Interciencia
versión impresa ISSN 0378-1844
INCI v.33 n.5 Caracas mayo 2008
Species diversity after prescribed burns at different intensities and seasons in a high altitude pinus hartwegii forest
Hortensia Catalina Martínez-Hernández and Dante Arturo Rodríguez-Trejo
Hortensia Catalina Martínez-Hernández. Forest Engineer and Graduate Student, Universidad Autónoma de Chapingo (UACH), Mexico.
Dante Arturo Rodríguez-Trejo. Forest Engineer, UACH, Mexico. M.Sc. in Silviculture and Forest Management, Colegio de Posgraduados, Mexico, Ph.D., University of Florida, USA. Professor, Researcher, UACH, Mexico. Address: División de Ciencias Forestales, UACH, Chapingo, Edo. de México, C.P. 56230, México. e-mail: dantearturo@yahoo.com
SUMMARY
In the Ajusco volcano, in Central Mexico, prescribed burnings of low and high intensity were applied in March and May 2002, along with one unburned control for March and another for May, considering conditions of open stands and closed stands, with the objective of evaluating the vegetation response of the Pinus hartwegii understory. Samplings were conducted every three months during one year. Number of herb and shrub species, density, covering, and frequency were recorded, and the Simpson diversity index was calculated. According to a principal component analysis, the March prescribed burnings of low and high intensity in open stands separate from the other treatments. The t test showed that the March low intensity burns in open stands yielded a higher Spring-diversity than the unburned control. For the shrub species diversity, the March and May high intensity burns in open stands formed a separated group. A c2 test revealed the species that appear preferably in burned areas. The results indicate that the use of prescribed burnings no later than March favor the richness and diversity of species of the understory.
Diversidad de especies después de quemas prescritas en diferentes épocas e intensidades en un bosque de pinus hartwegii de gran altitud
RESUMEN
En el volcán Ajusco, en México central, se aplicaron quemas prescritas de baja y de alta intensidad en marzo y mayo de 2002, dejándose un control no quemado para marzo y otro para mayo. También se consideraron condiciones de arbolado abierto y de arbolado cerrado. El objetivo fue evaluar la respuesta del sotobosque de Pinus hartwegii. Se hicieron muestreos cada tres meses durante un año, registrando el número de especies herbáceas y arbustivas, su densidad, cobertura y frecuencia, y se calculó el índice de diversidad de Simpson. De acuerdo con un análisis de componentes principales, las quemas prescritas en marzo, tanto a baja como alta intensidad, con arbolado abierto, formaron el grupo con mayores valores. La prueba t indicó que la diversidad en primavera fue mayor en el tratamiento de quemas prescritas en marzo a baja intensidad en masas abiertas, con respecto a su control no quemado. En el caso de arbustos el grupo con mayores valores correspondió a las quemas de alta intensidad en marzo y mayo en masas abiertas. La prueba de c2 reveló las especies que aparecen preferentemente en localidades quemadas. Los resultados indican que el uso de quemas prescritas no después de marzo favorece la riqueza y la diversidad de especies del sotobosque.
Diversidade de espécies depois de queimadas prescritas em diferentes épocas e intensidades em um bosque de pinus hartwegii de grande altitude
RESUMO
No vulcão Ajusco, no México central, se aplicaram queimadas prescritas de baixa e de alta intensidade em março e maio de 2002, deixando-se um controle na queimada para março e outro para maio. Também se consideraram condições de arvoredo aberto e de arvoredo fechado. O objetivo foi avaliar a resposta do sotobosque de Pinus hartwegii. Realizaram-se amostras cada tres meses durante um ano, registrando o número de espécies herbáceas e arbustivas, sua densidade, cobertura e freqüência, e se calculou o índice de diversidade de Simpson. De acordo com uma análise de componentes principais, as queimadas prescritas em março, tanto a baixa como alta intensidade, com arvoredo aberto, formaram o grupo com maiores valores. A prova t indicou que a diversidade na primavera foi maior no tratamento de queimadas prescritas em março a baixa intensidade em massas abertas, em relação a seu controle na queimada. No caso de arbustos o grupo com maiores valores correspondeu às queimadas de alta intensidade em março e maio em massas abertas. A prova de χ2 revelou as espécies que aparecem preferentemente em localidades queimadas. Os resultados indicam que o uso de queimadas prescritas não depois de março favorece a riqueza e a diversidade de espécies do sotobosque.
KEYWORDS / Fire Ecology/ Fire Effects/ Forest Fires/ Integrated Fire Management/ Mexico/
Received: 03/01/2007. Modified: 04/03/2008. Accepted: 04/10/2008.
The pine forests of tropical areas host a significant biodiversity. For instance, for Mexico, 47 species and 20 infraspecific taxa are reported (Farjon and Styles, 1997), which is almost half of the ~110 species reported in all the world. Also, some pines are among the tree species reaching the highest altitudes worldwide, and the displacement of the tree line at both high latitude and high altitude forests is an important indicator of global warming. Pinus hartwegii Lindl. is probably the pine reaching the highest altitude in America and one of the highest altitudes in the world. According to Perry (1991) and Farjon et al. (1997) it is found at 2200-4300masl in Mexico, while P. flexilis James reaches altitudes of 3800m in North America (Steele, 1990) and P. wallichiana A. B. Jacks may be found at 4000m at the Himalayas (Sargent et al., 1985).
In pine forests, fire is necessary for the promotion and conservation of richness of species and diversity (Van Lear et al., 1995; Brown, 2000), among other ecological functions. However, the fire responses of tropical pines and their associated species in most of the world are poorly known, including high altitude tropical pines. According to Rodríguez-Trejo and Fulé (2003) these are fire-dependant ecosystems, where the alteration of fire regimes by man through its exclusion (implying high intensity wildfires eventually) or excessive fire frequency coupled to rural socio-economic problems; both lead to deforestation, contributing to the reduction of biodiversity.
One alternative to counteract such alterations of fire regimes, is an integrated fire management, involving three key components: prevention and fighting of forest fires, community based-fire management (oriented to address the rural socio-economic issues), and ecological fire management (Rodríguez-Trejo, 2000; Myers, 2006). In order to accomplish integrated fire management, it is necessary to increase the use of prescribed burns coupled with research and monitoring of fire effects, particularly in countries like Mexico, where such information is scarce.
It is necessary to understand the positive effects of fire and make the best use of those effects to improve fire management and its subsequent benefits for the ecosystem and society. Based on the aforesaid, the objectives of the present study are to answer the following questions: a) what are the effects of prescribed fire and wildfires occurring in two different seasons and at two intensities on the richness and diversity of species of the understory of Pinus hartwegii ecosystems?; b) can different fire-treatments be classified in groups, based on principal components, with respect to their effects on the understory?; c) what are the indicative species of burned areas in the understory of P. hartwegii?; and d) what is the feasibility of fire use to promote diversity in these types of forests?
Study Area
The study area is located south of Mexico City, Distrito Federal, central Mexico, on the Ajusco volcano. The climate, according to the classification of Köppen modified by García (1981) is type C(w2)(w)(b)ig, semi cold, sub-humid, with rainfalls in the summer (Jun-Nov), mean annual temperature of 5-12ºC, and mean annual precipitation of 1138.62mm. Soils are andosols. The fire season spans winter (Jan) through spring (May). Research was conducted on a slope of the volcano, that has a North-West aspect, mean slope of 55% at 3553-3626masl. The area is characterized by a young forest stand (£8m high) of Pinus hartwegii, and belongs to the Comunidad de San Miguel y Santo Tomás Ajusco. The young forest stand has been there for 14-18 years. The adult trees that originated this cohort were killed during a windstorm. These pine forests are likely maintained by fire (Rzedowski, 1978). Representative genera in the understory include: Festuca, Muhlenbergia, Alchemilla, Geranium, Potentilla, Rumex, Lupinus, and Penstemon (Rzedowski, 1981). However, the effects of fire on plant responses at the community level are not fully understood.
Methodology
Establishment of the experiment
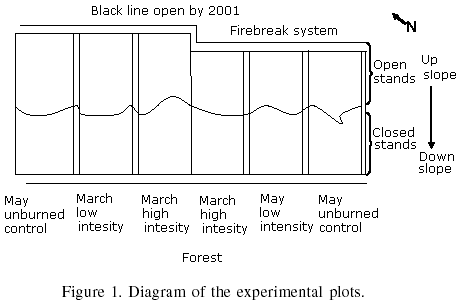
In March 21st and May 29th 2002, corresponding to mid-forest fire season and its final and severest part, prescribed burns were conducted, leaving non-burned controls for each date. Each plot was 0.6-0.75ha, making a total experimental area of 4.05ha. The coordinates of the Northwest vertex were 19°1258.8N and 99°1611.7W. Each plot had a 2m wide firebreak. No data was taken over a 5m wide perimeter belt on each plot, making an effective separation of 12m between plots (Figure 1).
The low intensity prescribed burns were initiated by 8:00am as backing fires (against wind and slope). The high intensity prescribed burns were initiated by 1:00pm as head fires (in favor of the wind and slope). The rate of spread was measured in 24 separate and randomly placed 10m long experimental units. Flame length was estimated visually with graduated 3m wooden rulers. The atmospheric data were obtained with pocket wind meters (wind speed), sling psychrometers (temperature, relative humidity), and compasses (wind direction). Fine fuels (grasses, needle litter) moisture content was estimated with Sackett tables modified by Burgan and Cohen (unpubl., referred by Rothermel, 1983) and using information on relative humidity, dry bulb temperature, month, fuels exposed or not exposed, time of the day, aspect and slope.
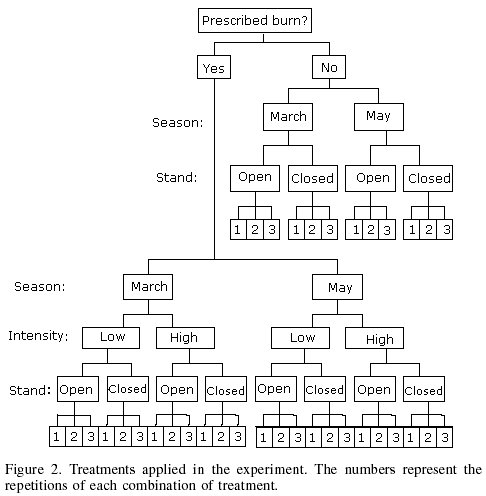
In each fire treatment area, approximately the lower half of the surface was covered with closed stands of juvenile pine trees (900-2500 trees/ha), while the upper half had open stands (300-700 trees/ha) with large gaps. Crown cover was 0-30% in the open stands, and 70-100% in the dense stands. Mean loading of surface fuels prior to fire were 13.2Mg·ha-1 in the open stands, and 11.35Mg·ha-1 in the dense stands. The experimental area was not fenced to exclude cattle grazing, because an objective was to study the vegetation response to fire under conditions generally occurring in Mexico. Figure 2 shows the different treatments utilized in the experiment.
Fire intensity was estimated from flame length using the models of Byram (1959) and Alexander (1982):
L= 0.0775·I0.46
I= exp((ln L - ln 0.0775)/0.46)
where L: flame length (m) and I: fire intensity (kW·m-1).
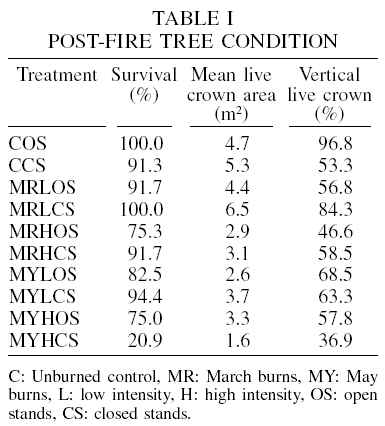
Post-fire tree condition (tree survival, mean live crown area and percentage of vertical live crown) was determined in another study in the same plot (Rodríguez-Trejo et al., 2207). In general, the values tended to be lower following May, high-intensity treatments and closed stands. In the unburned plots, first-year survival was equal or higher than 91.3%, while in the March low intensity (both densities) and March high or low intensity in both densities the survival was similar (larger or equal to 91.7%). The lowest survival corresponded to the May high intensity prescribed burn, with 20.9% (closed stands) and 75% (open stands). The mean live crown area by tree was 4.7-5.3m2 for the unburned controls, and 4.4-6.5m2 for the March low intensity open stand burn and the March low intensity closed stand burn, respectively. The lowest mean live crown area again was for the May high intensity burn in closed stands (1.6m2), with a low value for the May high intensity burn in open stands. A similar trend was found for the vertical live crown (Table I).
Vegetation sampling
Three months after application of the fire treatments, quadrats fitted and coincident in a vertex, were randomly delimited at each point. The quadrats were placed using a 1m coordinate system (X, Y) on each plot and random numbers for determining randomly such coordinates. The dimensions of these quadrats varied according to the life form of the understory species. For forbs and grasses, quadrats of 1m per side (1m2) were used, and for shrubs, quadrats of 4m per side (16m2). The information obtained was species, number of individuals and two perpendicular crown diameters (or covering in percentage in case of forbs and grasses). To determine the total number of species, extensive collecting was carried out in each treatment. Three quadrats of each dimension (two dimensions) per combination of treatment levels (12) were surveyed, which produced 72 quadrats. As these sites were sampled on four occasions (every three months), the total of surveyed quadrats was 288. Sampling intensity in herbaceous vegetation was 0.09%, while for shrubs it was 1.42%. With the field data, the Simpson diversity index (D) was calculated (Krebs, 1978) for forbs and grasses and for shrubs, separately, as
D= 1 - å(pi)2
pi= n/N
where pi: proportion of individuals of each species, n: number of individuals of each species, and N: number of total individuals of all the species.
The identification of species was performed at the Herbario de Preparatoria Agrícola, Universidad Autónoma Chapingo, México. Density, dominance (cover), frequency, as well as relative values of density, dominance, and frequency, were calculated to obtain importance values (Krebs, 1978). The fire treatments were applied in different months; therefore, the quarterly measurements did not correspond to the same time for the treatments of March when compared to those of May. The measurements of the March treatments correspond to Jun, Sep, Dec 2002, and March 2003, whereas those of May were conducted in Aug, Nov 2002, Feb and May 2003.
Statistical analysis
A principal components analysis was performed using as variables the average herb species diversities (summer, fall, winter and spring) and the shrub species diversities (corresponding to the same four seasons), in separate analysis, because of the different size of the experimental units. The data of the four samplings was used for the analysis. Additionally, a t test was carried out to compare the diversity of pairs of treatments. A non-parametric c2 test was employed to determine typical species of burned areas, comparing each treatment with the respective unburned control, and also burned vs non burned plots. The principal components analysis was performed with the procedure proc princomp, the t test with the procedure proc t test, and the non-parametric test with the procedure proc freq, all of SAS (v. 8.0 for microcomputers).
Results
Fire behavior
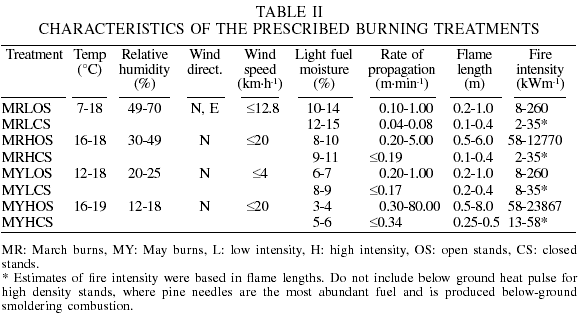
There was a high variability in the fire behavior for each treatment, as natural. High-intensity heading fires had a higher rate of propagation, and higher flame length and fire intensity than low-intensity backing fires. For example, the maximum rate of propagation in the March high-intensity heading fires in open stands was 5 times faster than in the March low-intensity backing fires in open stands. Also, the maximum flame length was 6 times longer and the fire intensity 49 times higher. May treatments also tended to show higher values in these parameters. Comparing the May high-intensity heading fire (that emulated a forest fire) with the March high-intensity heading fire, both in open stands, the former had a 16 times faster propagation rate, 33% larger maximum flame length and 87% higher maximum fire intensity. The fire behavior from each treatment is shown in Table II.
Species richness
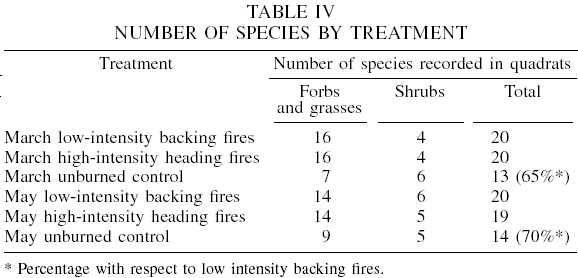
Table III lists the 52 species that were found. After one year following March low-intensity backing fires and high-intensity heading fires, 20 understory species were present in each treatment. In the March control area, there were a total of 13 species (65% of those in burned areas). After one year following the May, high-intensity heading fire, a total of 19 species were encountered, while a total of 20 species were present in areas burned using a May backing fire. By contrast, the May control plot had a total of 14 species (70% with respect to burned areas), showing less richness of species, especially of forbs, without fire (Table IV). Generally, low species richness is typical of high altitude sites like the present study area.
The families with the largest range of representation were asteraceae (21 species) and gramineae (5). The genus with the largest number of species (t) was Senecio.
Species diversity
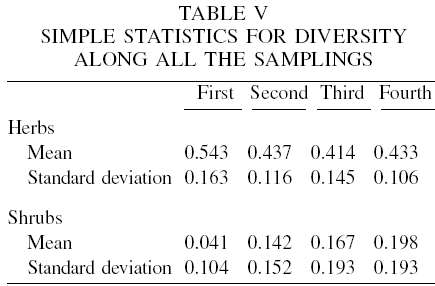
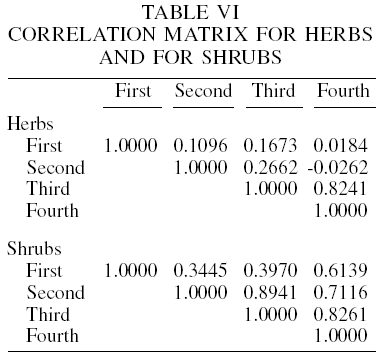
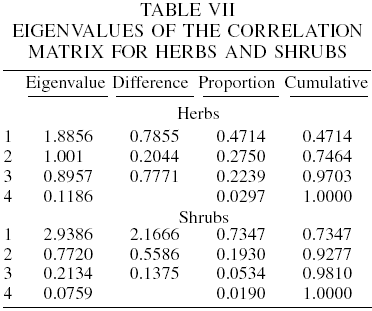
In the principal components analysis for herbs, eigenvalues indicate that three components (mostly the first) account for approximately 97% of the variance (with 47.1, 74.6 and 97.0%, respectively). The first eigenvector has larger loadings on the third and fourth sampling seasons (0.7051 and 0.6534). The second eigenvector has high loadings on the first and second sampling seasons. This suggests that the first principal component is primarily a measure of the two last sampling seasons (winter and spring) for diversity, and that the second principal component is mainly a measure of the first two sampling seasons (summer and fall). Tables V to VII show the mean and standard deviation of the variables used, the correlation matrix and its eigenvalues, respectively.
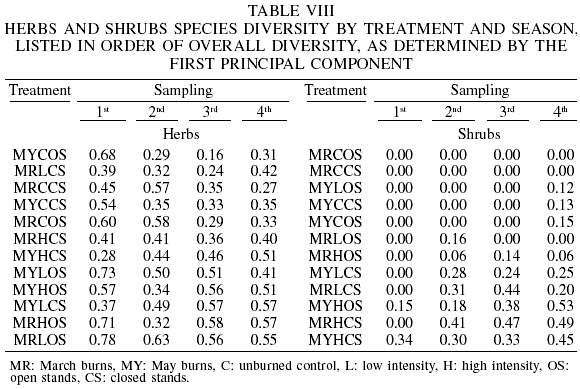
The species diversity for all the samplings, as determined by the first principal open condition, are part of the highest values correspond to March prescribed burns at low and high intensities, open condition, and to May low-intensity, open condition, (Table VIII).
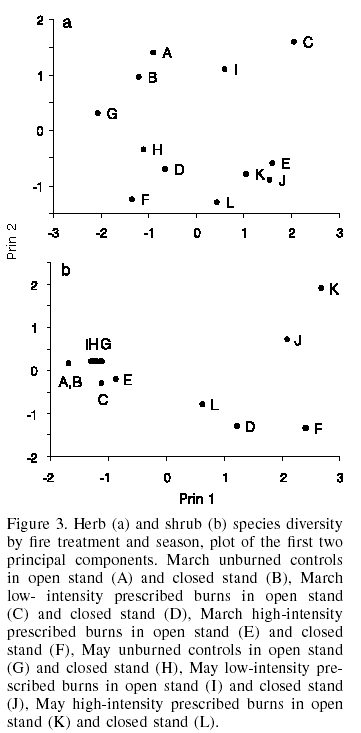
Figure 3A presents the component plot, showing the relationship between the first two components. Each observation corresponds to a prescribed fire treatment. The low-intensity prescribed burns in March, open condition, is the group with the highest values, while the group with the lowest values appears to be associated to the May unburned control in open stands. Other treatments that also have very high values in relation to the first component are the March high-intensity burns in open stands, and the May low-intensity burns in closed stands. However, in relation to the second principal component, the two last treatments have very low values, and the highest value is also associated to the March low-intensity prescribed burn in open condition, while the lowest value corresponds to the May high-intensity prescribed burning in closed stands.
The spring diversity (first sampling) was used in the t test because of its higher values. In this case, the only pair of treatments that exhibited statistically significant differences were the March low-intensity backing fire, when compared to their unburned control (p=0.0072).
Regarding the shrub species diversity, the first two components account for 93% of the variance (73.5 and 92.7%) and the first eigenvector has equally large loadings from the second to the fourth sampling seasons (fall, winter and spring, with 0.5174, 0.5462 and 0.5415, respectively). The first sampling (summer) had also a relatively high load (0.3753). For the second component, only the first sampling was relevant (0.8508).
The plot of the first two principal components (Figure 3) shows that the group with the highest values correspond to the May and March high-intensity burns in open and closed stands, respectively, followed by the group May low-intensity in closed stand, March low-intensity in closed stand and the May high-intensity burn in closed stand. Other groups are formed by the rest of treatments (see also Table VIII for the diversity values of each particular sampling). The values of treatments scatter in relation to the second principal component; however, the May high-intensity burns in open stands reach the highest value, and the lowest values correspond to the March burns (high and low intensity) in closed stands. According to the t test in this case the May high-intensity heading fire in open stands had higher spring-diversity than the May unburned controls in open stands.
Non-parametric analysis
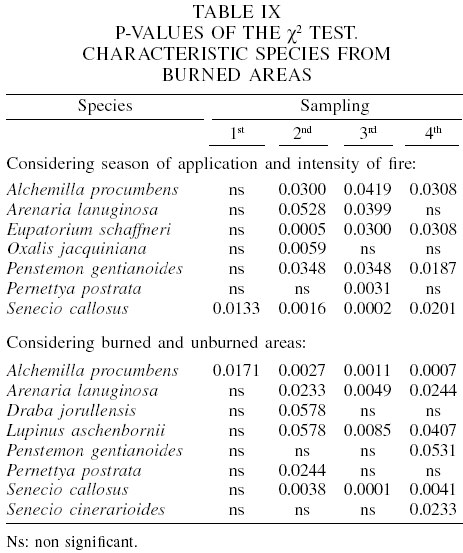
The species which appear preferably (p£0.05) in burned areas in different seasons (Table IX), for which they may be considered as indicators are A. procumbens, A. lanuginosa, E. schaffneri, O. jacquiniana, P. gentianoides, P. postrata, and S. callosus. Additionally, the non-parametric test was conducted without taking into account season nor intensity of fire; in other words, simply if it had been burned or not. The species which preferably (p£0.05) appear in burned areas (Table IX) are A. procumbens, A. lanuginosa, L. aschenbornii, P. gentianoides, P. postrata, D. jorullensis, S. callosus and S. cinerarioides. S. angulifolius is the species that is observed predominantly in unburned areas (considering burned and unburned areas), with the third and fourth samplings results only being significant for it (p=0.0396 in both cases).
Percentage of importance value
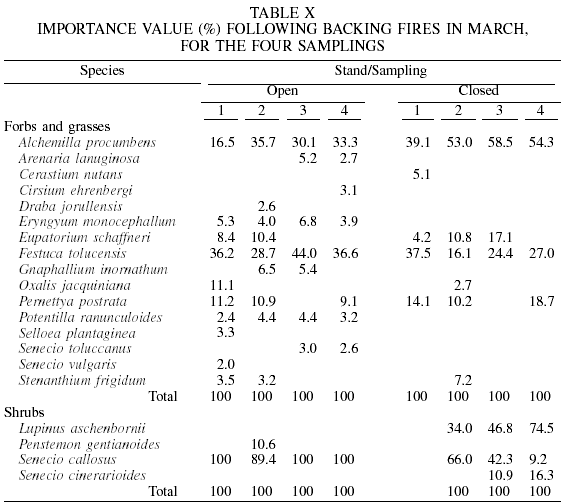
In the March high-intensity prescribed fire, the highest percentage of importance value (PIV) for the herbs corresponded to A. procumbens (values of 22.9-58.1) and F. tolucensis (14.9-45.2) in open as well as in closed condition, throughout the four samplings. In shrubs, S. callosus was the most important one (86.4-90.7 in open, and 24.9-100 in closed condition).
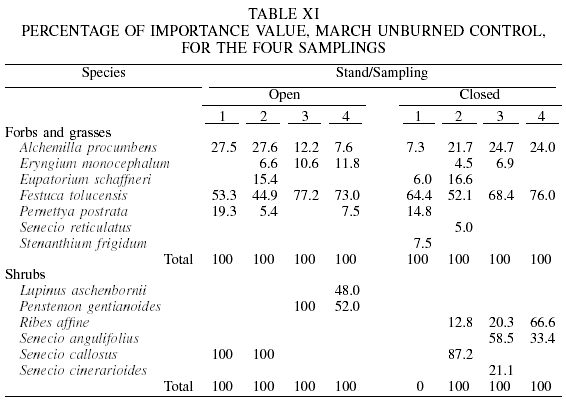
In the low-intensity prescribed burn of March, again A. procumbens and F. tolucensis prevailed among forbs and grasses, and S. callosus among shrub species (Table X). In the March unburned control, the herbaceous species cited previously dominated, and those which had greatest PIV among shrubs, were P. gentianoides and S. callosus (Table XI).
As for the May high-intensity burning, for herbaceous species F. tolucensis had values of 6.0-40.2, and A. procumbens of 27.0-64.2. With respect to shrub species, S. callosus (10.5-100) and P. gentianoides (27.5-64.8) were those with the highest values throughout the samplings.
With respect to low-intensity prescribed burning of May, F. tolucensis, A. procumbens, and E. schaffneri presented the highest PIV (19.1-43.9, 16.8-54.3 and 3-14.6, respectively), among herbaceous species. In shrubs, S. callosus prevailed with values of 85.4-100 in open condition, and 30-89 in closed condition.
In the May unburned control, again the herbaceous species F. tolucensis and A. procumbens were dominant, with PIV of 14.5-77.2 and 10.9-27.3, respectively. The shrubs P. gentianoides (41.9-100) and S. callosus (58.1-100) dominated in open condition, and Ribes affine (17.8-46.4) and S. angulifolius (50.5-58.5) in closed condition.
In the sites treated with fire, most of the species with greater PIV are typical of burned areas, as shown by the results of the non-parametric test. One of these species is A. procumbens, which, is present in the area of prescribed burns as well as in the control, both of March. In spite of the latter having fewer species, it reaches higher importance values in the first area. Similar behavior (PIV data not shown) is observed in other species, such as P. postrata and L. aschenbornii (March treatments) and A. procumbens, A. lanuginosa, E. schaffneri, L. aschenbornii, S. callosus and S. cinerarioides (May treatments).
Discussion
The increase in species richness and diversity documented in the burned areas is similar to that observed in North American pine forests of Pinus palustris Mill., Pinus ponderosa or Pinus elliottii var. elliotti (Wright and Bailey, 1982; Wade et al., 2000; Arno, 2000). Fire was shown to play an essential role in Pinus echinata Mill. ecosystems by creating and maintaining open canopy conditions that allow for relatively high diversity understory communities (Sparks, 1998). Periodic fire is considered a key factor for maintaining overall diversity and rare plant species in Pinus elliotii var. densa rockland ecosystems from South Florida (Snyder et al., 1992) and in the Ajusco study area (Table III) two species are rare (G. spathacea and S. paradoxa) and one is endemic (P. ranunculoides; Silva et al., 1999; NOM, 2001). García-Romero (2005) found that after forest fires in tropical high mountain grasslands on the Iztaccihuatl volcano, Mexico, Penstemon gentianoides dominates during the first two years, followed by Lupinus montanus 1.5 to 3 years after the fire, and eventually grasses replace forbs.
Increased richness in burned areas is attributed to species that not only re-sprout from root systems but also germinate from seeds. Furthermore, many of these species require direct solar radiation for germination and growth. Very likely, most of the species are present in seed banks prior to the fire (Whelan, 1997). Baskin and Baskin (1998) reported that fire is an environmental factor that alleviates physical dormancy of numerous species, including several species of Lupinus, and according to our study L. aschenbornii is an abundant species in burned sites. Martín-Martínez (2007) referred for Lupinus bilineatus Benth., also present in Pinus hartwegii forests in the region, 17.5% of germination for untreated seeds and 38.8% when treated with fire.
Biomass accumulation during the fire-free interval creates adverse space, nutrients, water, and light conditions for many associated species, leading to a reduction in the number of species (Grime, 1979). The burned areas in the present study had not been affected by fire for at least five years, which contributed to an increase in number of species after the application of the treatments. The prescribed fires likely reduced aerial competition for light by decreasing mostly the aboveground biomass of grasses, but also of herbs and shrubs, as well as the foliar area of tree crowns. These plants are also likely to depend on fire-induced inputs of nutrients. According to DeBano et al. (1998), ash is rich in cations. This is reminiscent of results after fire in Pinus ponderosa ecosystems in Oregon and Argentinian savannas (Kerns et al., 2006; Kunst et al., 2003), where increased herbs richness and diversity, likely related to post-fire increased resources availability after, respectively, fall burns and mid-fire season burns, in comparison to other seasons of burn and unburned controls.
Diversity appears to be increased by the fire, and withdrawing fire in many ecosystems reduces it (Keane et al., 2002). Likewise, the variability of the fire with respect to season, intensity, pattern, frequency and space creates the greatest diversity in components of the ecosystem (Collins and Gibson, 1990; Swanson et al., 1990; Brown, 2000), which helps explain the different responses found in this study. Moreover, according to Hiers et al. (2000) a variable fire season and other components of the fire regime help maintaining a greater variability of native species and, likely, are a key for conserving biodiversity in the Pinus palustris Mill. ecosystems in particular and in fire-dependent ecosystems in general.
In the same study area at the Ajusco volcano, Rodríguez-Trejo et al. (2007) pointed out that first year tree survival was 96% in the unburned control as well as after the March low-intensity prescribed burn, but it was only 48% after the May high-intensity fire.
The higher diversities associated to open conditions and March prescribed burns are related to the availability of direct solar radiation, nutrients and water because of the reduced competition, in comparison to the closed stands (Whelan, 1997). Also, the March burns corresponded to the beginning of spring, while the May burns corresponded to mid-spring, so that this last treatment implied a delay for the recuperation of the understory species (Brown, 2000).
The results of the present study show the value of using prescribed fire in March at low intensity in order to increase herbs diversity. Nonetheless, the results also point out the importance of varying fire intensity (and fire season) to yield higher shrub species richness. Under conditions common in Mexico, that include a very high incidence of human-caused forest fires, limited resources to suppress fires and with the fire season reaching the highest number of forest fires in April and May in the study area (Gobierno de la Ciudad de México, 2005), it seems most convenient to conduct prescribed fires no later than March. The results suggest that the objectives of increasing species diversity while limiting and reducing hazardous forest fuels can best met with prescribed fires before the end of March.
Conclusions
The areas prescribed-burned in March had more understory species and herbs diversity compared with the unburned control, due to the released growth space, the re-sprouting of many species, and the existing seed bank. The other burn treatments also resulted in an increased number of species, but there was a greater risk of fire escape (because of the season or the intensity of the fire) and tree mortality. The total number of species found (52) was relatively low, due to the area altitude.
Species indicative of burned sites, as indicated by the non parametric test, also presented a high percent importance value in the burned areas, although as the species in these areas are more numerous, lower values would be expected.
According to the principal component analysis, the herb species principal component values tended to be greater in the March-burned sites in open stands. In the case of shrubs, the highest values were obtained following the May and March high-intensity burns, in closed and open stands, respectively.
According to the results, and considering that the vast majority of forest fires in the region are human-caused and the relatively limited resources to fight wildfires, it may be a good strategy to conduct the prescribed burns no later than March. This may go in hand with other objectives like the reduction of hazardous fuels, that would limit the negative environmental impacts (tree mortality, erosion, pollution) of wildfires. Prescribed burning may also be compatible with moderate grazing.
Acknowledgements
The authors thank the communities of San Miguel and Santo Tomás Ajusco for the permission to conduct this work on their lands, to CONACYT installation project Nº I35626-B and to the University of Chapingo (Ajusco Project) for financial support, the herbarium of the Preparatoria Agrícola, UACH, for the invaluable support identifying the species, and CONAFOR and the Mexico City government for the authorizations and help for conducting this experiment. Finally, thanks to the reviewers. Finally, thanks to the reviewers. Their keen observations improved this work significantly.
References
1. Alexander ME (1982) Calculating and interpreting forest fire intensities. Can. J. Bot. 60: 349-357. [ Links ]
2. Arno SF (2000) Fire in Western forest ecosystems. In Brown JK, Smith JK (Eds.) Wildland fire in ecosystems: Effects of fire on flora. USDA Forest Service. Tech. Rep. RMRS-GTR-42. Vol. 2. pp. 97-120. [ Links ]
3. Baskin CC, Baskin JM (1998) Seeds: Ecology, biogeography, and evolution of dormancy and germination. Academic Press. San Diego, CA, USA. 666 pp. [ Links ]
4. Brown JK (2000) Ecological principles, shifting fire regimes and management considerations. In Brown JK, Smith JK (Eds.) Wildland fire in ecosystems. Effects of fire on flora. USDAFS. Gen. Tech. Rep. RMRS-GTR-42 vol. 2. pp. 185-203. [ Links ]
5. Byram GM (1959) Combustion of forest fuels. In Davis KP (Ed.) Forest fire: control and use. McGraw Hill. New York. pp. 61-80. [ Links ]
6. Collins SL, Gibson DJ (1990) Effects of fire on community structure in tallgrass and mixed grass prairie. In Collins SL, Wallace L (Eds.) Fire in North American Tallgrass prairies. University of Oklahoma Press. Norman, OK, USA. pp. 81-98. [ Links ]
7. De Bano L, Neary DG, Folliott PF (1998) Fire´s effects on ecosystems. Wiley. New York, NY, USA. 333 pp. [ Links ]
8. Farjon A, Styles BT (1997) Pinus (Pinaceae). Flora Neotropica. Monograph 75. New York Botanical Garden, New York. 291 pp. [ Links ]
9. Farjon A, Pérez de la Rosa JA, Styles BT (1997) A field guide to the pines of Mexico and Central America. Royal Botanical Gardens. Kew, UK. 147 pp. [ Links ]
10. García de Miranda E (1981) Modificaciones al sistema de clasificación climática de Köppen. S.E. México. 116 pp. [ Links ]
11. García-Romero A (2005) Dinámica del paisaje postfuego en el pastizal tropical de alta montaña: Volcán Iztaccihuatl, México. Interciencia, 29: 604-611. [ Links ]
12. Gobierno de la Ciudad de México (2005) Reporte de incendios forestales. Gobierno de la Ciudad de México. México. 15 pp. [ Links ]
13. Grime JP (1979) Plant strategies and vegetation processes. Wiley. Chichester, UK. 222 pp. [ Links ]
14. Hiers JK, Wyatt R, Mitchell RJ (2000) The effects of fire regime on legume reproduction in longleaf pine savannas: is a season selective?. Oecologia, 125: 521-530. [ Links ]
15. Keane RE, Ryan KC, Veblen T, Allen CD, Logan J, Hawkes B (2002) The cascading effects of fire exclusion in Rocky Mountains ecosystems. In Barron J (Ed.) Rocky Mountain futures: an ecological perspective. Island Press. Washington, DC, USA. pp. 133-152. [ Links ]
16. Kerns BK, Thies WG, Niwa CG (2006) Season and severity of prescribed burn in ponderosa pine forests: implications for understory native and exotic plants. Ecoscience, 13: 44-55. [ Links ]
17. Krebs C (1978) Ecology. Harper. New York, USA. 678 pp. [ Links ]
18. Kunst C, Moscovich F, Herrera J, Godoy J, Velez S (2003) Date of prescribed fire and herbaceous diversity of an Elionorus muticus (Spreng.) O. Kuntze savanna. Rev. Chil. Hist. Nat. 76: 105-115. [ Links ]
19. Martín-Martínez J (2007) Ecología de la semilla de Lupinus bilineatus Benth. Tesis. Universidad Autónoma Chapingo. Mexico. 70 pp. [ Links ]
20. Myers RL (2006) Living with fire. Sustaining ecosystems and livelihoods through integrated fire management: The Nature Conservancy. Tallahassee, FL, USA. 28 pp. [ Links ]
21. NOM (2001) Especies nativas de México de flora y fauna silvestres. Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio. Lista de especies en riesgo. Norma Oficial Mexicana NOM-059-ECOL-2001. Diario oficial de la Federación. 2ª sección. 03/06/2002. 85 pp. [ Links ]
22. Perry JP (1991) The pines of Mexico and Central America. Timber Press. Portland, OR, USA. 231 pp. [ Links ]
23. Rodríguez-Trejo DA (2000) Propuesta de manejo del fuego. In Rodríguez-Trejo DA, Rodríguez-Aguilar M, Fernández-Sánchez F, Pyne SJ (Eds.) Educación e incendios forestales. Mundi Prensa. México. 189-194. [ Links ]
24. Rodríguez-Trejo DA, Fulé PZ (2003) Fire ecology of Mexican pines and a fire management proposal. Int. J. Wild. Fire 12: 23-37. [ Links ]
25. Rodríguez-Trejo DA, Castro-Solís UB, Zepeda-Bautista M, Carr R (2007) First year survival of Pinus hartwegii following prescribed burns at different intensities and different seasons in Central Mexico Int. J. Wild. Fire 16: 54-62. [ Links ]
26. Rothermel RC (1983) How to predict the spread and intensity of forest and range fires. USDA FS Gen. Tech. Rep. INT-43. Orden, UT, USA. 161 pp. [ Links ]
27. Rzedowski J (1978) Vegetación de México. Limusa. México. 432 p. [ Links ]
28. Rzedowski J (1981) Comunidades vegetales. In Rzedowski J, de Rzedowski GC (Eds.) Flora fanerogámica del Valle de México. Vol I. Instituto de Ecología. México. pp. 47-54. [ Links ]
29. Sargent C, Sargent O, Parsell R (1985) The forests of Buthan: A vital resource for the Himalayas?. Trop. Ecol. 1: 265-286. [ Links ]
30. Silva LC, Romero FJ, Velázquez A, Almeida-Leñero L (1999) La vegetación de la región de montaña del sur de la Cuenca de México. In Velázquez A, Romero FJ (Comps.) Biodiversidad de la region de montaña del sur de la Cuenca de México: bases para el ordenamiento ecológico. Universidad Autónoma Metropolitana-Gobierno de la Ciudad de México. México. pp. 66-92. [ Links ]
31. Snyder JR, Herndon A, Robertson WB (1992) South Florida Rockland. In Myers RL, Ewel JJ (Eds.) Ecosystems of Florida. University of Central Florida Press. Orlando, FL, USA. pp. 230-276. [ Links ]
32. Sparks JC (1998) Effects of late growing-season and late dormant-season prescribed fire on herbaceous vegetation in restored pine-grassland communities. J. Veg. Sci. 9: 133-142. [ Links ]
33. Steele R (1990) Limber pine. In Burns RM, Honkala BH (Coords.) Silvics of North America. Vol. 1: Conifers. Agriculture Handbook 654. USDA Forest Service. Washington, DC, USA. pp. 348-354. [ Links ]
34. Swanson FJ, Franklin JF, Sedell JR (1990) Landscape patterns, disturbance and management in the Pacific Northwest, USA. In Zonneveld IS, Forman RTT (Eds.) Changing landscapes: an ecological perspective. Springer. New York, USA. pp. 191-213. [ Links ]
35. Van Lear DH, Carroll WD, Kapeluck PR, Johnson R (1995) History and restoration of the longleaf pine-grassland ecosystem: Implications for species at risk. For. Ecol. Manag. 211: 150-165. [ Links ]
36. Wade DD, Brock BL, Brose PH, Grace JB, Hoch GA, Patterson III WA (2000) Fire in eastern ecosystems. In Brown JK, Smith JK (Eds.) Wildland fire in ecosystems. Effects of fire on flora. Tech. Rep. RMRS-GTR-42. Vol. 2. USDA Forest Service. Washington, DC, USA. pp. 53-96. [ Links ]
37. Whelan RJ (1997) The ecology of fire. Cambridge University Press. Cambridge, UK. 346 pp. [ Links ]
38. Wright HA, Bailey AW (1982) Fire ecology: United States and Southern Canada. Wiley. New York, NY, USA. 501 pp. [ Links ]












 uBio
uBio