Interciencia
versión impresa ISSN 0378-1844
INCI v.30 n.11 Caracas nov. 2005
Primary and secondary somatic enbryogenesis in leaf sections and cell suspensins of coffea arabica cv. catimor.
Rafael Fernández-Da Silva, Luis Hermoso-Gallardo y Andrea Menéndez-Yuffá
Rafael Fernández-Da Silva. Biologist, Doctor in Biological Sciences. Universidad Central de Venezuela (UCV). Professor, Universidad de Carabobo, Venezuela.
Luis Hermoso-Gallardo. Biologist, UCV, Venezuela. Research Assistant, UCV, Venezuela.
Andrea Menéndez-Yuffá. Biologist, UCV, Venezuela. Magister in Technology of Recombinant DNA, Universidad de Cantabria, España. Doctor in Sciences, UCV, Venezuela. Professor Researcher, Laboratorio de Clonación y Genética Vegetal, Instituto de Biología Experimental, UCV, Venezuela Address: Laboratorio de Clonación y Genética Vegetal, Facultad de Ciencias Apartado 47114 Caracas 1041, Venezuela. e-mail: amenendez@cantv.net
Resumen
El principal objetivo de esta investigación fue caracterizar y optimizar el desarrollo de embriones somáticos secundarios en café (Coffea arabica cv. Catimor). Adicionalmente, dado que la formación de embriones somáticos primarios es la etapa previa a la diferenciación de embriones somáticos secundarios, se compararon tres métodos para su inducción: 1) medio sólido en dos etapas, primero un medio con 4,5µM de ácido 2,4-diclorofenoxiacético (2,4-D) y 35µM de benciladenina (BA), y luego un medio con 4,3µM de ácido naftalenoacético, 2) medio sólido con 4,4-53,3µM BA en una etapa, y 3) cultivos en suspensión. En este último tratamiento el callo se formó en dos etapas de medio sólido, un medio con 9,3µM de cinetina y 2,3µM de 2,4-D y un segundo medio con 22,2µM de BA, así como en un tercer medio líquido con 35µM de BA. Todos los tratamientos indujeron la embriogénesis somática primaria y secundaria. El máximo rendimiento de embriones somáticos primarios se obtuvo en suspensiones celulares. Los embriones somáticos secundarios se diferenciaron directamente a partir de células epidérmicas y subepidérmicas en la zona hipocotilar de los embriones somáticos primarios.
Summary
The main purpose of this research was to characterize and optimize the development of secondary somatic embryos in coffee (Coffea arabica cv. Catimor). In addition, as the primary somatic embryos formation is the previous stage to the differentiation of secondary somatic embryos, three methods for its induction were compared: 1) solid media in two stages, first in a medium with 4.5µM of 2,4-dichlorophenoxiacetic acid (2,4-D) and 35µM of benzyladenin (BA) and then in a medium with 4.3µM naphtaleneacetic acid, 2) solid media with 4.4-53.3µM BA in one stage, and 3) suspension cultures. In the latter treatment the callus was formed in two stages of solid media, one containing 9.3µM of kinetin and 2.3µM of 2,4-D, and a second one with 22.2µM of BA, and a third liquid medium with 35µM of BA. All the treatments induced primary and secondary somatic embryogenesis. The highest yield of somatic embryos was obtained in suspension cultures. The secondary somatic embryos differentiated directly from epidermal and subepidermal cells of the hypocotilar zone of the primary somatic embryos.
Resumo
O objetivo principal de este estudo foi caracterizar e melhorar u desenvolvimento dos embriões somáticos secundários no café (Coffea arabica cv. Catimor). Ademais, devido a que u desenvolvimento dos embriões somáticos primários e a primeira estágie da diferenciación dos embriões somáticos secundários, foram comparados três metodos para indução: 1) um meio solido em dois estágios, primeiro meio com 4,5µM do ácido 2,4-diclorofenoxiacetico (2,4-D) e 35µM do benzyladenine (BA) e segundo meio com 4,3µM do ácido naftalenoacético, 2) um meio solido em uma estágio (4,4-53,3µM BA), e 3) suspensões celulares. Em esse ultimo tratamento, u calo fui formado em dois estágies do meio solido, uma com 9,3µM do cinetine e 2,3µM da 2,4-D, e segundo com 22,2µM do BA, e um terceiro meio líquido com 35µM do BA. Todos os tratamentos induziram embriognese somática primária e secundária. O rendimento mais elevado dos embriões somáticos foi obtido em suspensões celulares. Os embriões somáticos secundários foram diferenciados na maneira direta das células epidérmicas e subepidérmicas da zona hipocotilar dos embriões somáticos primários.
KEY WORDS / Benzyladenine / Direct Somatic Embryogenesis / Histology / Indirect Somatic Embryogenesis / Leaf Explants / Primary Embryos / Secondary Embryos /
received: 01/06/2005. Modified: 07/04/2005. accepted: 07/20/2005.
Introduction
Coffee is a very important crop in the world as a source of a popular and stimulating beverage which is produced from its toasted and ground seeds. Its economic importance is evident through the 10 million US$ exports for the year 2000. It occupies second place in value of exports of main commodities for 1998, 1999 and 2000 (ICO, 2005).
The coffee plant belongs to the Rubiaceae family, being Coffea arabica the most cultivated species in the world. The methods for coffee regeneration using tissue culture are well established in the different species of this crop, and from almost all its organs excepting the root (Baumann and Neuenschwander, 1990). Coffee can be propagated through microcuttings or by somatic embryogenesis (Dublin, 1984), somatic embryogenesis being the most common technique because it presents the highest rate of multiplication. The first report of somatic embryogenesis in coffee was done by Staritsky (1970) induced from orthotropic stem sections; it was later determined that frequency of somatic embryos increases when leaf sections are used as explants (Söndhal and Sharp, 1977; Dublin, 1981; García and Menéndez, 1987; Neuenschwander and Baumann, 1992). Cell suspension cultures (Zamarripa et al., 1991; Hermoso-Gallardo and Menéndez-Yuffá, 2000) and temporary immersion cultures have also been established (Etienne-Barry et al., 1999) for scaling-up to mass-production. This brief outline evidences that in coffee several systems for the regeneration by somatic embryogenesis are well established. Nevertheless, none of the abundant reports on coffee somatic embryogenesis followed the process of secondary somatic embryogenesis in detail. Therefore, the present study was set out to establish and characterize morphologically and histologically the secondary somatic embryogenesis initiated from leaf sections and cell suspension cultures, because the secondary somatic embryogenesis is important in plant improvement programs using genetic engineering methods, as transgenic plants can be regenerated and mass propagated from potentially transformed tissues.
Materials and Methods
Plant material, disinfection and explant sowing
Plants of Coffea arabica cv. Catimor were used, a variety obtained by the cross between C. arabica cv. Caturra rojo x hybrid of Timor (a natural cross of C. arabica x C. canephora). This variety presents the remarkable characteristic of being resistant to coffee rust. Adult plants were maintained in soil-filled polypropylene bags in the gardens of the Institute of Experimental Biology, Universidad Central de Venezuela, in Caracas. Mature leaves without lesions were cut and disinfected as follows: leaves were washed in tap water and household soap, immersed in 10% (v/v) Betadine (10% povidone iodine from Laboratorios Farma, Caracas, Venezuela) where they were maintained 5min under continuous agitation. Leaf sections were immersed in 30% (v/v) commercial bleach (5,25% w/v of sodium hipochloride) for 30min with continuous agitation, followed by 3 rinses of 3min each with sterile distilled water. After disinfection, segments of 1cm2 were sown in tubes of 20cm length and 2.5cm diameter, placing the upper, adaxial, surface on the culture medium, which was made of half strength Murashige and Skoog (1962) salts, 29.6µM thiamine-HCl, 554.9µM myo-inositol, 222.1µM cysteine-HCl and 87mM sucrose; the pH was adjusted to 5,8 and 8g/l of agar were added to solidify the solution. The medium was sterilized in an autoclave at 121ºC for 15min. The medium also contained plant growth regulators, which were added before autoclaving and are specified in the next paragraphs.
Induction of primary and secondary somatic embryogenesis in solid media
The following treatments, varying mainly in the type and concentrations of plant growth regulators were evaluated. Treatment A: First stage in a basic medium with 4.5µM 2,4-dichlorophenoxiacetic acid (2,4-D) and 35µM benzyladenine (BA) during 12 weeks, and second stage in a medium with 4.3µM naphtaleneacetic acid (NAA; García and Menéndez, 1987). Treatment B: Culture in only one medium with 4.4, 13.3, 22.2, 26.6, 35.5, 44.4 or 53.3µM BA as the sole growth regulator. The cultures were incubated in the dark at room temperature (~25ºC).
Induction of and primary and secondary somatic embryogenesis in cell suspension cultures
Basically, the five-stage method reported by Hermoso-Gallardo and Menéndez-Yuffá (2000) was used with modifications, as follows.
Stage I. The leaves were disinfected as described above, and the leaf sections were cultivated in the basic culture medium with the following modifications: 4.9µM pyridoxine and 8.1µM nicotinic acid were added and the growth regulators used were 9.3µM kinetin and 2.3µM 2,4-D; the solidifying agent was 8g/l agar. The explants were incubated during 12 weeks in the dark at room temperature.
Stage II. The embryogenic calli were transferred to a medium with similar composition as in stage I, except that it contained 22.2µM BA as the sole growth regulator, and were maintained during 4 weeks in this medium, in the dark at room temperature.
Stage III. To establish cell suspensions the embryogenic calli were transferred to a liquid medium with similar composition as in stage II except it contained 35µM BA, and was maintained during 15 days with continuous shaking at 160rpm in the dark at room temperature. This procedure was used to disaggregate the calli.
Stage IV. The cultures were filtered through a 150 mesh steel sieve, and the obtained suspension was maintained in the same conditions as in stage III. The cultures were transferred to a fresh medium every 8 days. The embryogenic calli were induced in different media than that of the method in solid media, because when the system of cell suspensions was established (Hermoso and Menéndez, 2000), the yield in somatic embryos was higher when the embryogenic callus was induced with 2.3µM 2,4-D and 9.5µM kinetin, compared to 4.5µM 2,4-D and 35µM BA.
Morphoanatomical characterization
Leaf sections, embryogenic calli and somatic embryos obtained in the different treatments were fixed in 70% isopropyl alcohol. Free hand sections were stained with toluidine blue and methylene blue (1:1); and semi-permanent slides were prepared. Some tissues were also included in Paraplast X-TRA (Oxford Labware), sectioned in a rotatory microtome and stained with Orange G and Fast Green, according to Johansen (1940) modified by Menéndez-Yuffá and García (1997). Photographic records of the morphology and histological sections were done with a stereoscopic microscope and a light microscope with photographic accessories (Nikon).
Results and Discussion
In order to obtain secondary somatic embryos, primary somatic embryogenesis must be induced first. In the present study the results of comparing three methods to induce this process are presented first; then, the results of two methods used to obtain secondary somatic embryogenesis are described and discussed.
Primary somatic embryogenesis in solid media and cell suspensions
Embryogenic calli were observed earlier in the leaf sections cultivated in treatment A (4.5µM 2,4-D and 35µM BA) than in treatment B (medium with BA only). Treatment A induced the formation of abundant embryogenic calli in 100% of the explants, differing with treatment B, where only a little callus was formed only in 25 and 37% of the explants when cultured in media with 26.6 or 35µM BA, respectively. In treatment A after one week of culture, calli were developed in the cut edges of the explant and after 3 more weeks, abundant calli of white-beige color was present in 100% of the explants. Embryo differentiation was observed after 4 months in treatment A (first stage in a medium with two growth regulators and second stage in a medium with one growth regulator). In this treatment friable non-embryogenic calli with a rough surface could be clearly distinguished (Figure 1a). The embryogenic calli presented a smooth white surface, with somatic embryos on it, at different stages of development (Figure 1b). After 2 months of culture in different BA concentrations (treatment B), non-responding explants were observed as well as necrotic, brown friable calli (Figure 1d). This treatment also produced compact yellow embryos of smooth surface in the cut edges (Figure 1c) and individual or groups of globular somatic embryos in 10-15% of the explants. Embryo differentiation was observed two months after the initiation of the cultures in the media with only BA. After five months of culture somatic embryo aggregates, in different stages of development (globular, torpedo and cotiledonar) could be distinguished (Figure 1e).
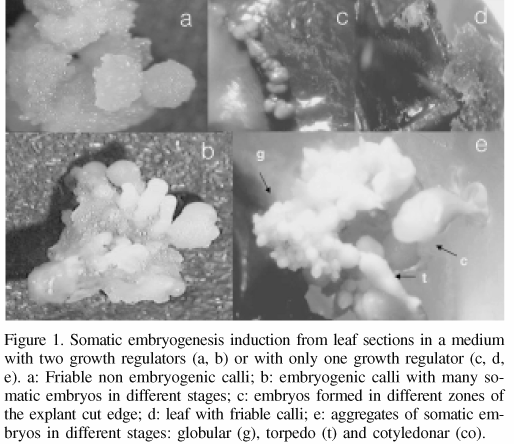
Histological sections of the embryogenic calli that were formed from leaf sections cultivated in treatment B showed little callus formation. The callus was formed by isodiametric cells organized in compact form and originating by the division of spongy and palisade parenquima. Embryos were formed from this callus, which is evidence of indirect somatic embryogenesis. Since callus formation was scarce, there is a lower risk of genetic variation in the somatic embryos regenerated. Skirvin and Janick (1976, cited by Skirvin et al., 1994) pointed out that callus is so often associated with somaclonal variation that some callus-derived variants have been called "calliclones" to denote their callus origin. "The relationship between callus and somaclonal variation is so strong that many commercial laboratories try to avoid callus development at any stage of propagation" (Skirvin et al., 1994).
The medium used to induce somatic embryogenesis in coffee usually contains a combination of an auxin (2,4-D) and a citokinin (kinetin, BA or 2-isopenteniladenin), the latter in higher concentration (García and Menéndez, 1987; Berthouly and Michaux-Ferriere, 1996). However, Yasuda et al. (1985) and Hatanaka et al. (1991,1995) succeeded in the induction of somatic embryogenesis in C. arabica and C. canephora using as the sole growth regulator either a citokinin, kinetin, BA or 2-isopenteniladenin respectively. The results of the present study confirmed that somatic embryogenesis can be induced in coffee using both media, one initially with two plant growth regulators (2,4-D and BA) and with one citokinin (BA) only. Several studies of somatic embryogenesis in two stages indicate that this occurs in an indirect way (Söndhal and Sharp, 1977; Dublin, 1981; Menéndez-Yuffá and García, 1997; Neuenschwander and Baumann, 1992); however, it is not clear whether the somatic embryogenesis induced in only one medium with citokinin is direct or indirect. The present observations indicate that the somatic embryogenesis induced in only one medium was indirect, with little callus formation, thus differing from Quiroz-Figueroa et al. (2002), who reported direct and indirect somatic embryogenesis from leaf sections of C. arabica cv. Caturra rojo.
Cell suspensions
Two weeks after the initiation of culture in liquid medium, it was observed after sieving that the suspension had the appearance of a fine cell suspension, beige in color. Two weeks later some somatic embryos could be observed, and 4 more weeks after that, a lot of embryos were present, most of them in torpedo shape stage. Embryogenic cultures derived from embryogenic calli displayed different types of cells. Large, elongated, thin-walled cells with little dense cytoplasm were observed in isolated form (Figure 2a) or in groups, and also smaller, rounded, thick-walled cells with dense cytoplasm, both forming groups or individually (Figure 2b). Smaller cells, considered as embryogenic, could be observed in different forms and patterns of division: lightly elongated cells with transverse division patterns, or symmetrical rounded cells with asymmetrical and transverse division (Figure 2c).
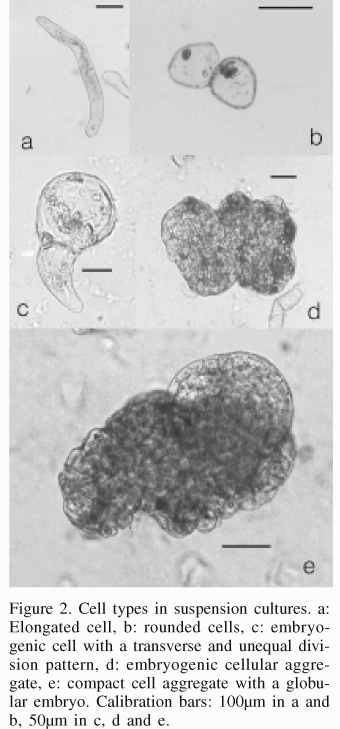
The somatic embryos originated from embryogenic cell aggregates (Figure 2d) or from only one isolated cell. Embryos with elongated form could be observed, apparently developed from elongated cells, while embryos with globular form (Figure 2e) and heart-shaped embryos with well defined uniseriated epidermis formed from compact embryogenic aggregates; likewise, some embryos developed from bodies with several fused embryos.
Secondary somatic embryogenesis in solid media with BA and in cell suspensions
The secondary somatic embryogenesis is the regenerative process whereby somatic embryos are developed from primary somatic embryos. It was characterized for leaf sections under treatment B (only one growth regulator) and for cell suspensions. Six months after placing the leaf sections in a culture medium with BA (treatment B), the differentiation of secondary somatic embryos was observed on the surface of the hypocotyl of primary somatic embryos (Figure 3a), which after 4 weeks reached the globular or torpedo stages (Figure 3b). The largest amount of secondary somatic embryos (58 embryos per explant in average) were differentiated in the medium with 26.6µM BA. Marques (1993) in Coffea eugenioides and Menéndez-Yuffá and García (1997) in C. arabica cv. Catimor mentioned the occurrence of secondary somatic embryogenesis in coffee. Bertrand-Desbrunais et al. (1988) described the regeneration of secondary somatic embryos of coffee after freezing in liquid nitrogen; these embryos formed on the surface of the primary embryos without precise localization. In Quercus suber secondary somatic embryos can be induced (Fernández-Guijarro et al., 1995) in a two-stage process in which the first culture was done in medium with an auxin (naphtaleneacetic acid) combined with a citokinin (BA). Choi et al. (1998) observed the differentiation of secondary somatic embryos on the surface of the primary somatic embryos differentiated in cell cultures of Acanthopanax koreanum in a medium with BA or Zeatin.
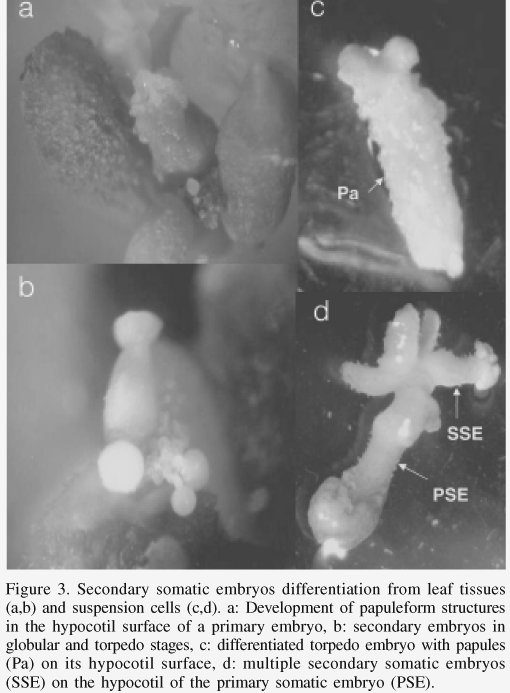
The formation of secondary somatic embryos was observed 4 months after the establishment of the cell suspension cultures of coffee. Their development was distinguished by the formation of elongated papilla structures at the level of the hypocotyl of the primary torpedo shaped embryos (Figure 3c, 3d). Later they could be seen as secondary somatic embryos in torpedo stage (Figure 3d), from which new embryos developed in its surface. Microscopical observations revealed that secondary somatic embryos developed directly by cell division at the epidermal and subepidermal levels (Figure 4a, 4b) in the hypocotyl of the primary somatic embryos, observed initially as papilla structures (Figure 3c, 4a), and later on as a few-celled embryo (Figure 4c), globular (Figure 4d) and more advanced stages. In species like Myrtus communis (Canhoto et al., 1999) and Solanum tuberosum (Seabrook and Douglass, 2001) secondary embryos can differentiate in different zones of the primary embryo, such as the base of the primary embryo hypocotyl and also zones close to the root pole. As in coffee, these secondary embryos are formed by cell divisions at epidermal and subepidermal levels. In coffee, it was frequently observed that embryos were fused in multiple embryogenic aggregates.

A high potential for repetitive embryogenesis was found in liquid media. The formation of primary and secondary embryos in solid media with BA always occurred in low numbers (10 primary and 58 secondary embryos in average per explant in 6 months), in comparison with primary and secondary somatic embryos formed in cell suspensions (3578 embryos per 100ml). The results provide evidence that the secondary somatic embryos of coffee can be formed in solid as well as liquid media, frequency being higher when the medium was liquid. Large masses of secondary and tertiary embryos were observed in cell suspensions, coinciding with observations in Manihot esculenta (Raemakers et al., 1993a, b) and Vitis rupestris (Martinelli et al., 2001), among others.
Finally, it is important to remark the great importance of the secondary somatic embryogenesis in programs for plant improvement using genetic transformation methods, since transgenic plants can be mass propagated from potentially transformed tissues, maintaining their genetic fidelity. This biotechnological potential has already been applied in coffee direction in our laboratory, obtaining transitory expression of the gus gene in plants regenerated by secondary somatic embryogenesis in tissues electroporated with the gus and bar genes (Fernández-Da Silva and Menéndez-Yuffá, 2003).
Concluding Remarks
The present research provides for the first time details about the process of secondary somatic embryogenesis in coffee, which occurs directly without callus formation. The results suggest that secondary somatic embryogenesis is a useful process for the regeneration of genetic transformation products, because it occurs in primary somatic embryos, which in coffee can be obtained in large numbers and can then be used as a subject for transformation processes. Availability of tissues with an elevated capacity for regeneration is an important condition for genetic transformation procedures, since secondary somatic embryogenesis occurs from the epidermal and subepidermal cells of the primary somatic embryos. These cells are the most susceptible ones to transformation in a process of bombardment and electroporation. Secondary somatic embryogenesis is a suitable means of regenerating cells transformed by these methods, as already was suggested for soybean (Droste et al., 2001) and coffee (Fernández-Da Silva and Menéndez-Yuffá, 2003).
Acknowledgements
The authors express their gratitude to Ana Herrera and Carlos Quirós for revision of the manuscript. This work received financial support from FONACIT, Venezuela (project Nº S1-098003209).
References
1. Baumann TW, Neuenschwander B (1990) Tissue culture in coffee biotechnology. Café Cacao Thé 34: 159-164. [ Links ]
2. Berthouly M, Michaux-Ferriere N (1996) High frequency somatic embryogenesis in Coffea canephora. Plant Cell Tiss. Org. Cult. 44: 169-176. [ Links ]
3. Bertrand-Desbrunais A, Fabre J, Engelmann F, Dereuddre J, Charrier A (1988) Reprise de lembryogenèse adventive à partir dembryons somatiques de caféier (Coffea arabica L.) après leur congélation dans lazote liquide. C. R. Acad. Sci. Paris 307 (Serie III): 795-801. [ Links ]
4. Canhoto JM, Lopes ML, Cruz GS (1999) Somatic embryogenesis and plant regeneration in myrtle (Myrtaceae). Plant Cell Tiss. Org. Cult 57: 13-21. [ Links ]
5. Choi YE, Yang DC, Park JC, Soh WY, Choi KT (1998) Regenerative ability of somatic single and multiple embryos from cotyledons of Korean ginseng on hormone-free medium. Plant Cell Rep. 17: 544-551. [ Links ]
6. Droste A, Pimentel LPC, Pasquali G, Mundstock EC, Bodanese-Zanettini MH (2001) Regeneration of soybean via embryogenic suspension culture. Scientia Agricola 58: 753-758. [ Links ]
7. Dublin P (1981) Embryogenèse somatique directe sur fragments de feuilles de caféier Arabusta. Café Cacao Thé 25: 237-242. [ Links ]
8. Dublin P (1984) Techniques de reproduction végétative in vitro et amélioration génétique chez les cafeiers cultivés. Café Cacao Thé 28: 231-244. [ Links ]
9. Etienne-Barry D, Bertrand B, Vásquez N, Etienne H (1999) Direct sowing of Coffea arabica somatic embryos mass-produced in a bioreactor and regeneration of plants. Plant Cell Rep. 19: 111-117. [ Links ]
10. Fernández Da Silva R, Menéndez-Yuffá A (2003) Transient gene expression in secondary somatic embryos from coffee tissues electroporated with the genes gus and bar. Electronic J. Biotechnol. 6: 29-38. [ Links ]
11. Fernández-Guijarro B, Celestino C, Toribio M (1995) Influence of external factors on secondary embryogenesis and germination in somatic embryos from leaves of Quercus suber. Plant Cell Tiss. Org. Cult. 41: 99-106 [ Links ]
12. García E, Menéndez A (1987) Embriogénesis somática a partir de explantes foliares del cafeto "Catimor". Café Cacao Thé 31: 15-22. [ Links ]
13. Hatanaka T, Arakawa O, Yasuda T, Uchida N, Yamaguchi T (1991) Effect of plant growth regulators on somatic embryogenesis in leaf cultures of Coffea canephora. Plant Cell Rep. 10: 179-182. [ Links ]
14. Hatanaka T, Sawabe E, Azuma T, Uchida N, Yasuda T (1995) The role of ethylene in somatic embryogenesis from leaf discs of Coffea canephora. Plant Sci. 107: 199-204. [ Links ]
15. Hermoso-Gallardo L, Menéndez-Yuffá A (2000) Multiplicación masiva del café (Coffea arabica L. cv. Catimor) mediante cultivo de suspensiones celulares embriogénicas. Acta Cient. Venez. 51: 90-95. [ Links ]
16. ICO (2005) Value of exports of main commodities. International Coffee Organization http://dev.ico.or/documents/exportvalues.pdf. [ Links ]
17. Johansen RA (1940) Plant microtechnique. McGraw-Hill. New York, USA. 523 pp. [ Links ]
18. Marques DV (1993) Induction of somatic embryogenesis on Coffea eugenioides Moore by in vitro culture of leaf explants. Café Cacao Thé 37: 251-255 [ Links ]
19. Martinelli L, Candioli E, Costa D, Poletti V, Rascio N (2001) Morphogenic competence of Vitis rupestris S. secondary somatic embryos with a long culture history. Plant Cell Rep. 20: 279-284 [ Links ]
20. Menéndez-Yuffa A, García EG (1997) Morphogenic events during indirect somatic embryogenesis in coffee "Catimor". Protoplasma 199: 208-214. [ Links ]
21. Murashige T, Skoog F (1962) A revised medium for rapid growth on bioassays with tabaco tissue culture. Physiol. Plant. 15: 473-497. [ Links ]
22. Neuenschwander B, Baumann T (1992) A novel type of somatic embryogenesis in Coffea arabica. Plant Cell Rep. 10: 608-612. [ Links ]
23. Quiroz-Figueroa FR, Fuentes-Cerda CFJ, Rojas-Herrera R, Loyola-Vargas VM (2002) Histological studies on the developmental stages and differentiation of two different somatic embryogenesis systems of Coffea arabica. Plant Cell Rep. 20: 1141-1149. [ Links ]
24. Raemakers CJJM, Amati M, Staritsky G, Jacobsen E, Visser RGF (1993a) Cyclic somatic embryogenesis and plant regeneration in cassava. Ann. Bot. 71: 289-294. [ Links ]
25. Raemakers CJJM, Bessembinder JJE, Staritsky G, Jacobsen E, Visser RGF (1993b) Induction, germination and shoot development of somatic embryos in cassava. Plant Cell Tiss. Org. Cult. 33: 151-156. [ Links ]
26. Seabrook JEA, Douglass LK (2001) Somatic embryogenesis on various potato tissues from a range of genotypes and ploidy levels. Plant Cell Rep. 20: 175-182. [ Links ]
27. Skirvin RM, McPheeters KD, Norton M (1994) Sources and Frequency of somaclonal variation. HortScience 29: 1232-1237. [ Links ]
28. Söndhal MR, Sharp WR (1977) High frecuency induction of somatic embryos in cultured leaf explants of Coffea arabica L. Z. Pflanzenphysiol. 81: 395-408. [ Links ]
29. Staritsky G (1970) Embryoid formation in callus tissues of coffee. Acta Bot. Neerl. 19: 509-514. [ Links ]
30. Yasuda T, Fujii Y, Yamaguchi T (1985) Embryogenic callus induction from Coffea arabica leaf explants by benzyladenine. Plant Cell Physiol. 26: 595-597. [ Links ]
31. Zamarripa A, Ducos JP, Bollon H, Dufour M, Petiard V (1991) Production dembryons somatiques de caféier en milieu liquide: effets du milieu. Café Cacao Thé 35: 233-244. [ Links ]












 uBio
uBio 
