Interciencia
versión impresa ISSN 0378-1844
INCI v.29 n.4 Caracas abr. 2004
MODELLING SHORT-TERM DYNAMIC BEHAVIOUR OF THE SURF CLAM (Mesodesma donacium)
FISHERY IN NORTHERN CHILE USING STATIC AND DYNAMIC CATCHABILITY HYPOTHESES
EDUARDO P. PÉREZ E. and JAVIER E. CHÁVEZ V.
Eduardo P. Pérez E. Marine Biologist, M.Sc. and Ph.D. Professor, Departament of Marine
Biology, Universidad Católica del Norte, Chile, and researcher, Centro de Estudios Avanzados en Zonas Áridas. Address:
Casilla 117 Coquimbo, Chile. email: eperez@ucn.cl
Javier E. Chávez V. Marine Biologist. Specialist, National Fishery Service, Coquimbo,
Chile. email: jchavez@sernapesca.cl
Abstract:
The catchability coefficient (q) is a technologically-related parameter that represents the proportion of individuals of a stock cuaght per unit of effort expended. This is frequently assumed to be constant, but there is evidence that in pelagic fishes the value of q may vary with the resource abundance. However, there are few records of q variability in benthic resources. This study evaluates two methods to estimate q: The first supposes the catchability coefficient to be constant over time, and the second supposes a density-dependent relation between q and the abundance of the resource. Both estimations were incorporated into a modified depletion model using data obtained from a local Mesodesma donacium fishery in Coquimbo Bay, Chile. Performance variables such as biomass, catch and catch per unit of effort were simulated and contrasted with observational data from the fishery. Results showed variability over time in the q value associated with the local abundance of the resource. However, the standard error associated to the fit caused the variability calculated for the performance variables to be higher in the case of a variable q than for those obtained with the static q model.
KEYWORDS: Assessment; Catchability; Mesodesma donacium; Standard Error of Regression; Stock
MODELAJE DEL COMPORTAMIENTO DINÁMICO A CORTO PLAZO DE LA PESQUERÍA DEL BIVALVO Mesodesma donacium
EN EL NORTE DE CHILE USANDO HIPÓTESIS DE CAPTURABILIDAD ESTÁTICA Y DINÁMICA
Resumen:
El coeficiente de capturabilidad (q) es un parámetro tecnológico que representa la proporción de individuos del stock que es removida por unidad de esfuerzo. Frecuentemente se asume como una constante, aunque hay evidencia que en peces pelágicos q puede variar en función de la abundancia del recurso. Sin embargo, para recursos bentónicos hay pocos registros de variabilidad de q. En este estudio se evaluaron dos métodos para estimar q: El primero supone una capturabilidad constante a través del tiempo, mientras que el segundo supone una relación de denso- dependencia entre q y la abundancia del recurso. Ambas estimaciones fueron incorporadas a un modelo de remoción modificado, utilizando datos obtenidos de la pesquería del bivalvo Mesodesma donacium en la Bahía de Coquimbo, Chile. Las variables de desempeño (biomasa, captura y captura por unidad de esfuerzo) fueron simuladas y contrastadas con valores observados en la pesquería. Los resultados mostraron que q varió a través del tiempo asociado a cambios en la abundancia local del recurso. Sin embargo, el error estándar asociado al ajuste generó que la variabilidad calculada para las variables de desempeño fueran más altas en el modelo basado en q variable que aquellas obtenidas con un q estático.
MODELAGEM DO COMPORTAMENTO DINÂMICO A CURTO PRAZO DA PESCARIA DO BIVALVO Mesodesma donacium NO
NORTE DO CHILE USANDO HIPÓTESES DE CAPTURABILIDADE ESTÁTICA E DINÂMICA
Resumo:
O coeficiente de capturabilidade (q) é um parâmetro tecnológico que representa a proporção de indivíduos do stock que é removida por unidade de esforço. Freqüentemente se assume como uma constante, ainda que há evidencia que em peixes pelágicos q pode variar em função da abundância do recurso. No entanto, para recursos bentônicos há poucos registros de variabilidade de q. Neste estudo se avaliaram dois métodos para estimar q: O primeiro supõe uma capturabilidade constante através do tempo, enquanto que o segundo supõe uma relação de denso-dependência entre q e a abundância do recurso. Ambas estimações foram incorporadas a um modelo de remoção modificado, utilizando dados obtidos da pescaria do bivalvo Mesodesma donacium na Bahia de Coquimbo, Chile. As variáveis de desempenho (biomassa, captura e captura por unidade de esforço) foram simuladas e contrastadas com valores observados na pescaria. Os resultados mostraram que q variou através do tempo associado a mudanças na abundância local do recurso. No entanto, o erro estandar associado ao ajuste gerou que a variabilidade calculada para as variáveis de desempenho foram mais altas no modelo baseado em q variável que aquelas obtidas com um q estático.
Received: 09/30/2003. Modified: 03/15/2004. Accepted: 03/31/2004
Ideally, fishery modeling may be used to predict the effects of management methods and regulations applied to a given fishery (Hilborn and Walters, 1992; Seijo et al., 1997). All models are, however, based on fundamental assumptions. One of these is related to the spatial/ temporal constancy of the catchability coefficient (q), defined as a technical coefficient that represents the proportion of individuals of a stock captured per unit effort applied (Peterman and Steer, 1981; Gulland, 1983).
From this point of view the catch per unit effort (CPUE) is the result of a relation between the level of biomass of the stock and the catchability coefficient (Arreguín-Sánchez, 1996). Thus, in absence of a direct assessment (fishing surveys, for example) the biomass of the population, an important variable in fisheries management that cannot be directly observed, may be inferred by means of an observable indicator such as the CPUE and from knowledge of the catchability coefficient (Richards and Schnute, 1986; Crecco and Overholtz, 1990; Ye and Mohammed, 1999). However, there have been failures with the assumption of constant catchability (Winters and Wheeler, 1985; Crecco and Overholtz, 1990). In fact, a large variability of this coefficient can be found in the literature due to, among other factors, the behavior of the resource in aspects such as reproductive aggregation, patterns of abundance in space and time (Ulltang, 1976; Peterman and Steer, 1981), changes in fishing power (Gulland, 1983) or in the distribution area (Winters and Wheeler, 1985) associated with environmental factors (Swain et al., 2000). These factors should influence the proportion of stock removed per unit of effort applied. Simultaneous space-time variability has been reported for q. Evidence was recently presented (Pérez and Defeo, 2003) which suggested spatial and temporal variability in q in the fishery of the nylon shrimp Heterocarpus reedi in Chiles northern zone. The implications of this variability of q in modelling of fisheries have not been evaluated as yet, although it has been proposed that the biomass would decrease at a higher rate than calculated following a model based on the supposition of a constant q (Pérez, 1996; Chávez, 2000). Also, the effect on estimates of the performance variables of the model based on the variability of the estimations of q (static or dynamic) needs to be evaluated. In general, only the trajectories of the performance variables which have been obtained by deterministic analyses have been reported, without considering errors associated with the models.
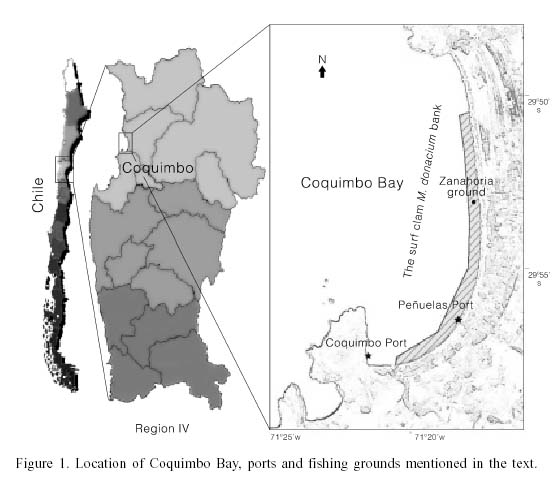
In order to evaluate the effect of uncertainty associated with the estimation of q on two indirect estimates of a stock of the bivalve Mesodesma donacium in Coquimbo Bay, Chile (29º55'S), two methods based on different hypotheses or assumptions about q were used: i) assumes this coefficient to be constant over time, and as such it is used to evaluate the dynamics of the stock, while ii) supposes a density dependence in the local abundance of the resource; that is, as the biomass of the resource declines, the catchability increases. The alternatives concerning the static or dynamic nature of the catchability were separately incorporated into a modified depletion model which utilized data from the M. donacium fishery. Finally, the expected values for the models based on each one of the hypothesis and its uncertainty associated with direct observations from the fishery under typical conditions in the field were compared.
Materials and Methods
M. donacium and its fishery
The surf clam Mesodesma donacium inhabits sandy beaches 0-5m deep. In Chile, fishermen operating from 24ft boats with outboard motors harvest the clam using a semiautonomous diving hookah. In many localities, in addition to divers, fishermen operating from the beach, called orilleros, exploit that fraction of the resource at depths of 0- 1.5m. The fishery operates under an open-access regime with one regulation: a minimum size restriction of 60mm of valve length. There is an exception, however, in Coquimbo Bay (Figure 1; Pérez, 1996), where the resource has been subject to a community based management since 1989 (Ariz et al., 1994). The community has established a license quota of 64 small-scale boats. This voluntary collective action has been recognised and backed by the New Law of Fishery and Aquaculture, in force since 1991 (Castilla, 1994). Nevertheless, the law establishes that this method of self-regulation can be legal only if there has been a technical analysis previously approved by authorities. As a consequence, some fleet dynamic analyses and sample programs have been implemented. In Coquimbo Bay the fleet operates an average of 3 days per week (156 days per year) with annual landings of around 1500tons per year. There is no information about the activity of orilleros, but total landings of 600ton per year are estimated (Pérez et al., 1998).
The area of distribution of the resource on the bank is approximately 1.5x106m2 (Ariz et al., 1994; Pérez, 1996; Chávez, 2000). The intensity of the fishery is spatially heterogeneous (Pérez, 1996) and different harvesting grounds can be clearly identified based on the CPUE of each area (Chávez, 2000). The method of operation of a diver includes collecting M. donacium individuals and placing them in tubular collector bags (chinguillos) which extend over the substrate as the diver advances. Once the bag is completely extended the diver returns to the initial position and repeats the collection in a different direction. The process is continued until the divers daily quota is filled. Pérez (1996) suggested that as the biomass diminished within a fishing ground, the area harvested by each diver was increased until a satisfactory capture was obtained; in this case the coefficient of catchability (sensu Baranov, 1918) increased as the resource decreased. This hypothesis was evaluated with the fishermen, and the length and width of the swept area by each diver was measured during his fishing operation. With this information it was possible to place a value on q over time in relation to the swept area, and thus obtain a value for q independent of its relation to either the CPUE or the biomass.
Dynamics of the CPUE with constant Catchability
If the catchability does not change over time, the biomass at each time t is given by
Bt+1= (Bt+Rt)exp-M - Ct (1)
where Bt: biomass (tons) and Ct: the previous capture at time t (in weeks, for this particular model), Rt: recruitment (in biomass terms) in time t, and M: natural weekly mortality as obtained by Pérez (1996) for the M. donacium bank in Coquimbo Bay (Ms=0.00865 per week). The use of the expression B exp–M/2 in equation (1) is based on the assumption that all individuals in the stock have an average length and weight. This lets the above expression be equivalent to ![]() , where
, where ![]() is the number of individuals of average length, and w is the average weight at that length. In this way equation (1) becomes equivalent to the usual expression in terms of number of individuals (Kirkwood et al., 2001; Restrepo, 2001).
is the number of individuals of average length, and w is the average weight at that length. In this way equation (1) becomes equivalent to the usual expression in terms of number of individuals (Kirkwood et al., 2001; Restrepo, 2001).
The catch is obtained as
Ct = q Et Bt (2)
where q: catchability (per hour) and Et: effort observed (hours of collection by diving) in time t.
The CPUE (kg per hour) is obtained using
CPUEt = Bt q (3)
The DeLury estimator was used for calculation of the static coefficient of catchability (Hilborn and Walters, 1992). This is based on a linear model to obtain the coefficient by relating the CPUE values observed and the cumulative effort observed by means of the expression
![]()
where ln(CPUEt): natural log of the observed CPUE (kg per hour of diving) at time t, q: slope, B1: biomass available before the first harvest, and ![]() cumulative effort observed (hours diving) of divers from port j at time t. This is required since two organizations of artisanal fishermen operate in Coquimbo Bay. The main group operates in the Peñuelas sector using approximately 50 boats; a second fleet of about 14 boats operates in the Coquimbo sector about 8km north from the first (Figure 1). The efforts of both fleets were summed and considered in the calculations.
cumulative effort observed (hours diving) of divers from port j at time t. This is required since two organizations of artisanal fishermen operate in Coquimbo Bay. The main group operates in the Peñuelas sector using approximately 50 boats; a second fleet of about 14 boats operates in the Coquimbo sector about 8km north from the first (Figure 1). The efforts of both fleets were summed and considered in the calculations.
Dynamic of the CPUE with variable catchability
The variability of the catchability coefficient was calculated weekly using the equation of Baranov (1918) which established that
qt = at / A (5)
where at: area harvested by the diver at time t. The expression A denotes the area of distribution of the resource. Caddy (1975) and Seijo et al. (1994) recommended this as a useful calculation tool for bottom-resource fisheries. Following this, the change in the value of q would be given by the function proposed by Pérez (1996), who defined the harvesting area of the diver as
at = (SLC · W) NCCt (6)
where SLC: standard length of the collector bag used by the diver, W: width of the strip harvested by the diver in meters (W= 1m; Pérez, 1996), and NCCt: number of changes in the position of the bag effected by the diver during his collection over time t. The data required for the calculations were obtained in coordination with divers according to a design agreed upon for collecting the information. The area of distribution of the resource (A) was taken from Ariz et al. (1994) and Chávez (2000).
In this case the dependency relationship between CPUE and catchability over time is given similarly to MacCall (1976), using CPUE as an indirect biomass index
qt = αCPUEt-β (7)
where α and β are parameters. Given that CPUEt is, in our interpretation, an indicator of relative abundance of the resource in time t, then the biomass at time t may be expressed as
Bt = CPUEt / qt (8)
Substituting Eq. (4) in (5) leads to
Bt = CPUEt / αCPUEt-β (9)
Similarly, in Eq. (2) and (4) the relation between biomass and catchability over time t can be directly modeled using equation (7) from MacCall (1976):
qt = χBt-δ (10)
where χ and δ are parameters.
Analogous to the dynamic biomass model described for the catchability constant (Eq. 1) the model that considers q as a variable, which is a function of the availability of biomass, would be given by
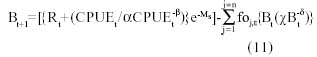
where foj,t: observed effort for the port j at time t. From Eq. (8) the CPUE can be estimated as
![]()
Thus, the term ![]() represents total catch at all ports j. The weekly catches, as well as the effort of both fleets were summed and considered in the calculation.
represents total catch at all ports j. The weekly catches, as well as the effort of both fleets were summed and considered in the calculation.
Inclusion of variability in estimations of the q parameter
The standard error (Zar, 1995) for the parameters of Eq. (4), (7) and (10) was calculated using non linear fit routines available in the SYSTAT 8.0 software. From these estimates of variability, 800 possible values of q were calculated by Monte Carlo analysis (Manly, 1991). Each of these values was entered into the respective simulation model, and the results were interpreted using box and whiskers plots.
Eq. (1) and (10) were parameterized using information based on the M. donacium fishery in Coquimbo Bay on the Northern Chilean coast (Figure 1).
More than 20 collecting grounds were identified within the bank (Pérez, 1996; Chávez, 2000). However, in order to evaluate the usefulness of Eq. (1) and (11) only one of the important grounds (termed Zanahoria by the fishermen, Figure 1) was selected for study, based on its area and number of boat trips observed (Chávez, 2000). Comparisons among the observed data and simulated output were made using percentiles and medians.
Results
Estimation of static and dynamic Catchability
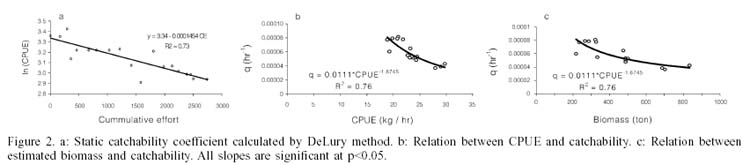
The analysis performed showed lower values when q was estimated through a dynamic approach. The DeLury method gave a catchability value of 0.0001454h-1 (Figure 2a), while the method based on Eq. (7) showed an inverse relation with lower values, between 0.00003 and 0.0008h-1 (Figure 2b). The same tendency was evident when contrasting catchability with estimated biomass (Figure 2c).
Error associated with the estimations
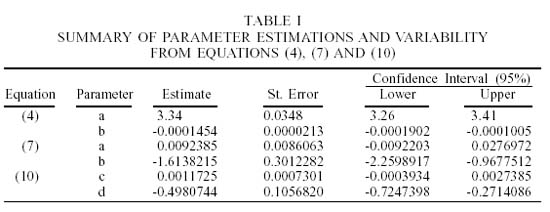
The standard error of the estimations of Eq. (4) was less (Table I) than those calculated for Eq. (7) and (10). Thus, for the slopes of these equations, the error with respect to the values estimated were 1% in the case of static q (Eq. 4) and 19% and 21%, respectively, for Eq. (7) and (10) in which a dynamic q was assumed.
As a result of the above, Eq. (7) and (10) produced negative values for the confidence interval, which was lower than the parameters a and c respectively (Table I). This implies a result of negative biomass, which is obviously without meaning. Of the 800 iterations, 38 values fell within this category for each week simulated. These values were omitted from all subsequent calculations.
Comparison between results from the models and observations
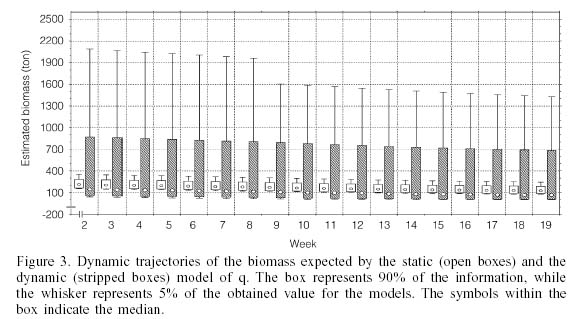
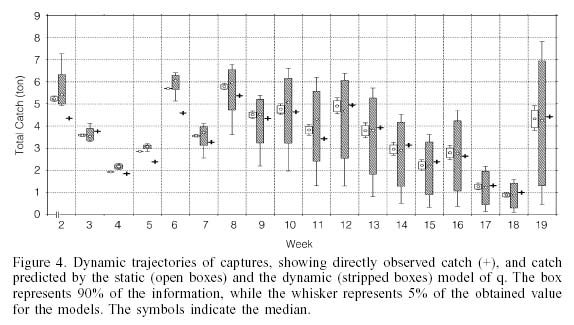
Biomass. Figure 3 shows the distribution of 95% of the calculated data. Each whisker represents 2.5% of the information, while the box represents the remaining 90%; within this is represented the median of the results obtained using the Monte Carlo simulation. A larger variability was observed in the model that included a dynamic q, with respect to the alternative model in which q was static. With the latter condition, the range of biomass values was 78-352tons (median=166ton), while with a dynamic q, this range was 2-5907tons (median= 103ton).
Catch. In most of the cases the catch observed was within the range of values expected from both models (Figure 4). As observed for biomass, the dynamic q model showed greater variation, while the variation was reduced with the static q model. Another aspect to note is that with the dynamic q model the tendency for the variability in the catch estimation increases from the 7th week, a fact which was not observed with the other model.
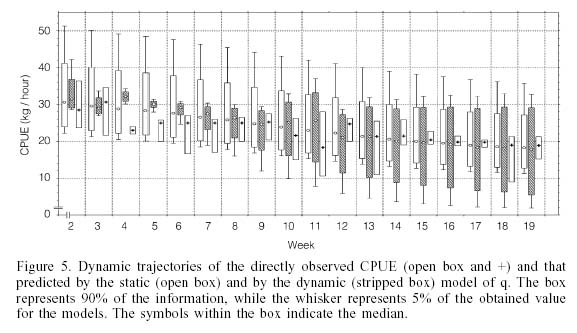
Catch per unit of effort. The range of values for the CPUE observed was within the range of variation expected from both models (Figure 5), although its variability was smaller. The tendency with this indicator showed that, in the case of the dynamic q model, its variability increased progressively beginning at the 5th week, a pattern that was not observed with the static q model.
Discussion
The results support the hypothesis of temporal variability in the catchability coefficient, associated with variations in local abundance of the resource, as higher q values were associated to lower biomass levels. This finding is against the general assumption that this coefficient is a constant. On the other hand, the standard error associated with the estimation of parameters by regression analysis caused the variability calculated for the performance variables to be higher with the variable q model than for those obtained with the static q model.
Although temporal variation in catchability has been demonstrated in diverse pelagic and demersal fisheries (MacCall, 1976; Peterman and Sterr, 1981; Bannerot and Austin, 1983; Crecco and Savoy, 1985; Gordoa and Hightower, 1991; Swain et al., 2000), and for crustaceans (Ye and Mohammed, 1999; Pérez and Defeo, 2003), we have found no references to the behavior of q in artisanal fisheries for benthic resources, specifically mollusks.
Various explanations have been proposed for the observed variability. Atran and Loesch (1995) carried out an analysis of weekly fluctuations in catchability of Brevoortia tyrannus, assuming that variations in the q coefficient could remain relatively constant when measured on an annual scale. This condition is generally violated, if ever fulfilled at all, when the analysis is carried out seasonally, as is verified in the present study by the finding of temporal variations in catchability when analyzed over short periods of time. This fact raises questions about the correct time scales needed to account for small variations in catchability which may affect the evaluation of the resource under study.
A second explanation has been associated with behavioral aspects of the resource (Arreguin-Sánchez, 1996, Godo et al., 1999; Swain et al., 2000). Species that form schools show strong inverse correlation between biomass and catchability (Paloheimo and Dickie, 1964; MacCall, 1976; Ulltang, 1976; Peterman and Steer, 1981; Crecco and Savoy, 1985; Angelsen and Olsen, 1987; Crecco and Overholtz, 1990; Hansen et al., 2000). Following drops in abundance per unit area after extraction efforts, the schools tend to regroup and maintain their density. When maintaining density with declining biomass, each unit of effort extracts a greater proportion of the remaining stock, so that as catchability increases, the stock is reduced. The preceding becomes evident as a strong density-dependent relationship.
In the case of M. donacium, however, the explanation seems to be related to the behavior of the divers, more than to the behavior of the resource. Pérez (1996) postulated a densitydependent effect between q and the abundance of this resource, since upon declines in abundance the diver must harvest a greater surface area to obtain the same catch. In this way the numerator in Eq. (11) increases, the denominator remaining constant. Thus, the catchability increases. This characteristic is present in the dynamic estimations of catchability in each fishery ground; as CPUE increases catchability decreases and vice-versa (Chávez, 2000).
Another aspect that emerges from the results obtained relates to the assumption that establishes that the CPUE would be a relative estimator of the resource abundance. Seijo et al. (1997) indicated that the assumption that the CPUE was a relative index of abundance for sedentary species would be limited, given changes in the value for catchability (Collins, 1987; Swain et al., 2000). Thus, in quantitative terms, the CPUE estimated on the basis of the dynamic model of q (Eq. 10) always showed a better fit of CPUE to the values obtained directly than did the CPUE obtained for these values using constant catchability. Thus, the CPUE may be considered as an efficient estimator of the abundance if and only if the b parameter in Eq. (7) is significantly less than and statistically different to zero. This conclusion is the same as that reached by Ulltang (1976) for Clupea harengus.
However, despite the above statements, the results suggest that although there was a significant inverse relation between biomass and catchability, the variability in the observations that serve as inputs to the regression equations cause the dynamic q model to have weaknesses (ie. negative biomass values) associated with the high standard error of the calculated parameters. In contrast, although the hypothesis of the constancy of q was falsified, the results of the model based on this assumption produced numbers within the range of values observed for the performance values (catch, CPUE) with greater precision than that of the model using dynamic q. As a consequence, for the case of the M. donacium fishery in Coquimbo Bay, it is possible to work with a biomass depletion model using a static q without making an important estimation error. In this sense, this result represents an advantage, as it is simpler to estimate this coefficient from routine information of fishery activities instead of producing new records for the area harvested by each diver. In relation to the variability in the harvested area, it is possible that within the fishery zone analyzed (known to the fishermen as Zanahoria) there are loci characterized by particular density and size structures. Loci were defined under the basic assumption that the stock can be subdivided into different such loci, each assuming different resource densities. A locus becomes the smallest geographical unit considered in which the population density can be considered effectively uniform (Caddy, 1975). Each locus would contain several age classes, all of which would have different densities. Thus, the distribution of the resource may be heterogeneous, even within the same fishery area, and may produce a high degree of variability within the area harvested by each diver and, therefore, a higher error in the model.
In a management context these results can be useful to make decisions with a relatively simple predictive model. This is particularly important when only historical data about CPUE are available for fisheries analysts. Furthermore, CPUE requires lower costs (in money and time consumed) compared with a more sophisticated statistical design. In order to evaluate the best tools to support management decisions, all these aspects must be considered in discussing a fishery monitoring system.
REFERENCES
1. Angelsen KK, Olsen S (1987) Impact of fish density and effort level on catching efficiency of fishing gear. Fish. Res. 5: 2171- 2178. [ Links ]
2. Ariz L, Jerez G, Pérez E, Potocnjack C (1994) Bases para la ordenación y desarrollo de las pesquerías artesanales del recurso macha (M donacium) en Chile Central. Informe Final. Instituto de Fomento Pesquero (IFOP): Valparaiso, Chile. 61 pp. [ Links ]
3. Arreguín-Sánchez F (1996) Catchability: a key parameter for fish stock assessment. Rev.Fish. Biol.Fisheries 6: 221-242. [ Links ]
4. Atran SM, Loesch JG (1995) An analysis of weekly fluctuations in catchability coefficients. Fish.Bull. 93: 562-567. [ Links ]
5. Bannerot SP, Austin CB (1983) Using frequency distribution of catch per unit of fishing effort to measure fish-stock abundance. Trans.Am.Fish.Soc. 112: 608-617. [ Links ]
6. Baranov TY (1918) On the question of the biological basis of fisheries. Proc.Inst.Icht.Invest. 1: 81-128. [ Links ]
7. Caddy JF (1975) Spatial models for an exploited shellfish population, and its application to Georges Bank scallop fishery. J.Fish.Res.Board.Can. 32: 1305-1328. [ Links ]
8. Castilla JC (1994) The Chilean small-scale benthic shellfisheries and the institutionalization of new management practices. Ecol.Internat.Bull. 21: 47-63. [ Links ]
9. Chávez J (2000) Análisis dinámico del coeficiente de capturabilidad y sus implicancias en la modelación de pesquerías: Mesodesma donacium en el banco de bahía Coquimbo, un estudio de caso. Tesis. Universidad Católica del Norte. Chile. 90 pp. [ Links ]
10. Collins JJ (1987) Increased catchability of the deep monofilament nylon gillnet and its expression in a simulated fishery. Can.J.Fish.Aquat.Sci. 44 ( Suppl. 2): 129-135. [ Links ]
11. Crecco VA, Overholtz W (1990) Causes of density- dependent catchability for Georges Bank haddock Melanogrammus aeglefinus. Can.J.Fish.Aquat.Sci. 47: 385-394. [ Links ]
12. Crecco VA, Savoy TF (1985) Density-dependent catchability and its potential causes and consequences on Connecticut River American Shad, Alosa sapidissima. Can.J.Fish.Aquat.Sci. 42: 1649-1657. [ Links ]
13. Godo OR, Walsh SJ, Engas A (1999) Investigating density dependent catchability in bottom trawl surveys. ICES. J.Mar.Sci. 56: 292- 298. [ Links ]
14. Gordoa A, Hightower JE (1991) Changes in catchability in a bottom-trawl fishery for Cape Hake (Merluccius capensis). Can.J Fish.Aquat.Sci. 48: 1887-1895. [ Links ]
15. Gulland JA ( 1983) Fish Stock Assessment. A manual for basic Methods. Wiley. New York, USA. 223 pp. [ Links ]
16. Hansen MJ, Beard TD, Hewett SW (2000) Catch rates and catchability of walleyes in angling and spearing fisheries in Northern Wisconsin lakes. North Am.J.Fish.Manag. 20: 109-118. [ Links ]
17. Hilborn RF, Walters CJ (1992) Quantitative fisheries stock assessment. Choice, dynamics and uncertainty. Routledeg, Chapman and Hall: New York, USA. 570 pp. [ Links ]
18. Kirkwood GP, Aukland R, Zara SJ (2001) Catch effort data analysis (CEDA),. Version 30 MRAG: London, UK.. [ Links ]
19. MacCall AD (1976) Density-dependence of catchability coefficient in the California Pacific sardine, Sardinops sagax caerulea, purse seine fishery. California Coop. Ocean. Fish.Inv.Rep. 18: 136-148. [ Links ]
20. Manly BFJ (1991) Randomization and Monte Carlo methods in biology. Chapman and Hall: New York, USA. 281 pp. [ Links ]
21. Paloheimo JE, Dickie LM ( 1964) Abundance and fishing success. In ICES Rapp. P. - v. Réun. 155: 152-162. [ Links ]
22. Pérez EP ( 1996) Análisis de la pesquería de Mesodesma donacium en el banco de Peñuelas (Chile, IV región), bajo condiciones de riesgo e incertidumbre. Thesis. CINVESTAVIPN. Merida, Yucatan. Mexico. 82pp. [ Links ]
23. Pérez EP, Defeo O (2003) Time-space variation in the catchability coefficient as a function of match per unit of effort in Heterocarpus reedi (Decapoda, Pandalidae) in North-Central Chile. Interciencia 28: 178-182. [ Links ]
24. Pérez E, Arias JL, Arias E, Defeo O, Stotz W, Valdebenito M (1998) Caraterización bioeconómica de la pesquería del recurso macha en la zona norte y centro sur de Chile. Final Report. Universidad Católica del Norte/Fondo de Investigación Pesquera (FIP): Coquimbo, Chile. 232 pp. [ Links ]
25. Peterman RM, Steer GJ (1981) Relation between sportfishing catchability coefficients and salmon abundance. Trans.Am.Fish.Soc. 114: 436-440. [ Links ]
26. Richards LJ, Schnute JT (1986) An experimental and statistical approach to the question: Is CPUE an index of abundance ?. Can.J.Fish.Aquat.Sci. 43: 1214-1227. [ Links ]
27. Restrepo VR ( 2001) Dynamic depletion models. In Regional Workshop on the assessment of the Caribbean spiny lobster (Panulirus argus). FAO Fisheries Report Nº619. Rome, Italy. pp. 345-356. [ Links ]
28. Seijo JC, Caddy JF, Euán J (1994) SPATIAL: space-time dynamics in marine fisheries. A software package for sedentary species. Comp. Inf. Ser. Fish. Nº6. FAO. Rome, Italy. 116 pp. [ Links ]
29. Seijo JC, Defeo O, Salas S (1997) Bioeconomía pesquera: Teoría, modelación y manejo. Documento técnico de pesca Nº 368. FAO. Rome, Italy. 176 pp. [ Links ]
30. Swain DP, Poirier GA, Sinclair AF (2000) Effect of water temperature on catchability of Atlantic cod Gadus morhua) to the bottomtrawl survey in the southern Gulf of St Lawrence. ICES J.Mar.Sci. 57: 56-68. [ Links ]
31. Ulltang Ø (1976) Catch per unit of effort in the Norwegian purse seine fishery for Atlanto- Scandinavian herring. Fao Fish.Tech.Pap. 155: 91-101. [ Links ]
32. Winters GH, Wheeler JP (1985) Interaction between stock area, stock abundance, and catchability coefficient. Can.J.Fish.Aquat.Sci. 42: 989-998. [ Links ]
33. Ye Y, Mohammed H (1999) An analysis of variation in catchability of green tiger prawn, Penaeus semisulcatus in waters off Kuwait. Fish.Bull. 97: 702-712. [ Links ]
34. Zar J (1995) Biostatistical Analysis.. 3rd ed Prentice-Hall. Englewood Cliffs: NJ, USA. 718 pp. [ Links ]












 uBio
uBio