Interciencia
versión impresa ISSN 0378-1844
INCI v.29 n.9 Caracas sep. 2004
Ileal amino acid digestibility and performance of pigs fed grain sorghum-based diets supplemented with phytase
Miguel Cervantes, Jorge Yánez, Miguel A. Barrera, José L. Figueroa, Noemí Torrentera and Willem Sauer
Miguel Cervantes. Zootechnical Engineer. Ph.D. in Non-Ruminant Nutrition, University of Kentucky, USA. Professor, Instituto de Ciencias Agrícolas, Universidad Autónoma de Baja California (ICA-UABC), Mexico. Address: Obregón y Julián Carrillo S/N. Colonia Nueva, Mexicali, BC, Mexico. e-mail: Miguel_Cervantes@uabc.mx
Jorge Yánez. M.Sc. in Animal Production Systems, ICA-UABC, Mexico. Professor, Universidad de Tlaxcala, Mexico.
Miguel A. Barrera Silva. M.Sc. in Animal Production Systems, ICA-UABC, Mexico. Manager, Swine Farm, Hermosillo, Sonora, Mexico.
José L. Figueroa Velasco. Agicultural Engineer. Ph.D. in Animal Nutrition, University of Nebraska, USA. Researcher, Colegio de Postgraduados, Montecillo, México.
Noemí Torrentera Olivera. Food Engineer and Master in Meat Sciences, Universidad Autónoma Metropolitana, Mexico. Researcher, ICA-UABC, Mexico.
Willem C. Sauer. Ph.D. in Animal Nutrition. Emeritus Professor, University of Alberta, Edmonton, Canada.
Resumen
Se realizaron dos experimentos para determinar el efecto de la adición de fitasa a dietas sorgo-pasta de soya en la digestibilidad ileal aparente (DIA) de aminoácidos (AA) y el comportamiento de cerdos en crecimiento. En un experimento, seis cerdos (peso promedio inicial 86,1kg) adaptados con una cánula simple tipo T en íleon terminal, fueron alimentados con tres dietas de acuerdo con un cuadro Latino repetido 3x3. La Dieta 1 fue la dieta base sorgo-pasta de soya; las Dietas 2 y 3 fueron la Dieta 1 adicionada con 500 y 1000 unidades de actividad de fitasa (FTU)/kg alimento, respectivamente. No se encontró efecto de la adición de fitasa en la DIA de AA (P>0,10). La DIA de arginina fue la más elevada y la de treonina la más baja. En otro experimento se distribuyeron aleatoriamente 28 cerdos (peso promedio inicial 22,9kg) en cuatro dietas (siete repeticiones/tratamiento) de acuerdo con un diseño de bloques completos al azar. Las dietas fueron: 1) dieta base sorgo-pasta de soya y 0,67% lisina digestible en íleon; 2) dieta base +350FTU/kg de alimento; 3) dieta base +700FTU; 4) dieta base +1050FTU. La dieta base cubría 100% el requerimiento de P disponible para cerdos de 20-50kg. Los análisis de regresión no mostraron efecto de la adición de fitasa en la ganancia diaria de peso (P=0,93); consumo de alimento, lisina y treonina (P=0,37); ni en la conversión alimenticia (P=0,54). Los cerdos alimentados con dieta base tendieron a tener una mayor ganancia de peso que los que recibieron la dieta 2 (P=0,09) o la dieta 3 (P=0,11). Los resultados indican que la fitasa no afecta el suministro de AA digestibles o, como consecuencia, el comportamiento de cerdos alimentados con dietas sorgo-pasta de soya.
Summary
Two experiments were conducted to determine the effect of phytase supplementation to sorghum-soybean meal diets on the apparent ileal digestibility (AID) of amino acids (AA) and performance of growing pigs. In one experiment, six pigs (average initial BW 86.1kg) fitted with a simple T-cannula at the distal ileum, were fed three diets in a repeated 3x3 Latin square design. Diet 1 was the basal sorghum-soybean meal diet. Diets 2 and 3 were the basal diet supplemented with phytase at 500 and 1000 units of phytase activity (FTU/kg diet), respectively. There was no effect of phytase supplementation on the AID of AA (P>0.10). Arginine AID was highest and threonine AID was lowest. In the second experiment, 28 pigs (average initial BW 22.9kg) were allotted to four diets (seven replicates/treatment) in a randomized complete block design. Diets were: 1) sorghum-soybean meal, basal diet, 0.67% apparent ileal digestible lysine; 2) basal diet +350FTU/kg diet; 3) basal +700FTU; 4) basal +1050FTU. The basal diet contained 100% of the available P requirement for pigs 20-50kg. Linear regression analyses showed no effect of phytase supplementation on average daily gain (ADG; P=0.93), feed (P=0.37), lysine (P=0.37) and threonine (P=0.37) intakes, or feed conversion (P=0.54). Pigs fed the basal diet tended to have higher ADG than those fed diet 2 (P=0.09) or diet 3 (P=0.11). Results indicate that phytase supplementation does not affect the supply of digestible AA or, as a consequence, the performance of pigs fed sorghum-soybean meal diets.
Resumo
Realizaram-se dois experimentos para determinar o efeito da adição de fitasa a dietas sorgo-pasta de soja na digestibilidade ileal aparente (DIA) de aminoácidos (AA) e o comportamento de cerdos em crescimento. Em um experimento, seis cerdos (peso médio inicial 86,1kg) adaptados com uma cânula simples tipo T em íleon terminal, foram alimentados com três dietas de acordo com um quadro Latino repetido 3x3. A Dieta 1 foi a dieta base sorgo-pasta de soja; as Dietas 2 e 3 foram a Dieta 1 adicionada com 500 e 1000 unidades de atividade de fitasa (FTU)/kg alimento, respectivamente. Não encontrou-se efeito da adição de fitasa na DIA de AA (P>0,10). A DIA de arginina foi a mais elevada e a de treonina a mais baixa. Em outro experimento se distribuiram aleatoriamente 28 cerdos (peso médio inicial 22,9kg) em quatro dietas (sete repetições/tratamento) de acordo com um desenho de blocos completos ao azar. As dietas foram: 1) dieta base sorgo-pasta de soja e 0,67% lisina digestiva em íleon; 2) dieta base +350FTU/kg de alimento; 3) dieta base +700FTU; 4) dieta base +1050FTU. A dieta base cobria 100% o requerimento de P disponível para cerdos de 20-50kg. As análises de regressão não mostraram efeito da adição de fitasa na ganância diária de peso (P=0,93); consumo de alimento, lisina e treonina (P=0,37); nem na conversão alimentícia (P=0,54). Os cerdos alimentados com dieta base tenderam a ter uma maior ganância de peso que os que receberam a dieta 2 (P=0,09) ou a dieta 3 (P=0,11). Os resultados indicam que a fitasa não afeta o subministro de AA digestivos ou, como conseqüência, o comportamento de cerdos alimentados com dietas sorgo-pasta de soja.
Keywords / Amino Acids / Ileal Digestibility / Phytase / Pigs / Sorghum /
Received: 04/01/2004. Modified: 08/02/2004. Accepted: 08/09/2004.
Introduction
Diets for pigs are usually formulated with cereal grains and soybean meal. Cereal grains, especially sorghum and corn as well as soybean meal, contain significant amounts of phytates, phosphorylated compounds which occur naturally in feedstuffs of plant origin (Maga, 1982). Phytic acid or myo-inositol hexa-phosphoric acid is an ester of phosphoric acid and inositol (Reddy et al., 1982), and is the most common form. Phytates also interact with other minerals such as Ca, Zn, Fe and Mg to form phytate-mineral complexes. According to Reddy et al. (1982) the phytate-mineral complexes interact with proteins forming phytate-mineral-protein complexes. In addition, Láztity and Láztity (1995) reported that phytates create electrostatic interactions with proteins, which result in the formation of protein-phytate complexes. Thus, phytates reduce the digestibility of P and some other minerals such as Ca in pigs (Cromwell, 1992) because they do not produce the enzyme required to hydrolyze the P-phytic acid linkages.
Several studies have demonstrated that the addition of microbial phytase releases P from the P-phytate complex, increasing its digestibility in pigs (Cromwell, 1992). On the other hand, since phytates also interact with proteins, an improvement in the digestibility of protein would be expected as well. Phytates are mainly concentrated in the aleurone layer and the germ of cereal grains (Bedford, 2000) which also contains most of the albumins and globulins (Stone and Savin, 1999). These are the best quality proteins in cereal grains because of their high contents of lysine and threonine (Tatham et al., 1995), the first and second limiting amino acid (AA) in sorghum. Thus, an improvement in protein digestibility upon phytase supplementation will also improve the digestibility of lysine and threonine, as well as the performance of growing-finishing pigs.
The object of this study was to determine the effect of phytase supplementation on the apparent ileal digestibility (AID) of AA and performance of pigs fed a sorghum-soybean meal diet.
Materials and Methods
Two experiments were conducted with crossbred (Landrace x Hampshire x Duroc) pigs at the Swine Experimental Unit of the Universidad de Baja California to determine the effect of phytase supplementation on the apparent ileal digestible (AID) aminoacids (AA) and on performance. The pigs were fed sorghum-soybean meal diets. The same batches of sorghum and soybean meal were used in the digestibility and performance studies. The phytase enzyme used was of microbial origin (Aspergillus niger phytase, Natuphos®, supplied by DSM Food Specialties 2600 MA, Delft, The Netherlands). The phytase activity was 10000FTU·g-1. An FTU is defined as the amount of phytase that liberates 1mMol of ortho-phosphate per min from Na-phytate 5.1mMol/l at pH 5.5 and 37ºC.
Experiment 1. Digestibility trial
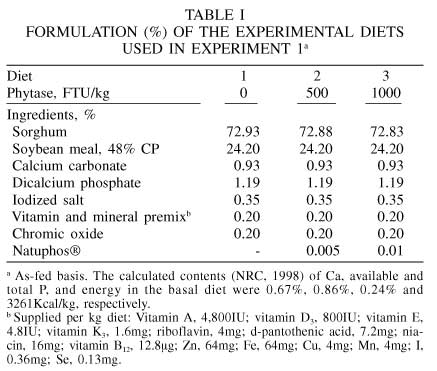
Six barrows with an average initial BW of 86.1kg, fitted with a simple T-cannula at the distal ileum, were used. The barrows were fitted with the canula when their average BW was 20.5 kg. A detailed description of pre- and post-operative care was previously given by Li et al. (1993). The cannulas were prepared from Tygon tubing (Norton Performance Plastics, Wayne, NJ, USA). The pigs were fed one of three experimental diets (Table I). Diet 1 consisted of a sorghum-soybean meal diet formulated to contain 18.2% crude protein (CP) and 0.77% AID lysine. Diet 2 was diet 1 supplemented with phytase at a rate of 500FTU/kg diet. Diet 3 was diet 1 supplemented with phytase at a rate of 1000FTU/kg diet.
The experiment was carried out according to a 3x3 repeated Latin square design (Steel and Torrie, 1980). Each experimental period comprised 12days, 8days of diet adaptation followed by 4 days of collection of ileal digesta. The barrows were fed equal amounts twice daily, at 07:00 and 19:00. The feed intake was limited to 3.3 times the DE requirement for maintenance (NRC, 1998) based on the average BW of the pigs at the start of each experimental period. The feed was mixed with water at a ratio of 1:1. Phytase was added and mixed with the feed at the same time water was added. The pigs consumed their allowed feed in 15min or less. Ileal digesta were collected in plastic bags tied to the barrel of the cannula. The bags were removed and replaced as soon as they were filled with digesta; no bag remained attached to the cannula longer than 20min. The collection of ileal digesta was initiated at 06:00 on day 9. Digesta were collected for 6h alternated with 6h periods when no digesta were collected. The last 6h collection of ileal digesta took place from 00:00 to 06:00 on day 12. Ileal digesta were stored immediately after collection at -20ºC.
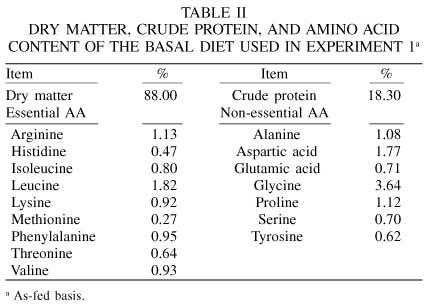
At the conclusion of the experiment, ileal digesta were thawed, pooled within pig and period for the same diet, and homogenized. A subsample of each homogenate was freeze-dried and ground through a 1mm mesh screen. Samples of diets and ileal digesta were analyzed for dry matter (DM) and (CP), which were determined according to AOAC (2000). Amino acid analyses were performed at the University of Missouri Experiment Station Chemical Laboratories in Columbia, MO, USA. Cysteine and tryptophan were not determined. The AA content of the basal diet is presented in Table II. Chromic oxide was analyzed according to Hill and Anderson (1958).
The data were analyzed according to a 3x3 replicated Latin Square using the GLM procedures (SAS, 1988). Regression analysis between the AID of CP and each AA and the rate of phytase supplementation to the basal diet were performed. In addition, the means of dietary treatments were compared by the following contrasts: C1, diet 1 vs diet 2; C2, diet 1 vs diet 3.
Experiment 2. Performance trial
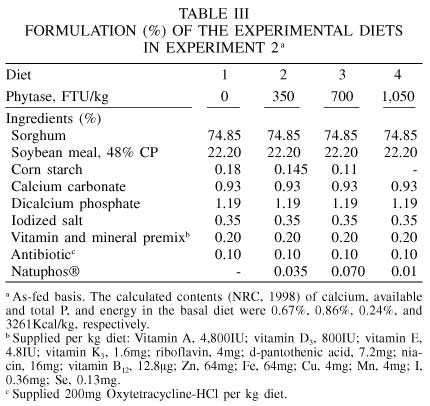
Twenty eight pigs, average initial BW of 22.9 ±3.8kg were used. The pigs were grouped by initial BW and randomly assigned to four experimental diets, based on sex, BW, age, and origin of litter. There were seven pigs (four barrows and three gilts) for each treatment and one pig per pen. Each pen (0.6x1.2m) was equipped with a single-hole feeder and a water nipple. The pigs were allowed to consume their diets ad libitum. They were weighed weekly and feed intake was also determined weekly. The experiment lasted 28 days. The average BW of the pigs at the conclusion of the experiment was 41.7kg.
The formulation of the experimental diets is presented in Table III. Diet 1 was similar to the basal diet used in Exp. 1. Diets 2, 3, and 4 were similar to diet 1 but supplemented with phytase at rates of 350, 700, and 1050FTU/kg diet. All diets contained 0.67% apparent ileal digestible lysine (17.4% CP); 0.10% percentage units below the requirement as specified by NRC (1998) for pigs 20-50kg BW. It was assumed that phytase might increase the digestibility of lysine and, as a result, the supply of digestible lysine would also increase. Thus, the increase in lysine supply because of the phytase supplementation was expected to improve pig performance. Dicalcium phosphate was added to all diets in order to meet 100% of the requirement of available P.
Data were analyzed according to a randomized complete block design (Steel and Torrie, 1980) with four treatments in seven blocks, using the GLM procedures (SAS, 1988). Regression analyses were performed to determine linear relationships. Additionally, treatment means were compared by the following contrasts: C1, diet 1 vs diet 2; C2, diet 1 vs diet 3; C3, diet 1 vs diet 4.
Results and Discussion
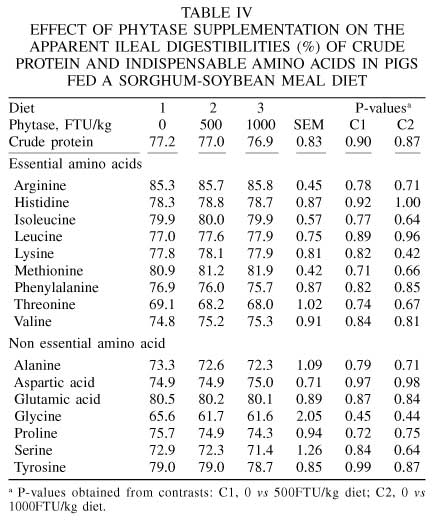
The AID values of the essential AA are presented in Table IV. There was no effect of phytase supplementation to the sorghum-soybean meal diet on the AID of CP or AA (P>0.10). The results are in agreement with those of Traylor et al. (2001). These authors reported that supplemental phytase had very little, if any, effect on the AID of CP and AA in growing pigs fed cornstarch-based dehulled soybean meal diets. They reported slight numerical increases in the apparent and true ileal digestibilities of most AA when phytase (500FTU/kg diet) was supplemented. The improvement in the average digestibility of the essential AA was 0.8 percentage units (pu).
Mroz et al. (1994) and Kemme et al. (1999) reported small increases in the AID of AA upon phytase supplementation to diets for growing pigs. Mroz et al. (1994) reported significant increases in the AID of arginine (2.5pu) and methionine (3.9pu) when phytase (800FTU/kg diet) was supplemented to a corn-tapioca-barley-peas-soybean meal diet. The average of the AID of the indispensable AA increased by 0.7pu. Kemme et al. (1999) reported significant increases in the AID of isoleucine (2.1pu), lysine (2.4pu), threonine (2.9pu) and tryptophan (4.4pu) upon supplementation of phytase (900FTU/kg diet) to a corn-soybean meal diet. The average of the AID of the indispensable AA increased by 2.2%pu. Although the majority of the studies show that supplementing diets with microbial phytase increases or tends to increase the AID of AA, the magnitude of the overall improvement was only about 1 to 2pu.
Based on the present and the aforementioned studies, it can be concluded that phytase supplementation usually results in small numerical increases in the AID of AA. Sometimes, the increases may reach significance even though they are of a small magnitude. The magnitude of the response to phytase supplementation is likely to depend on the phytate content and the activity of intrinsic phytase in the diet (Liao et al., 2002). The largest response would be expected when the diet is high in phytate and has a low activity of intrinsic phytase.
According to Honig and Wolf (1991) phytic acid and phytate may form complexes with protein and free AA at pH values normally occurring in the gastrointestinal tract of the pig. Insoluble complexes of phytate-protein or phytate-AA may be formed, which are less susceptible to hydrolysis by digestive enzymes. Knuckles et al. (1989) conducted an in vitro study in rats and found that phytate and its hydrolysates significantly reduced the digestion of casein and bovine serum albumin by pepsin. However, trypsin digestion of casein and serum albumin was not affected. Another in vitro study showed that phytate binds free AA. Liao et al. (2002) indicated that pre-incubation of phytate with phytase may prevent the formation of protein- or AA-phytate complexes. Also, Reddy et al. (1982) suggested that phytate may inhibit the activity of digestive enzymes via direct binding or by indirect chelation of cations that are necessary for enzymatic activity. Singh and Krikorian (1982) reported that phytates at concentrations 10-100mM substantially reduced the activity of trypsin. In theory (Liao et al., 2002), phytase may cleave the protein- and AA-phytate bond resulting in the release of protein and AA. However, the results from this and other in vivo studies (Liao et al., unpublished data; Traylor et al., 2001) are not consistent with these in vitro results. This discrepancy may arise from the fact that the protein- and AA-phytate complexes are stabilized by weak electrostatic interactions (Láztity and Láztity, 1995), which in theory can be disrupted by pH alterations such as those occurring in the gastrointestinal tract of pigs, without the need of any specific enzyme.
The AID value of arginine was highest, followed by lysine. Threonine had the lowest AID value, whereas the remainder of the AA had intermediate AID values (Table IV). In most studies, including this one, threonine had a lower digestibility value than the other AA, as a result of its relatively high content in endogenous protein (Sauer and Ozimek, 1986; Montagne et al., 2000). Protein in mucin, a large molecular weight glyco-protein that is highly concentrated in endogenous protein, is of low digestibility (Moughan and Schuttert, 1991).
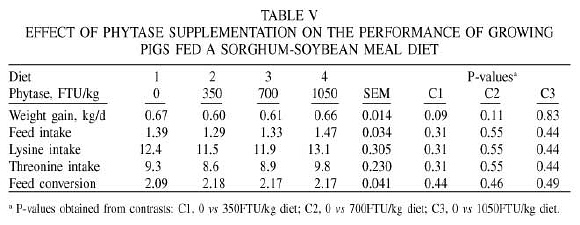
The results of the performance trial are presented in Table V. Linear regression analyses showed no effect of phytase supplementation on the average daily gain (ADG; P=0.93), feed intake (P=0.37), lysine intake (P=0.37), threonine intake (P=0.37) or feed conversion (P=0.54). Moreover, comparisons made by contrasts showed that pigs fed the basal diet tended to have higher ADG than pigs fed the basal diet supplemented with phytase at a rate of 350 (P=0.09) or 700 (P=0.11) FTU/kg diet. Daily gain was not affected (P=0.83) by the supplementation of phytase at a rate of 1050 FTU/kg diet. Feed conversion was not affected by the supplementation of phytase at rates of 350 (P=0.44), 700 (P=0.046) or 1050 (P=0.49) FTU/kg. The lack of response in ADG and feed conversion to the supplementation of phytase to the sorghum-soybean meal diet is consistent with the lack of effect of phytase supplementation on AA digestibility.
The higher ADG of pigs fed the basal diet and the basal diet supplemented with phytase at a rate of 1050FTU/kg compared to the other diets appears to be related to numerical differences in the consumption of lysine and threonine rather than to phytase. This response may not be surprising since lysine and threonine are the first two limiting AA in sorghum-soybean meal diets for pigs (Lewis, 2000), and the diets used in this experiment were purposely formulated to be deficient in these two AA.
The supplementation of phytase to corn- or sorghum-soybean meal diets has consistently shown to increase the availability of P in growing-finishing pigs. Accordingly, the improvements in the performance of pigs fed diets containing low levels of inorganic P, as phytase was supplemented have been reported (Lei et al., 1993; Young et al., 1993; Cromwell et al., 1995a, b). However, there is no report in the literature showing the effect of phytase supplementation on the performance of growing-finishing pigs, beyond those reported as a result of the improvement in P availability.
Conclusions
The results of this study indicate that the supplementation of microbial phytase to sorghum-soybean meal diets does not affect the apparent ileal digestibility of amino acids and the performance of growing pigs. The lack of response may be explained as the result of a possible low content of phytates in the ingredients used in the experimental diets, or that phytates do not affect the ileal apparent digestibility of amino acids.
REFERENCES
1. AOAC (2000) Official Methods of Analysis (17th ed.). Association of Official Analytical Chemists. Gaithersburg, MD, USA. 2200 pp. [ Links ]
2. Bedford MR (2000) Exogenous enzymes in monogastric nutrition- their current value and future benefits. Anim. Feed Sci. Technol. 86: 1-13. [ Links ]
3. Cromwell GL (1992) The biological availability of phosphorous in feedstuffs for pigs. Pig News and Information 13: 75N-78N. [ Links ]
4. Cromwell GL, Coffey RD, Monegue HJ, Randolph JH (1995a) Efficacy of low activity microbial phytase in improving the bioavailability of phosphorus in corn-soybean meal diets for pigs. J. Anim. Sci. 73: 449-456. [ Links ]
5. Cromwell GL, Coffey RD, Parker GR, Monegue HJ, Randolph JH (1995b) Efficacy of a recombinant-derived phytase in improving the bioavailability of phosphorus in corn-soybean meal diets for pigs. J. Anim. Sci. 73: 2000-2008. [ Links ]
6. Hill FN, Anderson DL (1958) Comparison of metabolizable energy and productive energy determination with growing pigs. J. Nutr. 64: 587-603 [ Links ]
7. Honig DH, Wolf WJ (1991) Phytate-mineral-protein composition of soybeans: gel filtration studies of soybean meal extracts. J. Agric. Food Chem. 39: 1037-1042. [ Links ]
8. Kemme PA, Jongbloed AW, Mroz Z, Kogut J, Beynen AC (1999) Digestibility of nutrients in growing-finishing pigs is affected by Aspergillus niger phytase, phytate and lactic acid levels. 2. Apparent total tract digestibility of phosphorus, calcium and magnesium and ileal degradation of phytic acid. Livest. Prod. Sci. 58: 119-127. [ Links ]
9. Knuckles BE, Kuzmicky DD, Gumbmann MR, Betschart AA (1989) Effect of Myo-inositol phosphate esters on in vitro and in vivo digestion of protein. J. Food Sci. 54: 1348-1350. [ Links ]
10. Láztity R, Láztity L (1995) Phytic Acid in Cereal Technology. In Pomeranz Y (Ed.) Advances in Cereal Science and Technology. American Association of Cereal Chemists. St. Paul, MN, USA. pp. 309-372. [ Links ]
11. Lei XG, Ku PK, Miller ER, Yokoyama MT (1993) Supplementing corn-soybean meal diets with microbial phytase linearly improved phytate phosphorus utilization by weaning pigs. J. Anim. Sci. 71: 3359-3367. [ Links ]
12. Lewis AJ (2000) Amino acids in swine nutrition. In Lewis A, Southern JL (Eds.) Swine Nutrition. 2nd ed. CRC Press. New York, NY, USA. pp 151-186. [ Links ]
13. Li S, Sauer WC, Fan MZ (1993) The effect of dietary protein level on ileal and fecal amino acid digestibility in early-weaned pigs. J. Anim. Physiol. Nutr. 70: 117-128. [ Links ]
14. Liao S, Sauer WC, Kies A (2002) Supplementation of microbial phytase to swine diets: Effect on utilization of nutrients. In Nakano S, Ozimek L (Eds.) Food Science and Technology Product. Research Singpost. Kerala, India. pp 199-227. [ Links ]
15. Maga JA (1982) Phytate: Its chemistry, occurrence, food interactions, nutritional significance, and methods of analysis. J. Agr. Food Chem. 30: 1-9. [ Links ]
16. Montagne L, Toullec R, Lalles JP (2000) Calf Intestinal Mucin: Isolation, Partial Characterization, and Measurement in Ileal Digesta with an Enzyme-Linked Immunosorbent Assay. J. Dairy Sci. 83: 507–517. [ Links ]
17. Moughan PJ, Schuttert G (1991) Composition of nitrogen-containing fractions in digesta from the distal ileum of pigs fed a protein-free diet. J. Nutr. 121: 1570-1574. [ Links ]
18. Mroz Z, Jongbloed AW, Kemme PA (1994) Apparent digestibility and retention of nutrients bound to phytate complexes as influenced by microbial phytase and feeding regimen in pigs. J. Anim. Sci. 72: 126-132. [ Links ]
19. NRC (1998) Nutrient Requirements of Swine. 10th ed. National Academy Press. Washington, DC, USA. 183 pp. [ Links ]
20. Reddy NR, Sathé SK, Salunkhe DK (1982) Phytates in legumes and cereals. Adv. Food Res. 28: 1-92. [ Links ]
21. SAS (1988) SAS/STAT users guide: Statistics. Release 6.03. SAS Institute, Inc. Cary, NC, USA. 956 pp. [ Links ]
22. Sauer WC, Ozimek L (1986) Digestibility of amino acids in swine: Results and their practical applications. A Review. Livest. Prod. Sci. 15: 367-388. [ Links ]
23. Singh M, Krikorian AD (1982) Inhibition of trypsin activity in vitro by phytate, J. Agric. Food Chem. 30: 799-800. [ Links ]
24. Steel RGD, Torrie JH (1980) Principles and Procedures of Statistics: A Biomedical Approach. 2nd ed. McGraw-Hill. New York, USA. 622 pp. [ Links ]
25. Stone PG, Savine R (1999) Grain quality and its physiological determinants. In Satorre EH, Slafer GA (Eds.) Wheat: Ecology and Physiology of Yield Determination. Food Products Press. Binghampton, NY, USA. pp. 3-12. [ Links ]
26. Tatham AS, Shewry PR, Belton PS (1995) Structural studies of cereal prolamines, including wheat gluten. In Pomeranz Y (Ed.) Advances in Cereal Science and Technology. American Association of Cereal Chemists. St. Paul, MN, USA. pp. 18-78. [ Links ]
27. Traylor SL, Cromwell GL, Lindeman MD, Knabe DA (2001) Effects of level of supplemental phytase on ileal digestibility of amino acids, calcium, and phosphorus in dehulled soybean meal for growing pigs. J. Anim. Sci. 79: 2634-2642. [ Links ]
28. Yi Z, Kornegay ET, Ravindran V, Lindemann MD, Wilson JH (1996) Effectiveness of Natuphos phytase in improving the bioavailabilities of phosphorus and other nutrients in soybean meal-based semi-purified diets for young pigs. J. Anim. Sci. 74: 1601-1611. [ Links ]
29. Young LG, Leunissen M, Atkinson JL (1993) Addition of microbial phytase to diets for young pigs. J. Anim. Sci. 71: 2147-2150. [ Links ]












 uBio
uBio