present in the apical membrane of the proximal renal tubule. An increase in the BBMV fluidity is suggested by the decrease in the BBMV maltase and alkaline phosphatase transition temperature and by the increase in the unsaturated fatty acids content of BBMV phospholipids. These results may explain the increase in SGLT1 affinity for glucose evidenced by the decrease in the Kmof the BBMV [14C]-glucose uptake and the Kd of the BBMV [3H]-phlorizin binding caused by TAA intoxication.
(Key words: Rat, thioacetamide, carcinogens, glycosuria, SGLT1)
Recibido: 23/11/07 - Aprobado: 21/01/08
Introduction
Thioacetamide (TAA) was originally used as a fungicide (Vadi and Neal, 1981). Thioacetamide is a weak carcinogen that mainly affects liver and kidney (Kleinfeld, 1957; Bruck et al., 2007; Hasegawa et al., 2007; Rekha et al., 2008) and causing the latter histological alterations in the proximal renal tubule (Baker and Smuckler, 1974), an increase in the size of the cortical kidney nuclei and nucleoli and a decrease in the glucose-6-phosphatase activity of the nuclear envelope (Inciarte and González-Mujica, 1988). At present, it is not clear if TAA has a general effect on macromolecules synthesis and/or degradation; however it has been shown that its administration reduces the expression of liver cytochrome P450 (Sathyabama and Padmanaban, 1984) and decreases the amount of liver nuclear envelope glucose-6-phosphatase (González-Mujica et al., 1993).
Lundsgaard (1933) postulated the phosphorylation/dephosphorylation theory to explain the glucose changes during its intestinal absorption; this theory claims the participation of both the hexoquinase and glucose-6-phosphatase enzymes. However, the phlorizine dose required to inhibit the glucose phosphorylation/dephosphorylation is large in comparison to the amount needed to produce glycosuria (Taggart, 1958); furthermore the lack of glycosuria in patients with Von Gierke disease (Cori, 1954) permits to rule out the phosphorilation/dephosphorilation theory. However, recently it has been proved that in animals that do not express the glucose transporter 2 (GLUTS 2), glucose undergoes phosphorylation/dephosphorylation during its intestinal absorption (Stümpel et al., 2001). It is interesting to point out that the intestinal absorption and kidney reabsorption of glucose occur by a similar if not identical mechanism.
In view of the above results and of our findings, in preliminary experiments in which TAA treatment produced glycosuria in rats, we studied the effects of TAA on some kidney functions and on the Na+/glucose co-transporter (SGLT1) of the proximal renal tubule by measuring the BBMV D-[14C]-glucose uptake and the BBMV [3H]-phlorizin binding in order to estimate the amount of SGLT1 in the proximal tubule brush border membrane, also some lipid characteristics and, the fluidity of the BBMV were studied.
Materials and Methods
Animals and treatments
Male Sprague-Dawley rats weighing 200-250 g and located at the Instituto de Medicina Experimental, Facultad de Medicina, Universidad Central de Venezuela, were used. The experimental group received a dose of TAA of 75 mg/kg of body weight; (Koch Ligh Lab, England) dissolved in 0.9% NaCl, at a concentration of 10 mg/mL, by subcutaneous injection during 3 consecutive days. The control group received an equivalent volume of a 0.9% NaCl solution. The rats were used 24 h after the last TAA dose was given. Two to four rats were kept in metallic cages with free access to food and water. The number of animals per experiment varied.
Kidney function
Rats were anesthetized by intraperitoneal injection of penthobarbital (60 mg/kg of body weight). Catheters were placed in the jugular vein, the carotid artery and the urinary bladder, respectively. Glomerular filtration rate was measured using inulin clearance. Animals were injected continuously by the vein with 28 mL/min. of 5% inulin in 0.9% NaCl. Forty minutes after the surgery was finished, samples of blood (250 µL) and urine (all the volume present at that time) were collected every 20 minutes, up to a total of 5 samples. Inulin in blood and urine samples, was estimated as described by Heyrovsky, (1956).
To measure the tubular glucose reabsorption, rats were injected continuously through the vein with 28 mL/min. of increasing concentrations of glucose (5-30%). Blood and urine samples were collected as before. In blood and urine samples, glucose concentration was estimated by the glucose oxidase method (Lott and Turner, 1975). A glucose titration curve was constructed, following Robson et al. (1968).
Urinary elimination of several ions and compounds was carried out as follows: Na+ and K+ by flame spectrophotometer; phosphate (Fiske and Subbarow, 1925), H+ potenciometricaly, and amino acids by colorimetric technique (Henry, 1969).
Also, the blood pressure was measured by placing a catheter in the carotid artery connected to a pressure transducer attached to a polygraph (Grass, model 7-B)
D-[U-14C]-glucose uptake by brush border membrane vesicles (BBMV) from proximal renal tubule
The BBMV were purified following Malathi et al. (1979). The relative purity of the fraction was estimated by electron microscopy (TEM Hitachi 500) and by measuring the activity of the following enzymes: succinate-cytochrome C reductase, NADH-cytochrome C reductase and b-glucuronidase for mitochondria, microsomes and lysosomes, respectively (Bergmeyer, 1963); Na+- K+-ATPase for basolateral membrane (Cohen et al., 1986), maltase (De Sa Moita et al., 1989) and alkaline phosphatase (Andersch and Szcypinski, 1947) as BBMV markers.
The maltase and alkaline phosphatase enzymes were also determined, using control and TAA treated BBMV at different temperatures (from 0 to 40 ºC) in order to draw an Arrhenius plot and calculate the activation energy (AE) and the transition temperature. Proteins were measured by Lowry et al. (1951).
The D-[U-14C]-glucose (2.5 mCi/mM Commisariat a Lenergie atomique, France) uptake by BBMV obtained from control and TAA-treated rats was carried out as described by Malathi et al. (1979). The radioactivity was measured using a liquid scintillation counter (LKB Rackbeta) with 75% efficiency.
BBMV density
BBMV were placed on the top of a linear sucrose gradient (density 1.10-1.30 g/cm3) in 10 mM Tris-HCl pH: 7.4 and centrifuged at 50000 g for 16h at 4ºC. For each gradient fraction, the 280 nm absorption and the refraction index were recorded.
Lipid extraction and separation
BBMV total lipids were extracted following Folchs method (Folch et al., 1975), the phospholipids were separated by silica G thin layer chromatography using petroleum ether: diethyl ether: acetic acid in a proportion of 90:10:1. The phospholipids phosphorus was estimated (Chen et al., 1956) after hydrolysis with concentrated perchloric acid at 180ºC for 2 h. The methyl esters of fatty acid phospholipids were obtained (Kates, 1964). The fatty acid methyl esters were separated using a Hewlett Packard 4880 gas-liquid chromatographer with a glass column (0.4 x 184 cm) with diethylglycol adipate as stationary phase and nitrogen as mobile phase at a rate flow of 30 mL/min and a temperature of 200 ºC.
[3H]-phlorizin binding to BBMV from the proximal renal tubule
The [3H]-phlorizin binding to BBMV was used to measure the amount of SGLT1 present in the brush border membrane renal tubule. [3H]-phlorizin 35-55 Ci/mmol. (New England Nuclear. USA) binding to BBMV obtained from control and TAA-treated rats was carried out as described elsewhere (Silverman and Black, 1975). From the free and bound [3H]-phlorizin values, Scatchard plots were constructed in order to calculate the number of binding sites and their affinity. The radioactivity was measured using a LKB Rackbeta liquid scintillation counter with 35% efficiency.
Statistical analysis
All data are expressed as mean ± standard error, and the statistical significance were performed by the Student t test.
Results
TAA effects on arterial pressure and renal function.
At shown in Table 1, TAA administration to rats produced a significant increase in glucose (P<0.05), sodium (P<0.05) and phosphate (P<0.01) urinary fractional excretion. This compound also caused a 42% decrease in the glomerular filtration rate and a 7.7% decrease in the arterial pressure (Table 1). On the other hand, there were no changes in the urinary excretion of potassium, amino acids, H+ nor in the blood glucose due to TAA administration.
Table 1. TAA effects on glycaemia, blood pressure and some renal function parameters
| Parameter | N | Control | TAA-treated |
| GFR (mL/min.) | 10 | 1.78 ± 0.25 | 1.03 ± 0.10 ** |
| Glucose UFE (%) | 8 | 0.004 ± 0.002 | 13.19 ± 5.22 ** |
| Phosphate UFE (%) | 6 | 0.051 ± 0.012 | 3.93 ±1.66 ** |
| Sodium UFE (%) | 6 | 3.19 ± 0.94 | 6.11 ± 2.64 * |
| Potassium UEF (%) | 6 | 18.33 ± 6.32 | 21.90 ± 7.89 |
| Amino acid (mg/mL) | 10 | 0.031 ± 0.2 | 0.020 ± 0.018 |
| Urinary pH | 10 | 6.60 ± 0.14 | 6.56 ± 0.09 |
| Glycaemia (mg %) | 10 | 82.56 ± 3.60 | 82.30 ± 4.64 |
| Blood pressure (mm Hg) | 6 | 110.24 ± 1.21 | 101.80 ± 3.47 * |
The following parameters were measured for control and TAA-treated rats (75 mg/Kg body weight): glomerular filtration rate (GFR), urinary fractional excretion (UFE) of glucose, phosphate, sodium and potassium, urinary amino acid excretion, urinary pH, glycaemia and blood pressure, as described in Materials and Methods. The values correspond to the means ± standard error. N corresponds to the number of the experiments done, * and ** represent statistically significant differences at P<0.05 and P<0.01, respectively
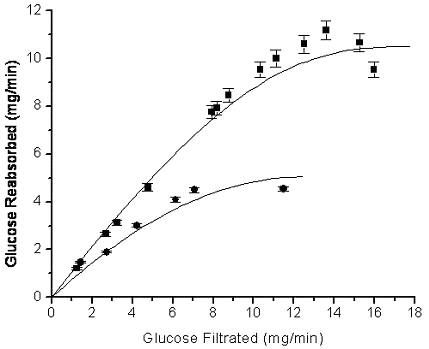
TAA effects on glucose tubular reabsorption
The glucose titration curves for control and TAA-treated rats are shown in Figure 1. It could be observed that in treated animals, an increase in the load of filtered glucose was accompanied by a decrease in tubular reabsorption; this was in contrast to what was observed in controls, where an increase in the filtered glucose load occurred simultaneously with an increase in the tubular reabsorption. In controls, the Tmax G and Tmin G were of 10.77 mg/min and 8.91 mg/min, respectively. On the other hand, in the TAA-treated group the Tmax G and the Tmin G were of 4.27 mg/min (60.35% decrease in relation to control) and 2.25 mg/min (74.75% decrease in relation to control), respectively.
Figure 1. TAA effects on glucose titration curve. To measure the tubular glucose reabsorption, control (![]() ) and TAA-treated (
) and TAA-treated (![]() ) rats were injected in the vein with 28 mL/min. of increasing concentrations of glucose (5 – 30 %). In blood and urine samples, glucose concentration was estimated by the glucose oxidase method (Lott and Turner, 1975). Glucose titration curves were done following Robson et al. (1968). Values represent the average ± standard errors of 18 separate experiments
) rats were injected in the vein with 28 mL/min. of increasing concentrations of glucose (5 – 30 %). In blood and urine samples, glucose concentration was estimated by the glucose oxidase method (Lott and Turner, 1975). Glucose titration curves were done following Robson et al. (1968). Values represent the average ± standard errors of 18 separate experiments
BBMV relative purity
As shown in Table 2, the BBMV fraction was enriched with the enzymes maltase and alkaline phosphatase and presented a low amount of the marker enzymes of other organelles; this result was similar to that reported by others (Malathi et al., 1979). It is interesting to point out that TAA treatment did not affect the BBMV enzymatic profile (results not shown). The electron microscopy showed vesicles of different sizes, with little or no electrondense material inside (results not shown).
Table 2. BBMV enzimatic characteristics
| Enzyme | Homogenate | BBMV | Relative activity | % Recuperation |
| Maltase | 126.4 ± 0.2 | 1180.5 ± 0.3 | 9.4 ± 0.2 | 27.4 ± 1.2 |
| Alkaline phosphatase | 9,1 ± 2.1 | 63.8 ± 8.0 | 7.0 ± 1.8 | 26.0 ± 1.4 |
| NADH Cyt- c reductase | 47.5 ± 0.3 | 4.5 ± 0.6 | 0.09 ± 0.03 | 0.25 ± 0.03 |
| b-glucuronidase | 29.5 ± 0.1 | 10.6 ± 0.5 | 0.35 ± 0.1 | 1.2 ± 0.1 |
| Na+/K+ ATPase | 101.9 ± 0.4 | 42.7 ± 0.5 | 0.45 ± 0.1 | 0.9 ± 0.1 |
| Succinate Cyt-c reductase | 37.4 ± 0.2 | 1.6 ± 0.2 | 0.04 ± 0.02 | 0.1 ± 0.09 |
The BBMV were prepared following Malathi et al., (1979) and the enzymatic assays were carried out as described in Material and Methods. The values represent the means ± standard error of 3 to 5 separate experiments. The enzymatic activities are expressed as follow:
Maltase: nmol of glucose produced/mg protein/min
Alkaline phosphatase: mmol of p-nitrophenil phosphate/mg protein/min
NADH Cit-C reductase: nmol Cit reduced/mg protein/min
b-glucuronidase: mg phenolftalein liberated/mg protein/h
Succinate Cyt-C reductase: nmol of Cyt-c reduced/mg protein/min
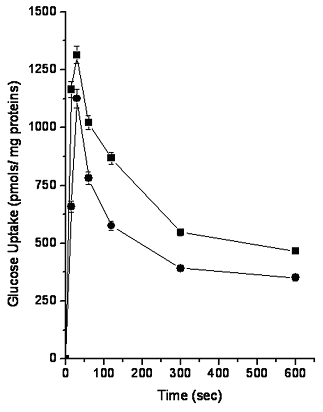
TAA effects on kinetic D-[U-14C]-glucose uptake by BBMV
As shown in Figure 2, controls and TAA-treated BBMV [14C]-glucose uptake exhibited an overshoot at 30 sec and afterwards it declined in a similar way to that reported elsewhere (Malathi et al., 1979). The BBMV [14C]-glucose overshoot was 1350 ±38 and 1143 ± 40 pmol of glucose/mg of proteins for control and TAA-treated rats, respectively, with difference at P<0.01.
Figure 2. TAA effect on D-[14C]-glucose uptake by BBMV. BBMV obtained from control (![]() ) and TAA-treated (
) and TAA-treated (![]() ) rats were incubated with 0.2 mM D-[14C]-glucose (1 mCi/mmol) and the reaction was terminated by the fast filtration method as described Malathi et al. (1979). The radioactivity was measured using a liquid scintillation counter (LKB Rackbeta). The values represent the average of 4 separate experiments ± standard errors
) rats were incubated with 0.2 mM D-[14C]-glucose (1 mCi/mmol) and the reaction was terminated by the fast filtration method as described Malathi et al. (1979). The radioactivity was measured using a liquid scintillation counter (LKB Rackbeta). The values represent the average of 4 separate experiments ± standard errors
As shown in Table 3, the [14C]-glucose uptake by BBMV kinetic parameters were similar to that reported by others (Turner and Silverman, 1977), and it could be observed that TAA treatment produced almost a 63% decrease in the Vmax and also a decrease of nearly 82% in the Km (both differences statistically significant at P<0.01).
Table 3. TAA effects on Na+/glucose cotransporter kinetic parameters
|
| KM (mM) | VMAX (nmol/mg protein x min) |
| Control | 4.16 ± 1.18 | 12.63 ± 3.57 |
| TAA treated | 0.73 ± 0.03 * | 4.69 ± 0.51 * |
BBMV from control and TAA-treated rats (3 doses of 75 mg/Kg body weight) were incubated for 10 sec. at 20 ºC in 0.1 M NaCl, 0.1 M manitol, 5 mM HEPES-Tris pH 7.5 and increasing glucose concentrations (0.05-0.25 mM) in the presence of 0.1 mCi/assay of [14C]-glucose. The reaction was stopped by fast filtration with nitrocellulose membranes, and the radioactivity was counted. The Km and VMAX were calculated from the double reciprocal plots. The values represent the means of 3 experiments ± standard errors. *Statistically significant difference at p<0.01
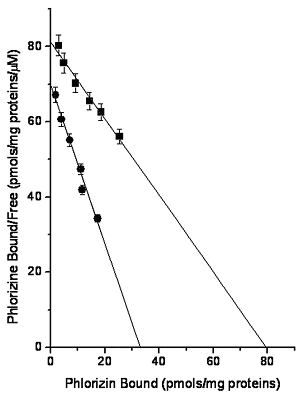
TAA effects on [3H]-phlorizin binding to BBMV
From the Scatchard plot shown in Figure 3 the following constant for [3H]phlorizin binding to control BBMV were calculated: Kd 0.98 mM, and the number of binding sites of 79.66 pmols/mg of proteins, values that were similar to earlier reports (Olianova et al., 2001). TAA treatment decreased by 52% the Kd (to 0.47 mM), and by almost 59% the number of binding sites (to 33.02 pmol/mg of proteins).
Figure 3. TAA effects on [3H]-phlorizin binding to BBMV. BBMV obtained from control (![]() ) and TAA-treated (
) and TAA-treated (![]() ) rats were incubated with increasing concentrations of [3H]-phlorizin (4.5 to 22.5 pmol) as described by Silverman and Black (1975). From the free and bound [3H]-phlorizin values, Scatchard plots were constructed. Each value represents the average of 4 separate experiments ± standard error
) rats were incubated with increasing concentrations of [3H]-phlorizin (4.5 to 22.5 pmol) as described by Silverman and Black (1975). From the free and bound [3H]-phlorizin values, Scatchard plots were constructed. Each value represents the average of 4 separate experiments ± standard error
TAA effects on BBMV fluidity
The study of the thermotropic effect of the maltase and alkaline phosphatase, obtained from the Arrhenius plots, showed the following results:
For maltase, in controls, the transition temperature was 29.6 ºC; the Activation Energy (AE) was 10.1 Kcal/mol, below the transition temperature and 7.0 Kcal/mol above it, corresponding to a 30% decrease. In membranes from TAA-treated animals, the transition temperature, for maltase, was 27.8º C; and the AE were of 7.1 and 5.21 Kcal/mol below and above the transition temperature respectively, showing a decrease of 26.8%.
For alkaline phosphatase, in controls, the transition temperature was 29.5ºC; the AE was 7.24 Kcal/mol below the transition temperature and 3.23 Kcal/mol above it, showing a decrease of 44.6%. In BBMV from TAA-treated animals the transition temperature, for alkaline phosphatase was 28.7ºC; and the AE were 6.97 and 3.3 Kcal/mol below and above the transition temperature, respectively, showing a decrease of 44.5%.
In control animals, the phospholipid/protein relationship for BBMV was 465±14 nmol/mg; and for TAA-Treated rats, it was 378±11 nmol/mg, a significant difference at P<0.01.
Table 4 illustrates the percentage of the different fatty acids present in BBMV phospholipids; there is an increase in the unsaturated fatty acids by TAA treatment (unsaturated/saturated 1.01), in comparison with the controls (unsaturated/saturated 0.86) with a greater difference in C 20:3, which increase 42.6% with drug administration.
Table 4. TAA effects on the fatty acid composition of phospholipids from BBM
| Fatty acid | Controls | TAA Treated | Statistic Significance |
| C 16 : 0 | 22.2 ± 0.7 | 19.9 ± 0.6 | p<0.02 |
| C 17 : 0 | 0.6 ± 0.05 | 0.7 ± 0,06 | NS |
| C 18 : 0 | 20.0 ± 0.4 | 18.0 ± 1.0 | p<0.01 |
| C 18 : 0 | 10.6 ± 0.2 | 10.1 ± 0.2 | NS |
| C 18 : 2 | 9.5 ± 0.6 | 9.6 ± 0.5 | NS |
| C 20 : 3 | 10.8 ± 1.0 | 15.4 ± 1.5 | p<0.01 |
| C20 :4 | 22.2 ± 0. | 21.7 ± 0.5 | NS |
| C 20 : 5 | 1.2 ± 0.06 | 0.6 ± 0.06 | p<0.05 |
| C 22 : 6 | 2.1 ± 0.09 | 2.0 ±0.08 | NS |
BBMV lipids from control and TAA-treated rats were separated on silica G using petroleum ether: diethyl ether: acetic acid in a proportion of 90:10:1. The fatty acids present in phospholipids were separated by gas chromatography. Data are expressed in percentages and represent the means ± standard errors of 12 separate assays with 3 rats in each. NS: not significant difference.
The flotation density of the BBMV was not affected by TAA treatment, being 1.15 g/cm3.
Discussion
It has been reported that TAA is nephrotoxic, however to cause kidney damage, either chronic (six months) exposure (Fleck et al., 1988) to the compound or a larger dose than the one used in this investigation is required (Waters et al., 2005). Furthermore, the histological examination of the kidney (results not shown) only showed an increase in the nuclei and nucleoli size of the proximal renal tubule cells, in agreement to our earlier report (Inciarte and González-Mujica, 1988).
The decrease in the glomerular filtration rate, produced by TAA administration (Table 1), could be due to the decrease in blood pressure induced by the drug. TAA also produces glycosuria without changes in glycaemia with an increase in the fractional excretion of Na+ and phosphate. It has been reported (Baker and Kleinman, 1974) that an increase in the osmolar clearance of glucose increases the sodium fractional excretion. Probably, the increases in the Na+ and phosphate fractional excretion are due to the increases water and glucose excretion and not to a direct effect of TAA, because the function of the solute cotransporters, such as Na+/glucose, are directly coupled to the water flow in the kidney tubule (Loo et al., 1994). Lötscher et al., (1997) suggest that the Na+/phosphate cotransporter is transported from the intracellular compartment to the brush border membrane of the proximal renal tubule, in response to changes in plasma phosphate concentration. In addition, in rats, when the extracellular volume increases, the urinary elimination of Na+ and phosphate also increases due to changes in the Na+/phosphate cotransporter that are related to transduction signals including cytoskeleton proteins such as: ezrin, moesin and PDZ-dcl (Puschett et al., 2003). At present, there is no available information regarding the possibility that cytoskeleton proteins regulate the amount of the Na+/glucose cotransporter present in the brush border membrane of the proximal renal tubule.
It has been suggested that the height of the glucose uptake by BBMV (overshoot) depends on the membrane permeability to the anion that accompanies the cation sodium (Kessler et al., 1978; Toggemburger et al., 1978). TAA administration produces a substantial decrease in the glucose overshoot that suggests changes in the membrane permeability that affect the transmembrane movement of Cl- and/or Na+ in these vesicles.
The glucose titration curves (Figure 1) prove that the glycosuric effect of TAA is due to a decrease in the glucose reabsorption capacity; furthermore the treated animals showed a lower Tmin G with respect to controls. Elsas and Rosemberg (1969) suggest that a decrease in the Tmin G indicates alterations in the kinetics parameter that in fact occur and is evidenced by a decrease in the SGLT1 Vmax induced by the TAA treatment (Table 3). The decrease in the Na+/glucose cotransporter Vmax implies an increase in glucose osmolar load that might explain the increase in the Na+ and phosphate urinary fractional excretion.
It has been suggested that Na+ induced conformational changes in the glucose binding site of the Na+/glucose cotransporter and that both sites are in the same polypeptide (Peerce and Wright, 1984;1985). If TAA alters the Na+ binding to the cotransporter, its capacity to bind glucose will be reduced.
The Km and Vmax values obtained for control BBMV Na+/glucose cotransporter (Table 3) are similar to the system of low affinity and high capacity that operates in the first portion of the proximal renal tubule described by Turner and Moran (1982). The reduction of the SGLT1 Vmax produced by TAA administration could be due to a noncompetitive inhibition effect of the drug; however, this alteration was not observed when BBMV controls were incubated in vitro, with different concentration (1-5 mg%) of TAA (results not shown). Also, the concomitant reduction of the BBMV [3H]-phlorizin binding sites, strongly suggests that TAA acute intoxication produces a decrease in the amount of the Na+/glucose cotransporter present in the brush border membrane of the proximal renal tubule. This result adds evidence that TAA acts by decreasing the expression of some proteins, such as cytochrome P 450 (Sathyabama and Padmanaban, 1984) and glucose-6-phosphatase present in the rat liver nuclear envelope (González-Mujica et al., 1993).
The decrease in the Km of the Na+/glucose cotransporter (Table 3) and the decrease in the Kd of the binding of [3H]-phlorizin to BBMV (Figure 3) produced by TAA treatment could be due to the increase in the membrane fluidity as a consequence of the drug administration; this change in the membrane fluidity was evidenced by the increase in the relationship between unsaturated/saturated BBMV phospholipids and fatty acids and in the decrease in the transition temperature and the AE of maltase and alkaline phosphatase produced by TAA administration. It is interesting to point out that Scheel et al. (1982) reported a decrease in the Km without change in the V with and increase in the membrane fluidity.
Acknowledgment
The collaboration of the following persons is highly appreciated: MSc. Norma Motta for her advise in the development of this work; Dr. Atahualpa Pinto for performing the histological examination; and Dra. Kay Tarble de Scaramelli for correcting the manuscript.
References
1. Andersch, M.A.; Szcypinski A.J. 1947. Determination of alkaline phosphatase in serum with p-nitrophenylphosphate. Am. J. Clin. Pathol., 17:571-574. [ Links ]
2. Baker, E.; Smuckler, E. 1974. Non hepatic thioacetamide injury. II. The morphological features of proximal renal tubular injury. Am. J. Pathol., 74:575-590. [ Links ]
3. Baker, J.; Kleinman, L. 1974. Relationship between glucose and sodium excretion in the new-born dog. J. Physiol., 243:45-61. [ Links ]
4. Bergmeyer, H. 1963. Methods of enzymatic analysis. Academic Press, New York and London. [ Links ]
5. Bruck, R.; Weiss, S.; Traister, A.; Zvibel, I.; Aeed, H.; Halpern, Z.; Oren, R. 2007. Induced hypothyroidism accelerates the regression of liver fibrosis in rat. J. Gastroenterol. Hepatol., 22:2189-2194. [ Links ]
6. Chen, P.S.; Toribara, T.; Warner, H. 1956. Microdetermination of phosphorus. Anal. Chem., 28:1756-1758. [ Links ]
7. Cohen, M.; Klepser, H.; Shapiro, E. 1986. Insulin stimulate renal glomerular sodium-potasium triphosphatase activity. Biochim. Biophys. Acta, 856:182-184. [ Links ]
8. Cori, G. 1954. Glycogen structure and enzyme deficiences in glycogen store disease. Harvey Lect., 48:145-171. [ Links ]
9. De Sa Moita, R.; Pradere, J.D.; González-Mujica, F. 1989. Metabolismo de la glucosa durante la intoxicación con tioacetamida. Rev. Fac. Med. UCV., 12:39-47. [ Links ]
10. Elsas, L.; Rosenberg, L. 1969. Familial glycosuria: A genetic reappraisal of hexose transport by kidney and intestine. J. Clin. Invest., 48:1845-1853. [ Links ]
11. Fiske, D.; Subbarow, P. 1925. The colorimetric determination of phosphorous. J. Biol. Chem., 66:375-382. [ Links ]
12. Fleck, C.; Zimmermann, T.; Franke, H.; Braunlich, H.; Dargel, R. 1988. Relation between renal and hepatic excretion of drugs: VII. Hepatic and renal excretion of phenol red in thioacetamide-induced acute and chronic liver damage. Exp. Pathol., 33: 47-54. [ Links ]
13. Folch, C.H.; Less, W.; Sloan, G. 1975. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem., 226:497-509. [ Links ]
14. González-Mujica, F.; Motta, N.; Waddell, I.; Burchell, A. 1993. Nuclear envelope glucose-6-phosphatase from control and thioacetamide treated liver. Biochem. Soc. Trans., 22:40S. [ Links ]
15. Hasegawa, M.; Ide, M.; Takenaka, S.; Yamate, J.; Tsuyama, S. 2007. Urinary metabolic fingerprinting for thioacetamide - induced rat acute hepatic injury using fourier transformion cyclotron resonance mass spectrometry (FT-ICR MS), with reference to detection of potential biomarkers for hepatotoxicity. Toxicol. Pathol., 35:570-575. [ Links ]
16. Henry, R. 1969. Clinical Chemistry. Principle and Techniques. Hoeber-Harper, New York. First Edition. [ Links ]
17. Heyrovsky, A. 1956. A new method for the determination of inulin in plasma and urine. Clin. Chem. Acta, 1:470-474. [ Links ]
18. Inciarte, H.; González-Mujica, F. 1988. Efectos de la tioacetamida sobre la envoltura nuclear de las células de la corteza renal de ratas. Inv. Clin., 29: 121-140. [ Links ]
19. Kates, P. 1964. Simplified procedures for hydrolysis or methanolysis of lipids. J. Lipid Res., 15:132-135. [ Links ]
20. Kessler, M.; Acuto, O.; Storelli, C.; Murer, H.; Müller, M.; Semenza, G. 1978. A modification procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membrane. Their use in investigation. Some properties of D-glucose and choline transport system. Biochem. Biophys. Acta. 506: 136-154. [ Links ]
21. Kleinfeld, R.G. 1957. Early changes in rat liver and kidney cells induced by thioacetamide. Cancer Res., 17: 954-961. [ Links ]
22. Loo, D.; Zeuthen, T.; Chandy, G.; Wright, E. 1994. Cotransport of water by the Na+/glucose cotransporter. Proc. Natl. Acad. Sci., 92:13367-13370. [ Links ]
23. Lötscher, M.; Kaissling, B.; Biber, J.; Levi, M. 1997. Role of microtubules in the rapid regulation of renal phosphate transport in response to acute alteration in dietary phosphate content. J. Clin. Invest., 99:1302-1312. [ Links ]
24. Lott, J.; Turner, K. 1975. Evaluation of Trinders glucose oxidase method for measuring glucose in serum and urine. Clin. Chem. Acta., 21:1754-1760. [ Links ]
25. Lowry, O.; Rosebrough, N.; Farr, A.; Randal, R. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem., 193: 265-275. [ Links ]
26. Lundsgaard, E. 1933. Hemmung von esterifizierungsvorgangenals unsache der phlorrhizinwirking. Biochem. Z., 264:209-220. [ Links ]
27. Malathi, P.; Preiser, H.; Fairclough, P.; Mallet, P.; Crane, R. 1979. A rapid method for the isolation of kidney brush border membrane. Biochem. Biophys. Acta, 554:259-273. [ Links ]
28. Olianova, N.; FalKm S.; Bertelot, A. 2001. Two step mechanism of phlorizin binding to the SGLT1 protein in the kidney. J. Membrane Biol., 179:223-242. [ Links ]
29. Peerce, B.; Wright, E. 1984. Sodium-induced conformational change in the glucose transporter of the intestinal brush border. J. Biol. Chem., 259:14105-14112. [ Links ]
30. Peerce, B.; Wright, E. 1985. Evidence of tyrosyl residue at the Na+ site of the intestinal Na+/glucose cotransporter. J. Biol. Chem., 260:6026-6031. [ Links ]
31. Puschett, J.; Whitbred, J.; Ionosi-Irimie, M.; Vu, H.; Rabon, E.; Robinson, J.; Deininger P. 2003. Molecular effects of volume expansion on the renal sodium phosphate cotransporter. Am. J. Med. Sci., 336:1-8. [ Links ]
32. Rekha, R.D.; Amali, A.A.; Her, G.M.; Yeh, Y.H.;Gong, H.Y.; Hu, S.Y.; Lin, G.H.; Wu, J.L. 2008. Thioacetamide accelerates steatohepatitis, cirrhosis and HCC by expressing HCV core protein in transgenic zebrafish Danio rerio. Toxicology, 243:11-22. [ Links ]
33. Robson, A.; Srivastava, P.; Bricker, N. 1968. The influence of saline loading on renal glucose reabsorption in rat. J. Clin. Invest., 47:329-335. [ Links ]
34. Sathyabama, S.; Padmanaban, G. 1984. Effects of thioacetamide on cytochrome P-450 synthesis in rat liver. Biochem. J., 218:371-377. [ Links ]
35. Scheel, G.; Acevedo, E.; Conzelman, E.; Nehrkorn, H.; Sandhoff, K. 1982. Model for the interaction of the membrane-bound substrate and enzyme. Hydrolysis of ganglioside GD1a by sialidase of neuronal membrane isolated from calf brain. Eur. J. Biochem., 127:245-253. [ Links ]
36. Silverman, M.; Black, J. 1975. High affinity phlorizin receptor sites and their relation to the glucose transport mechanism in proximal tubule of dog kidney. Biochim. Biophys. Acta. 394:10-30. [ Links ]
37. Stümpel, F.; Burcelin, R.; Jungermann, K.; Thorens, B. 2001. Normal kinetics of intestinal glucose absorption in the absence of GLUT 2: Evidence for a transport pathway requiring glucose phosphorylation and transfer into the endoplasmic reticulum. Proc. Natl. Acad. Sci., 98:11330-11335. [ Links ]
38. Taggart J, 1958. Mechanism of renal tubular transport. Am. J. Med., 24:774-784. [ Links ]
39. Toggemburger, G.; Kessler, N.; Rothatien, A.; Semenza, G.; Tannenbaun, C. 1978. Similarity in effects on Na+ gradients and membrane potentials on D-glucose transport and phlorizin binding to vesicles derived from brush-borders of rabbit intestinal mucosal cells. J. Membrane Biol., 40:269-290. [ Links ]
40. Turner, R.J.; Moran, A. 1982. Heterogeneity of sodium-dependant D-glucose transport sites along the proximal tubule: evidence from vesicles studies. Am. J. Physiol., 242: F406-F416. [ Links ]
41. Turner, R.J.; Silverman, M. 1977. Sugar uptake into brush border vesicles from normal human kidney. Proc. Natl. Acad. Sci., 74:2825-2829. [ Links ]
42. Vadi, H.V.; Neal, R.A. 1981. Microsomal activation of thioacetamide-S-oxide to a metabolite(s) that covalently binds to calf thymus DNA and other polynucleotides. Chem. Biol. Interactions, 35:25-38. [ Links ]
43. Waters, N.J.; Waterfield, C.J.; Farrant, R.D.; Holmes, E.; Nicholson, J.K. 2005. Metabonomic deconvolution of embedded toxicity: application to thioacetamide hepato- and nephrotoxity. Chem. Res. Toxicol., 18:639-654. [ Links ]