Interciencia
versión impresa ISSN 0378-1844
INCI v.32 n.1 Caracas ene. 2007
Methane soil-vegetation-atmosphere fluxes in tropical ecosystems
Eugenio Sanhueza
Doctor in Sciences, Universidad de Chile. Researcher, Instituto Venezolano de Investigaciones Científicas (IVIC), Venezuela. Address: Atmospheric Chemistry Laboratory, IVIC. Apdo. 22117. Caracas 1020A, Venezuela. e-mail: esanhuez@ivic.ve
SUMMARY
Recently, a surprising discovery indicated that, by an unknown process, vegetation emits methane to the atmosphere. This finding could have serious implications in atmospheric chemistry, climate, and mitigation of global change. In order to evaluate the magnitude of the tropical vegetation source, a re-evaluation of results obtained at various Venezuelan ecosystems is made. CH4 fluxes from the soil-grass system in savanna ecosystems indicate that grasses produce CH4 at ~10ng·m-2·s-1. Furthermore, CH4 accumulation within the nocturnal mixing layer at the Guri site, which is affected by savanna and forest emissions, was used to make a rough upper limit estimation of <70ng·m-2·s-1 for CH4 emission from forest vegetation. These estimates are likely to be somewhat low as they do not take into account the light-induced production of CH4 by the vegetation. Global extrapolation of these fluxes indicates that, ignoring the possible stimulating effects of solar radiation, savanna and forest vegetation result in CH4 emissions of ~5Tg·yr-1 and <22Tg·yr-1, respectively. These estimates are in agreement with the lower estimates based on laboratory CH4 flux measurements, reported in the literature. On the other hand, the global extrapolation of the atmosphere-soil uptake fluxes results CH4 sinks of ~1.3Tg·yr-1 in savannas and of 3.3Tg·yr-1 in forests. In conclusion, Venezuelan field measurements support the discovery that vegetation emits CH4. However, global extrapolation indicates that tropical vegetation would contribute modestly to global methane emission, which, additionally, is offset in part by savanna and forest CH4 soil uptake. Most likely, carbon sequestration benefits from forestation should not be significantly affected by CH4 emissions by trees.
Flujos de metano del sistema suelo-vegetación-atmósfera en ecosistemas tropicales
RESUMEN
Recientemente, un sorpresivo descubrimiento mostró que, por un proceso no establecido, la vegetación emite metano. Esto podría tener serias implicaciones en química atmosférica, el clima, y las actividades de mitigación del cambio global. Para evaluar la magnitud de la vegetación tropical como fuente de CH4, se re-evalúan los resultados obtenidos en varios ecosistemas venezolanos. Los flujos a la atmósfera desde el sistema suelo-pasto en la sabana indican que los pastos producen CH4 a una velocidad de ~10ng·m-2·s-1. Además, la acumulación de CH4 dentro de la capa de mezcla nocturna en el Guri, lugar afectado por las emisiones de los ecosistemas de sabana y bosque, permite hacer una primera estimación del límite superior de la emisión de CH4 de la vegetación de bosque de <70ng·m-2·s-1. Estos valores podrían estar subestimados pues no incluyen el efecto de la radiación solar sobre la producción de CH4 por la vegetación. Ignorando el posible efecto de la radiación solar, la extrapolación global de estos flujos producen una emisión de CH4 de ~5Tg·año-1 y <22Tg·año-1, para la vegetación de sabana y bosque, respectivamente, valores que concuerdan con estimaciones más bajas reportadas en la literatura, basadas en mediciones de flujos en laboratorio. Por otra parte, la extrapolación global del consumo de CH4 por los suelos produce un sumidero de ~1,3Tg·año-1 para sabanas y 3,3Tg·año-1 para bosques. En conclusión, mediciones de campo en Venezuela apoyan el descubrimiento que la vegetación emite metano. Sin embargo, la extrapolación global indica que la vegetación tropical haría una modesta contribución a la emisión global, la cual adicionalmente sería compensada por el consumo de CH4 en los suelos. Los beneficios del secuestro de carbono por forestación no serían significativamente afectados por la emisión de CH4 por los árboles.
Fluxos de metano do sistema solo-vegetação-atmosfera em ecossistemas tropicais
RESUMO
Recentemente, um surpreendente descobrimento mostrou que, por um processo não estabelecido, a vegetação emite metano. Isto poderia ter serias implicações em química atmosférica, o clima, e as atividades de mitigação da mudança global. Para avaliar a magnitude da vegetação tropical como fonte de CH4, se reavaliam os resultados obtidos em vários ecossistemas venezuelanos. Os fluxos para a atmosfera desde o sistema solo-pasto na savana indicam que os pastos produzem CH4 a uma velocidade de ~10ng·m-2·s-1. Além disso, a acumulação de CH4 dentro da capa de mistura noturna em Guri, lugar afetado pelas emissões dos ecossistemas de savana e bosque, permite fazer uma primeira estimação do limite superior da emissão de CH4 da vegetação de bosque <70ng·m-2·s-1. Estes valores poderiam estar subestimados pois não incluem o efeito da radiação solar sobre a produção de CH4 pela vegetação. Ignorando o possível efeito da radiação solar, a extrapolação global destes fluxos produzem uma emissão de CH4 de ~5Tg·ano-1 e <22Tg·ano-1, para a vegetação de savana e bosque, respectivamente, valores que concordam com estimações mais baixas relatadas na literatura, baseadas em medições de fluxos em laboratório. Por outra parte, a extrapolação global do consumo de CH4 pelos solos produz um sumidouro de ~1,3Tg·ano-1 para savanas e 3,3Tg·ano-1 para bosques. Em conclusão, medições de campo na Venezuela apóiam o descobrimento de que a vegetação emite metano. No entanto, a extrapolação global indica que a vegetação tropical faria uma modesta contribuição à emissão global, a qual adicionalmente seria compensada pelo consumo de CH4 nos solos. Os benefícios do seqüestro de carbono por florestação não seriam significativamente afetados com a emissão de CH4 pelas árvores.
KEY WORDS / Global Methane Budget / Methane from Vegetation / Methane in Tropical Ecosystems /
Received: 08/11/2006. Modified: 12/11/2006. Accepted: 12/12/2006.
Atmospheric methane is an important greenhouse gas whose radiative properties and atmospheric chemistry affect both climate and stratospheric ozone. Recently, researchers at the Max Plank Institute for Nuclear Physics in Heidelberg made a surprising discovery: by an unknown process, dead and live vegetation emit CH4 to the atmosphere. The first field measurements supporting the finding came from studies made in Venezuelan ecosystems (Crutzen et al., 2006; Sanhueza and Donoso, 2006), indicating that tropical vegetation produces methane. Also, CH4 emissions from vegetation may explain recent satellite observations over the Amazon forest, indicating high atmospheric levels of methane (Frankenberg et al., 2005, 2006). The discovery is surprising because chemistry in plants is generally thought as an aerobic process and methane production is mainly associated with anaerobic environments (e.g., wetlands, enteric fermentation).
The global extrapolation made by Keppler et al. (2006), based on laboratory measurements of CH4 fluxes from plants and net primary productivity (NPP) of ecosystems, indicates CH4 quantities that appear to be very significant (up to 30%) for the global budget. Although the budget has some significant uncertainties (Prather and Ehhalt, 2001) this emission from plants would be difficult to incorporate without some significant re-evaluation of the sources already evaluated. As expected, the Keppler et al. (2006) article created an intense debate and concern, not only in the scientific community but also in policy makers, due to the spin given by certain media coverage (NIEPS, 2006); a significant emission of methane from trees would have serious implications in offsetting greenhouse emissions by reforestation, promoted by the Kyoto protocol.
Kirschbaum et al. (2006), using the same basic CH4 flux information produced by Keppler et al. (2006) presented alternative extrapolations, based on foliage biomass and photosynthetic rates, which indicate much lower global plant CH4 emissions. Ferretti et al. (2006) tested the global production reported by Keppler et al. (2006) against ice core records of atmospheric CH4 concentrations and stable carbon isotope ratios (d13CH4) over the last 2000 years and also concluded that global emission from plants must be much lower than the one proposed by these authors. In the past, our research group measured methane soil-vegetation-atmosphere fluxes at various ecosystems in Venezuela, which are re-analyzed and summarized in this article. It is concluded that, in agreement with Keppler et al. (2006), tropical vegetation would emit methane; however, the magnitude of this source should be much lower than the one estimated by these authors, in concordance with Kirschbaum et al. (2006) and Ferretti et al. (2006) calculations. Global emissions of CH4 from tropical savannas and forests would be readily reconciled within the uncertainties in the established methane budget.
Field measurements
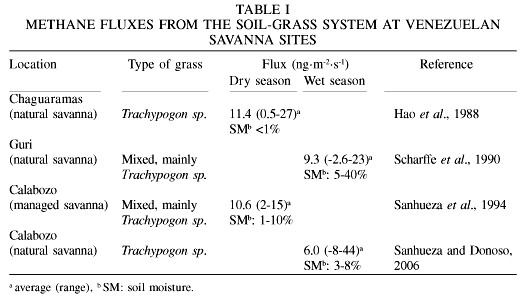
Measurements were made during dry and wet seasons at various sites in the Venezuelan savanna climate region (Table I). A map indicating the locations of the sites (i.e., Chaguaramas, Guri, Calabozo) is found in Sanhueza et al. (2000). Two well defined climatic periods occur in the region: a dry season from Dec to Apr and a rainy season from May to Nov. Surface annual temperatures range from 24 to 28ºC. The amplitudes of daily temperatures are 10-15ºC, with larger deviations occurring during the dry season (Sanhueza et al., 1988). The annual rainfall is 900-2100mm, with only ~10% occurring during the dry season. Soils are acidic and poor in nutrients, with a low rate of mineralization. They support gramineous grasses interrupted by trees and shrub. In the northern part of the Guayana shield (Guri site), relatively exuberant patches of forest occurs. Physical and chemical properties of soils and other local characteristics of the study sites are found in references listed in Table I.
Fluxes from savanna soil-grass systems and forest soils were measured using the static chamber technique. CH4 was analyzed by gas chromatography, using a flame ionization detector. Other experimental/analytical details are given by Scharffe et al. (1990). Gravimetric soil moistures were measured in samples of 2cm depth and soil temperatures, at 1cm depth, were recorded continuously with a thermocouple during the flux measurements.
At the Guri site, which was selected in order to study "simultaneously" fluxes of trace gases from forest and savanna soils, atmospheric concentrations of methane were also taken at 2m height, at locations shown in Sanhueza et al. (1990). Based on calibration gas measurements, under field conditions, the precision of CH4 measurements was 1.0 ±0.5% (n= 175), with an accuracy of 2%.
Results and Discussion
Methane uptake by Venezuelan savanna and forest soils
Oxidation of atmospheric CH4 by methylotrophic bacteria in aerated soils (Conrad, 1996) is a significant (~5%) global methane sink (Prather and Ehhalt, 2001). Methylotrophic bacteria are those aerobic bacteria that utilize one-carbon compounds as a source of carbon and energy and assimilate formaldehyde as a major source of cellular carbon (Hanson and Hanson, 1996). In general, different soils exhibit different CH4 oxidation responses with respect to temperature, indicating that populations of methanotrophs in nature could adapt to different temperatures (Hanson and Hanson, 1996). The CH4 supply to CH4-oxidizing organisms is diffusion controlled and decreases under high soil moisture conditions, and oxidation rates are lower (Striegl, 1993). When soils are too wet, soil micro-sites become anaerobic and CH4 is produced rather than consumed; wetlands are the major global source of methane (Prather and Ehhalt, 2001).
Savanna soils. CH4 flux measurements were made in unperturbed and cleared plots in a Trachypogon sp. savanna in Calabozo (Sanhueza and Donoso, 2006). In cleared plots standing grasses were clipped to just above soil surface and plant litter removed from the soil surface. Average soil moistures during the measurement period ranged from 3 to 8%; therefore, there should not be any gas transport limitation between atmospheric CH4 and the soil bacteria. The results indicate that Trachipogon sp. savanna soils in Calabozo consume methane under wet season conditions, at a rate of -4.7ng·m-2·s-1 (Sanhueza and Donoso, 2006). This flux is in the range of consumption reported by Seiler et al. (1984) in soils of a broad-leafed savanna in South Africa. On the other hand, under very dry conditions, the consumption of atmospheric methane by savanna soils would be negligible during the dry season, as suggested by the results reported by Hao et al. (1988) and Zepp et al. (1996), most likely due to an inhibition of the soil microbial processes under dry conditions (Schnell and King, 1996).
Forest soils. Soil flux measurements made in the forest at Guri show an average consumption of ~10ng·m-2·s-1 (Scharffe et al., 1990). This value is in agreement with fluxes found in tropical forests in Costa Rica (Keller and Reiners, 1994) and Australia (Kiese et al., 2003). At both Costa Rica and Australia sites, rates of CH4 uptake during the less wet periods were higher, as compared to the wetter periods.
Methane emission from savanna grasses
Confusing and controversial results, respectively, were reported in studies of CH4 surface fluxes in the Venezuelan savanna region (Hao et al., 1988; Scharffe et al., 1990; Sanhueza et al., 1994). On average, a net emission of CH4 was reported. However, quite often consumption was observed in individual plots (see Table I). According to Sanhueza et al. (1994) these results contrast with the general belief that non-flooded soils of temperate, subtropical, and tropical regions only act as sinks for atmospheric CH4. Sanhueza et al. (1994) speculated that, by an unknown mechanism, it is possible that the CH4 emitted in the Venezuelan savanna region was produced by biogenic activity. Now, after the publication of the Keppler et al. (2006) paper, showing that both live plants and plant debris produce CH4, it is clear that the soil-grass system is more complex than assumed in the past. Therefore, to understand and explain the CH4 flux variability, the presence of live or dead plants should be taken into consideration in addition to soil processes. Measurements made in savannas of Brazil (Poth et al., 1995) and South Africa (Zepp et al., 1996) also indicated emission of CH4 to the atmosphere from the soil-grass system. The average emissions from the soil-grass system in global tropical savanna ecosystems may range from 5 to 11ng·m-2·s-1 (Sanhueza and Donoso, 2006).
Recently, the analysis of measurements made during the 1990 wet season in Calabozo (Estación Biológica de los Llanos, Guárico state) indicated that the emissions of CH4 originating from the soil-grass system is actually due to production of CH4 by grasses (Sanhueza and Donoso, 2006). Measurements were made in unperturbed plots and plots where standing dry and green Trachypogon sp. grasses were clipped just above the soil surface. The results indicate that plots with grass emit CH4, whereas those without grass consume CH4. From the difference between plots with and without grass it was calculated that the dry/green mixture of grasses produce CH4 at a rate of ~10ng·m-2·s-1. Fluxes from the soil-grass system obtained during the 1988 dry season in Chaguaramas (Hao et al., 1988), are in good agreement with the ones observed in Calabozo during the wet season; in this case CH4 consumption by dry soils should be negligible (Sanhueza and Donoso, 2006). The results also suggest that both dry and green grasses should produce CH4 at similar rates. These results were obtained using opaque chambers to measure fluxes. Additional research is required to better quantify the effect of solar radiation enhancing the fluxes, as found by Keppler et al. (2006).
The relatively low production of CH4 from tropical savanna grasses is in agreement with results obtained using opaque chambers to measure the fluxes in temperate grasslands (Mosier et al., 1991, 1997) and tropical pastures (Keller and Reiners, 1994; Mosier and Delgado, 1997). These studies mainly indicated that the soil-grass system of these ecosystems consume CH4. In these ecosystems, CH4 soil uptake would predominate over CH4 production by grasses.
Methane emission from tropical forests
Satellite measurements using the SCHIAMACHY instrument indicated high CH4 levels over the Amazon forest, exceeding modeled concentrations and revealing that emissions inventories underestimated CH4 sources of the region (Frankenberg et al., 2005). Methane emission from the vegetation, not included in the models, was suggested to be responsible for the discrepancy (Keppler et al., 2006). As discussed below, our results suggest that forest vegetation emits CH4, but its magnitude would be much lower than needed to explain the discrepancy between satellite measurements and underestimated emission inventories.
Increasing concentrations of CH4 during nighttime in the northern part of the Guayana shield, Venezuela, during the 1988 wet season, suggest the presence of a substantial methane source (Scharffe et al., 1990). The site is within the savanna climate region, but it is also affected by emissions from a nearby forest. Using the CH4 accumulation within a 100m deep nocturnal boundary layer (NBL), the authors estimated a CH4 flux of 130ng·m-2·s-1, which extrapolated to a corresponding global savanna source of ~60Tg·yr-1. A dispersed source of CH4 (i.e., termites, small tract of flooded soils) was invoked to account for these results. Now, with the new available information, a fraction of this emission could be due to the vegetation present at the site.
A re-interpretation of the Guri data was made by Crutzen et al. (2006). These authors concluded that the Guri field data is indicative of large CH4 production rates from tropical vegetation. These authors suggest that it is quite feasible that, in addition to wetlands, tropical ecosystems produce some 30-60Tg·yr-1, mostly coming from the vegetation, with some uncertain contribution from termites, which for the whole globe may amount to 20Tg·yr-1 (Frankenberg et al., 2005). The authors recognize that the main uncertainties of the new analysis arise from the extrapolation of measurements at one site with savanna and forest vegetation, to a surface equivalent of the global savanna, and from the numerical evaluation of the CH4 fluxes in the NBL.
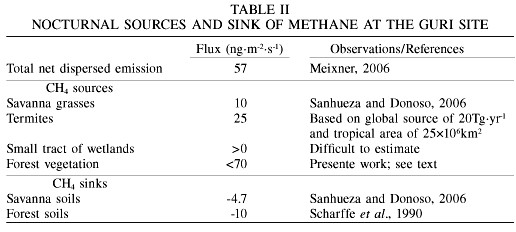
Here, in order to be able to make a first approach to the emission of CH4 from tropical rainforest vegetation, a detailed evaluation/estimation of sources and sinks of CH4 at the Guri site is made. Since nighttime vertical profiles of O3 within the NBL at the Guri site (Sanhueza et al., 2000) indicate that gases (including CH4) are not uniformly mixed in a 100m deep NBL, it is likely that there is a vertical gradient of CH4 from the surface to the top of the NBL. Assuming a linear decrease of the concentration and integrating between the surface (0m) and the top of the NBL (100m), which could also be visualized as an "imaginary nocturnal box" of 50m of height (Denmead et al., 1996), a "total" CH4 flux coming from dispersed savanna and forest sources of 57ng·m-2·s-1 was estimated by Meixner (2006). This flux is in good agreement with the estimated nocturnal emissions of CH4 produced by a "dispersed source" (termites, anaerobic decay of waterlogged wood, poorly drained patches of soils, thick moss mats, cavities of tank bromeliads) in the Amazon forest, which range from 23 to 230 (most likely 75) ng·m-2·s-1 (do Carmo et al., 2006).
Nocturnal sources and sinks of methane at the Guri site are summarized in Table II. Considering that measurements were made near the border of the savanna/forest ecosystems, an equal contribution (by unit area) from savanna and forest ecosystems was assumed in the calculations. As discussed in the previous section, an emission of 10ng·m-2·s-1 is due to savanna grasses, which is in agreement with the CH4 fluxes measured from the soil-grass system at the Guri site (Scharffe et al., 1990). Assuming that tropical emissions of methane from termites (~20Tg·yr-1) are homogenously distributed, a flux of ~25ng·m-2·s-1 could be derived for this source. It is very difficult to estimate the contribution of "small wetland pockets", however, since the region is quite hilly, emission of CH4 from this source should be likely small, but >0. The only nocturnal CH4 sinks would be microbial consumption in soils and the fluxes used in the calculation are those discussed in a previous section. Assuming that the contribution from scarce savanna trees is negligible, the emission of CH4 from the forest vegetation could be estimated using the values given in Table II and the relation
Net dispersed source = vegetation emissions + other emissions - soil consumption
where the net dispersed source equals 57ng·m-2·s-1, the soil consumption (assumed to be the average of CH4 consumption by savanna and forest soils, respectively) is 7.5ng·m-2·s-1, and other sources (termites + wetland pockets) are estimated to be >25ng·m-2·s-1. Therefore, the vegetation emission, considered to originate from savanna grasses and forest vegetation, will result in <40ng·m-2·s-1.
Therefore, an upper limit for the nocturnal forest vegetation emission of <70ng·m-2·s-1 is calculated. However, considering that solar radiation stimulates methane emissions from vegetation, it is likely that the estimated nighttime emission rate somewhat underestimates the CH4 emission throughout the day. Clearly, there are large uncertainties in this estimate, easily by a factor of 2.
Global extrapolations
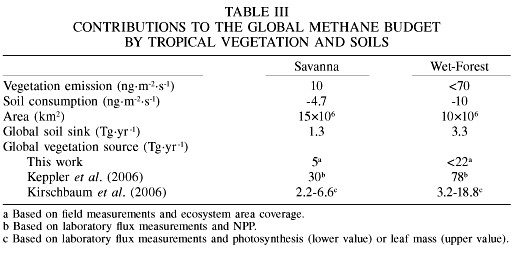
Assuming that the estimated fluxes presented in previous sections are representative of world savannas and wet forests, a first approach to the global contribution of these ecosystems to the global CH4 budget can be made. The appropriate/pertinent information and results of the extrapolation are summarized in Table III.
The global CH4 sink by savanna soils is ~1.3Tg·yr-1 (7 months wet season) and 3.3Tg·yr-1 by forest soils. This corresponds to only ~0.9% of the total global sink of 576Tg·yr-1 (Prather and Ehhalt, 2001); most of CH4 is removed from the atmosphere by reaction with the OH radical. Ignoring the effects of light on methane emissions from vegetation, the global CH4 emissions from savanna and forest vegetation are ~5 and <22Tg·yr-1, respectively. Considering that the total global source is 598Tg·yr-1 (Prather and Ehhalt, 2001) the contribution of tropical vegetation would be <5%.
As shown in Table III, the global tropical vegetation source of CH4 estimated in this work, based on field measurements and ecosystem area coverage, is much lower than the value given by Keppler et al. (2006), which is based on laboratory experiments (mostly individual plants) and ecosystem net primary productivities (NPP). However, our estimates are within the range produced by the alternative calculations, based on foliage biomass and photosynthetic rates, made by Kirschbaum et al. (2006).
Considering that tropical ecosystems cover a large fraction of world continents, it is likely that the global emissions of methane from vegetation from all ecosystems should be rather low. As mentioned, the soil-grass system of temperate grasslands (Mosier et al., 1991, 1997) and tropical pastures (Keller and Reiners, 1994; Mosier and Delgado, 1997) ecosystems consume CH4. Kirschbaum et al. (2006) and Ferretti et al. (2006) found that vegetation makes a small contribution to the global emission of CH4. Therefore, carbon sequestration (consumption of atmospheric CO2 by growing trees) due to forestation should outweigh the radiative forcing (warming) produced by CH4 tree emissions. A quite similar conclusion was reached by NIEPS (2006).
Conclusions
Field measurement results indicate that tropical vegetation (savanna and wet-forest) emits CH4, supporting the surprising discovery made by Keppler et al. (2006). However, the global extrapolation of Venezuelan ecosystems field data indicate that tropical vegetation is likely to make a modest contribution to the global emission of CH4. Furthermore, vegetation emissions are in part additionally offset by savanna and forest soils uptake. It is most likely that the carbon sequestration benefits from forestation should not be significantly affected by CH4 emissions from trees. All pertinent estimates have large uncertainties especially with respect to effects of solar radiation on the emissions. Further field studies are needed to better define methane emissions from plants.
References
1. Conrad R (1996) Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol. Rev. 60: 609-640. [ Links ]
2. Crutzen PJ, Sanhueza E, Brenninkmeijer CAM (2006) Methane production from mixed tropical savanna and forest vegetation in Venezuela. Atmos. Chem. Phys. Discuss. 6: 3093-3097. www.copernicus.org/EGU/acp/acpd/published_papers.htm [ Links ]
3. Denmead OT, Raupach MR, Dunin FX, Cleugh HA, Leuning R (1996) Boundary layer budgets for regional estimates of scalar fluxes. Global Change Biol. 2: 255-264. [ Links ]
4. do Carmo JB, Keller M, Díaz JD, Camargo PB, Crill P (2006) A source of methane from upland forests in the Brazilian Amazon. Geophys. Res. Lett. 33: L04809, doi:10.1029/2005GL025436, 2006. [ Links ]
5. Ferretti DF, Miller JB, White JWC, Lassey KR, Lowe DC, Etheridge DM (2006) Stable isotopes provide revised global limits of aerobic methane emissions from plants. Atmos. Chem. Phys. Discuss. 6: 5867-5875. www.copernicus.org/EGU/acp/acpd/ published_papers.htm [ Links ]
6. Frankenberg C, Meirink JF, van Weele M, Platt U, Wagner, T (2005) Assessing methane emissions from global space-borne observations. Science 308: 1010-1014. [ Links ]
7. Frankenberg C, Meirink JF, Bergamaschi P, Goede APH, Heiman M, Körner S, Platt U, van Weele M, Wagner T (2006) Satellite chartography of atmospheric methane from SCIAMACHY on board ENVISAT: Analysis of the years 2003 and 2004. J. Geophys Res. 111: DO7303, doi:10.1029/2005JD006235, 2006. [ Links ]
8. Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol. Rev. 60: 439-471. [ Links ]
9. Hao WM, Scharffe D, Crutzen PJ, Sanhueza E (1988) Production of N2O, CH4 and CO2 from soils in the tropical savannah during the dry season. J. Atmos. Chem. 7: 93-105. [ Links ]
10. Keller M, Reiners WA (1994) Soil-atmosphere exchange of nitrous oxide, nitric oxide, and methane under secondary succession of pasture to forest in the Atlantic lowlands of Costa Rica. Global Biogeochem. Cycles 8: 399-409. [ Links ]
11. Keppler F, Hamilton JTG, Brass M, Roeckmann T (2006) Methane emissions from terrestrial plants under aerobic conditions. Nature 439: 187-191. [ Links ]
12. Kiese R, Hewett B, Graham A, Butterbach-Bahl K (2003) Seasonal variability of N2O emission and CH4 uptake by tropical rainforest soils of Queensland, Australia. Global Biogeochem. Cycles 17: 1004, doi:10.1029/2002GB002014 [ Links ]
13. Kirschbaum MUF, Bruhn D, Etheridge DM, Evans JR, Farquhar GD, Gifford RM, Paul KI, Winters AJ (2006) A comment on the quantitative significance of aerobic methane release by plants. Funct. Plant Biol. 33: 521-530. [ Links ]
14. Meixner F (2006) Interactive comments on "Methane production from mixed tropical savanna and forest vegetation in Venezuela by Crutzen et al." Atmos. Chem. Phys. Discuss. 6: S1717-S1722. www.copernicus.org/EGU/acp/acpd/published_papers. htm [ Links ]
15. Mosier AR, Delgado JA (1997) Methane and nitrous oxide fluxes in grasslands in western Puerto Rico. Chemosphere 35: 2059-2082. [ Links ]
16. Mosier A, Schimel D, Valentine D, Bronson K, Parton W (1991) Methane and nitrous oxide fluxes in native, fertilized and cultivated grassland. Nature 350: 330-332. [ Links ]
17. Mosier AR, Parton WJ, Valentine DW, Ojima DS, Schimel DS, Heinemeyer O (1997) CH4 and N2O fluxes in the Colorado shortgrass steppe 2. Long-term impact of land use change, Global Biogeochem. Cycles 11: 29-42. [ Links ]
18. NIEPS (2006) Do recent scientific findings undermine the climate benefits of carbon sequestration in forest? An expert review of recent studies on methane emissions and water tradeoffs. Nicholas Institute for Environmental Policy Solutions. Duke University, USA. Feb. 9, 2006. www.env.duke.edu/institute/methanewater.pdf [ Links ]
19. Poth M, Anderson IC, Miranda HS, Miranda AC, Riggan PJ (1995) The magnitude and persistence of soil NO, N2O, CH4, and CO2 fluxes from burned tropical savanna in Brazil. Global Biogeochem. Cycles 9: 503-513. [ Links ]
20. Prather M, Ehhalt D (2001) Atmospheric chemistry and greenhouse gases. In Climate Change 2001. The Scientific Basis. Cambridge University Press. Cambridge, UK. pp. 239-287. [ Links ]
21. Sanhueza E, Donoso L (2006) Methane emission from tropical Trachypogon sp. grasses. Atmos. Chem. Phys. 6: 5315-5319. www.copernicus.org/EGU/acp/acpd/ published_papers.htm [ Links ]
22. Sanhueza E, Cuenca G, Gómez MJ, Herrera R, Ishizaki Ch, Martí I, Paolini J (1988) Characterization of Venezuelan environment and its potential for acidification. In Rodhe H, Herrera R (Eds.) Acidification in Tropical Countries. Wiley. Chichester, UK. pp. 197-255. [ Links ]
23. Sanhueza E, Hao WM, Scharffe D, Donoso L, Crutzen PJ (1990) N2O and NOx emissions from soils of the northern part of the Guayana shield, Venezuela. J. Geophys. Res. 95D: 22481-22488. [ Links ]
24. Sanhueza E, Cárdenas L, Donoso L, Santana M (1994) Effect of plowing on CO2, CO, CH4, N2O and NO fluxes from tropical savanna soils. J. Geophys. Res. 99D: 16429-16434. [ Links ]
25. Sanhueza E, Fernández E, Donoso L, Romero J (2000) Boundary layer ozone in the tropical American northern hemisphere region. J. Atmos. Chem. 35: 249-272. [ Links ]
26. Scharffe D, Donoso L, Crutzen PJ, Sanhueza E (1990) Soil Fluxes and atmospheric concentrations of CO and CH4 in the northern part of the Guayana shield, Venezuela, J. Gephys. Res. 95D: 22475-22480. [ Links ]
27. Schnell S, King GM (1996) Response of methanotrophic activity in soil and cultures to water stress. Appl. Environ. Microbiol. 62: 3203-3209. [ Links ]
28. Seiler W, Conrad R, Scharffe D (1984) Field studies of methane emission from termite nests into the atmosphere and measurements of methane uptake by tropical soils. J. Atmos. Chem. 1: 171-186. [ Links ]
29. Striegl RG (1993) Diffusional limits to the consumption of atmospheric methane by soils. Chemosphere 26: 715-720. [ Links ]
30. Zepp RG, Miller WL, Burke RA, Dirk A, Parsons B, Scholes MC (1996) Effects of moisture and burning on soil-atmosphere exchange of trace carbon gases in a southern African savanna. J. Geophys. Res. 101: 23699-23706. [ Links ]












 uBio
uBio