Interciencia
versión impresa ISSN 0378-1844
INCI v.34 n.9 Caracas sep. 2009
Importance of the extraction method in the quantification of total phenolic compounds in Phaseolus Vulgaris L.
María Virginia Mujica, Marisela Granito and Naudy Soto
María Virginia Mujica. Chemical Engineer, Universidad Nacional Experimental Politécnica "José Antonio Sucre", Venezuela. M.Sc. in Food Sciences, Universidad Simón Bolívar (USB), Venezuela. Professor, Universidad Centroccidental Lisandro Alvarado (UCLA), Venezuela. Address: Departamento de Procesos Agroindustriales, UCLA. 3001, Barquisimeto, Venezuela. e-mail: mvmujica@ucla.edu.ve
Marisela Granito. Biologist and Doctor in Food Sciences, USB, Venezuela. Professor, USB, Venezuela.
Naudy Soto. Agroinduastrial Technician, UCLA, Venezuela. Laboratory Assistant, UCLA, Venezuela.
SUMMARY
In order to study the effects of the type of solvent and extraction technique on the quantification of the total phenolic compounds in samples of Phaseolus vulgaris, four techniques of methanol extraction (plate stirring, wrist-action shaking, sonication and homogenization-sonication) and two procedures of sequential extraction, were evaluated. With the various techniques of methanol extraction, significantly different results were obtained. The highest content of total phenolic compounds was obtained with plate stirring. Comparing the six tested procedures, the highest concentrations of total phenolic compounds were obtained with the sequential extraction in water-NaOH 0.2N-methanol. The results showed that the extraction of phenolic compounds of P. vulgaris depends to a large extent on the analytic technique and that the type of solvent used, and the methanol extraction-alkaline hydrolysis-ethylacetate extraction is the method that enables the best quantification. Thus, its use can be recommended for analytical purposes.
Importancia del método de extracción en la cuantificación de compuestos fenólicos totales en Phaseolus Vulgaris L.
RESUMEN
Se estudió el efecto del tipo de solvente y de la técnica de extracción sobre la cuantificación de los compuestos fenólicos totales de una muestra de Phaseolus vulgaris. Para ello se evaluaron cuatro técnicas de extracción en metanol (agitación en plancha, agitador de muñeca, sonicación y homogenización-sonicación) y dos procedimientos de extracción secuencial. Con las técnicas de extracción metanólica se obtuvieron resultados significativamente diferentes, y el mayor contenido de compuestos fenólicos totales se obtuvo con la agitación en plancha. Al comparar los seis procedimientos ensayados, las concentraciones de compuestos fenólicos totales más elevadas se obtuvieron con la extracción secuencial en agua-NaOH 0,2N-metanol. Los resultados mostraron que la extracción de los compuestos fenólicos de P. vulgaris depende en buena medida de la técnica analítica y del tipo de solvente utilizado, y que la extracción metanólica-hidrólisis alcalina-extracción con acetato de etilo es el método que permite una mejor cuantificación, por lo que se puede recomendar su uso para propósitos analíticos.
Importância do método de extração na quantificação de compostos fenólicos totais em Phaseolus Vulgaris L.
RESUMO
Estudou-se o efeito do tipo de solvente e da técnica de extração sobre a quantificação dos compostos fenólicos totais de uma amostra de Phaseolus vulgaris. Para isto foram avaliadas quatro técnicas de extração em metanol (agitação mecânica, agitador manual, sonicação e homogeneização-sonicação) e dois procedimentos de extração sequencial. Com as técnicas de extração metanólica obteve-se resultados significativamente diferentes, e o maior conteúdo de compostos fenólicos totais se obteve com a agitação mecânica. Ao comparar os seis procedimentos ensaiados, as concentrações de compostos fenólicos totais mais elevadas foram obtidas com a extração sequencial em água-NaOH 0,2N-metanol. Os resultados mostraram que a extração dos compostos fenólicos de P. vulgaris depende principalmente da técnica analítica e do tipo de solvente utilizado, e que a extração metanólica-hidrólise alcalina-extração com acetato de etilo é o método que permite uma melhor quantificação, por tanto pode ser recomendado seu uso para propósitos analíticos.
KEYWORDS / Extraction Method / Phaseolus vulgaris / Total Phenolic Compounds /
Received: 10/20/2008. Accepted: 09/02/2009.
Introduction
Phenolic compounds act as essential metabolites for plant growth and reproduction, and as protecting agents against pathogens. In addition, they are related to the sensorial properties of food of vegetal origin, mainly regarding color.
These compounds constitute a large group of about 8000 compounds with varied structures and chemical properties (Robbins, 2003). In general, they are substances containing one or more aromatic rings with one or more hydroxyl groups and can be classified in three main categories: simple phenols, which include phenolic acids, polyphenols constituted by flavonoids and tannins; and a miscellaneous group that comprises compounds such as coumarins, stilbenes and lignans. Phenolic acids, flavonoids, stilbenes and lignans are the most abundant phenolic compounds in plants (Vermerris and Nicholson, 2006).
These compounds can be found in a free state, conjugated with sugars or esters, or polymerized. They are not evenly distributed in tissues or cells, and can be associated to components of the cell wall such as polysaccharides and proteins. In addition, their stability under thermal and oxidative damage is very variable (Nackz and Shahidi, 2004).
An appropriate analysis of phenolic compounds depends on multiple factors, such as their chemical nature, sample particle size, storage time and conditions, extraction and quantification methods, choice of standards, and presence of interferences (Shahidi and Nackz, 2004). Thus, it is necessary to adjust sample preparation procedures to achieve the best possible estimates of phenolic compounds content in different foods.
Currently, there is a growing interest in the study of foodstuffs as a source of phenolic compounds, and many in vivo and in vitro assays have shown that those present in fruits, vegetables and legumes can reduce the risk of chronic illnesses such as cancer, and heart and neurodegenerative diseases (Anderson et al., 1984; Geil and Anderson, 1994; Auger et al., 2004; Iriti and Faoro, 2006; Kumar and Surh, 2008; Parkar et al., 2008).
Phaseolus vulgaris is one of the most consumed legumes on a global scale, and in addition to being an important source of proteins and of complex carbohydrates, it can be considered as a functional foodstuff due to its content of soluble fibre and of phenolic compounds, with their corresponding antioxidant activity (Aparicio-Fernández et al., 2005; Granito et al., 2007).
There is wide variation in the phenolic contents reported for P. vulgaris (Heimler et al., 2005; Espinosa-Alonso et al., 2006; Anton et al., 2007; Granito et al., 2007; Ranilla et al., 2007; Boateng et al., 2008), which is due on the one hand to differences inherent to the analyzed variety and the conditions of cultivation and storage, and on the other hand to the different extraction and quantification procedures used.
Luthria (2006) states that the preparation of the sample in the analysis of phenolic compounds is often underestimated and considers it as "a means to an end" and, despite the great advances in chromatographic and spectroscopic instrumentation for the separation and identification of phenolic compounds, sample preparation has received little attention. In this respect, this author recommends a four-step scheme for the analysis of this type of compounds: i) assess the existence of multiple forms of the analytes of interest, ii) select the most efficient extraction technique, iii) evaluate the extraction with several solvents or solvent mixtures and iv) optimise the extraction conditions (i.e. temperature, number of cycles, sample particle size and sample:solvent ratio).
In this study, six procedures for the extraction of the total phenolic compounds of a black variety of Phaseolus vulgaris were compared using the Folin-Ciocalteu reagent for quantification. In addition, in one of the procedures used, the effect of the extraction time was assessed.
Materials and Methods
Samples
A two-month post-harvest black variety of Phaseolus vulgaris L. was used. Whole grains were ground and the flour was passed through a 20mesh sieve. The ground sample was stored frozen in glass containers wrapped with aluminum foil.
Extraction procedures
Six procedures for the extraction of total phenolic compounds were used. In the first four, only one solvent, methanol-water 80:20 v/v acidified with 0.1% HCl was used, and different extraction techniques were used. The two remaining procedures consisted of sequential extractions with different solvents. The extraction techniques were:
Plate stirring. A sample (1g) was suspended in 25ml methanol-water 80:20 v/v acidified with 0.1% HCl, and stirred on a plate (Corning, model PC 420, USA) for 2h at room temperature. Later, the mixture was centrifuged at 1800g for 15min, the methanol was decanted and the residue was re-extracted with 25ml of fresh methanol. It was centrifuged again and the extracts were combined.
Wrist-action shaking. A sample (1g) was suspended in 25ml of methanol-water 80:20 v/v acidified with 0.1% HCl and shaken for 2h in a wrist-action shaker (Burrel, USA) at maximum speed. Subsequently, the same procedure described above was followed.
Sonication. 1 g of sample was suspended in 25ml of methanol-water 80:20 v/v acidified with 0.1% HCl and sonicated (Bransonic, Branson 2510, USA) for 15min at room temperature. Subsequently, the same procedure described in 2.2.1 was followed.
Homogenisation-sonication. A sample (1g) was suspended in 25ml of methanol-water 80:20 v/v acidified with 0.1% HCl and homogenised for 60s at 15rpm with an homogenizer (Kinematica, Polytron 3100, USA) and later sonicated for 15min. at room temperature. Subsequently, the same procedure described above was followed.
Sequential extractions with water, NaOH 0,2N and methanol 50%. According to the method of Singleton and Rossi (1965) modified, 2g of sample were extracted with 100ml of N2 saturated water, plate stirred and centrifuged. In this step, three extraction times were tested: 1, 14 and 20h. To the residue, 50ml of NaOH 0.2N were added, plate-stirred for 30min and centrifuged. Again, to this residue, 50ml of 50% methanol were added, it was plate-stirred for 30min and centrifuged. This last step was repeated once more, and then the supernatants were combined.
All centrifugations were performed at 1800g for 15min, and the supernatants were set aside for the quantification of the total phenolic compounds.
Methanol extraction followed by alkaline hydrolysis and extraction with ethylacetate. This was performed according to the method of Luthria and Pastor-Corrales (2006) modified. The methanol extraction described above was performed, and the resulting residue was hydrolysed with 25ml of NaOH 2N, stirring on a plate for 1h. The reaction mixture was then acidified with 7ml HCl 7.2N. The phenolic compounds released were extracted with ethylacetate (2´32ml). The organic layers were combined and evaporated at 45°C under vacuum. The residue was dissolved in 25ml methanol-water 80:20 v/v with 0.1% HCl.
Quantification method
Gallic and tannic acids were used as standards for the quantification. The calibration curves had a concentration ranging 0.01-0.6mg·ml-1 and the results were expressed as mg GAE (gallic acid equivalent)/100g of sample and as mg TAE (tannic acid equivalent)/100g of sample. The quantification of the total phenolic compounds was based on the Folin-Ciocalteu reaction, according to the method of Singleton and Rossi (1965), measuring the absorbance at 765nm.
Statistical analysis
All the extractions were made in triplicate. A one-way variance analysis was applied with the Statgraphics Plus 4.0 software to determine significant differences (a=0.05) among the levels of the variables studied. For comparison of the means, Duncans multiple range test (a=0.05) was applied.
Results and Discussion
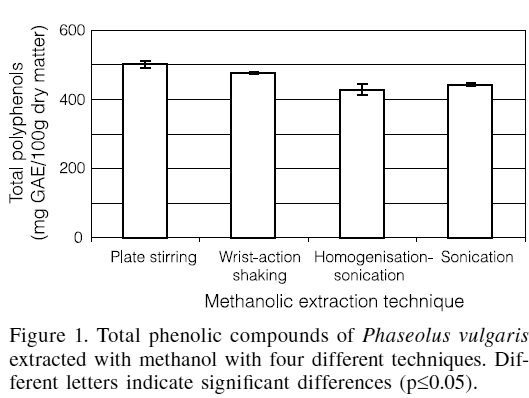
In Figure 1, the content of the total phenolic compounds obtained with each of the methanol extraction techniques tested, which varied from 428 to 501mg GAE/100g or from 470 to 555mgTAE/100g, is shown. The extraction with plate stirring for 2h produced the highest content of phenolic compounds, with no significant differences found (p£0.05) between the sonication and the homogenisation-sonication techniques. From this result it can be inferred that homogenisation with the homogenizer does not increase the extraction of phenolic compounds. These results are convenient from a practical point of view, since plate stirring is the simplest technique among the ones tested.
Luthria and Mukhopadhyay (2006) found that plate stirring with methanol 80% resulted in a better extraction for the analysis of phenolic acids in an eggplant sample, compared to sonication with pure methanol and to extraction with 85 and 100%acetone using a wrist-action shaker.
Espinosa-Alonso et al. (2006) reported 98 at 155mg GAE/100g in black varieties of P. vulgaris after extraction with 80% methanol. Boateng et al. (2008) found about 800mg GAE/100g for a spotted brown variety using 80% ethanol extraction, while Anton et al. (2007) quantified 192mg FAE (ferulic acid equivalent)/100g for the same variety using a wrist-action shaker and acidified methanol (methanol:water:HCl, 80:10:1 v/v).
Besides the genetic variability and cultivation and storage conditions, the differences previously mentioned in the total phenolic compounds content of P. vulgaris could be due to the different techniques and solvents used for extraction. Nevertheless, the values are of the same order of magnitude and, thus, it could be inferred that the extraction with the polar solvents used by the cited authors give comparable results.
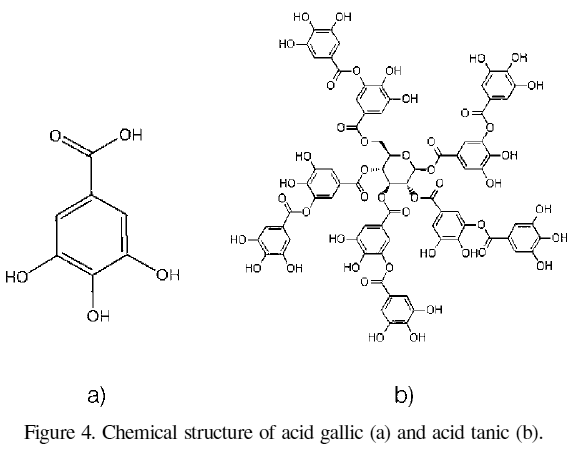
Regarding the use of two different standards for the quantification of the total phenolic compounds, in Figures 2 and 3 are shown the calibration curves for the gallic and tannic acids, respectively. The slope of the gallic acid curve has is higher, which could be interpreted as this compound having a higher reducing power since a higher absorbance is obtained for the same concentration, which could be due to the differences in chemical structure of the two acids (Figure 4), given that the tannic acid is an hydrolysable tannin while the gallic acid is a simple phenol.
Gallic acid is mostly used to express the content of phenolic compounds in the majority of foods, including legumes like P. vulgaris; however, the most appropriate would be to use ferulic acid because it is the most abundant phenolic acid in P. vulgaris (Garcia et al., 1998; Luthria and Pastor-Corrales, 2006). In this respect, Anton et al. (2007) used ferulic acid as standard to evaluate the effect of dehulling of this species on the content of total phenolic compounds and of tannins.
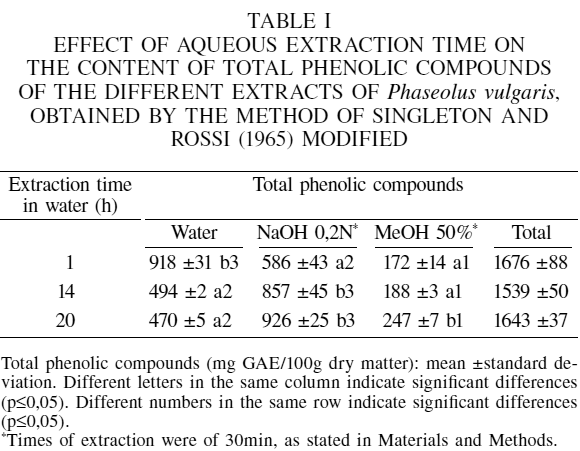
The contents of total phenolic compounds in the aqueous, alkaline and methanolic extracts of P. vulgaris obtained following the method of Singleton and Rossi (1965) is shown in Table I. According to the results, a statistically significant effect of time on each type of extract was found. In the aqueous extract, the highest content of phenolic compounds was obtained with 1h stirring, with no significant differences between 14 and 20h. In the alkaline extract, after extraction with water at the three studied times, the highest concentration was reached with 20h stirring, and no significant differences were found respect to the 14h value. Regarding the methanol extracts, the highest concentration of phenolic compounds was achieved with 20h of extraction, and no significant differences were found between 1 and 14h.
Previous studies have shown that the variation in the content of total phenolic compounds with time of extraction depends on the type of solvent used (Akowuah et al., 2005; Lapornik et al., 2005; Turkmen et al., 2007). Lapornik et al. (2005) found that the phenolic compounds of the aqueous extracts of strawberries decreased with the time of extraction, whereas they increased in the methanol and ethanol extracts.
With the sequential extraction process used, it is possible to obtain contents of total phenolic compounds in the same order of magnitude for all the times evaluated. In Table I it can be seen that the total content of phenolic compounds varied in the range of 1539 to 1676mg GAE/100g or 1797 to 1867mg TAE/100g, which allows to suggest 1h as the most convenient time of extraction in water for the purpose of speed of analysis.
For Shahidi and Naczk (2004) the extraction period is another variable that affects the recovery of the phenolic compounds, and it can vary from 1min to 24h; however, as time increases, the possibility of oxidation of the phenolic compounds also increases, unless reducing agents are added. The latter is not possible if the total phenolic compounds are quantified with the Folin-Ciocalteu reagent, as this method is based on the reduction of the phosphomolybdic acid by the phenolic compounds and therefore, other reducing compounds could be detected.
When evaluating the results for the various extraction times it is found that the concentrations of total phenolic compounds for the different types of extract were significantly different. With 1h extraction, the highest concentration was in the aqueous extract, whereas with 14 and 20h of extraction, the highest concentration was obtained with the alkaline extract, followed by the aqueous one.
These results are similar to those reported by Granito et al. (2007), who used the method of Singleton and Rossi (1965) to quantify the total phenolic compounds of a black variety of P. vulgaris, obtaining 1917mg TAE/100g. However, it is important to note that these results are much higher than those obtained with the methanol extraction techniques initially assayed. This is due to the fact that with the Singleton and Rossi (1965) method the total phenolic compounds of three different extracts are quantified, while with the other methods, only methanol extracts are performed.
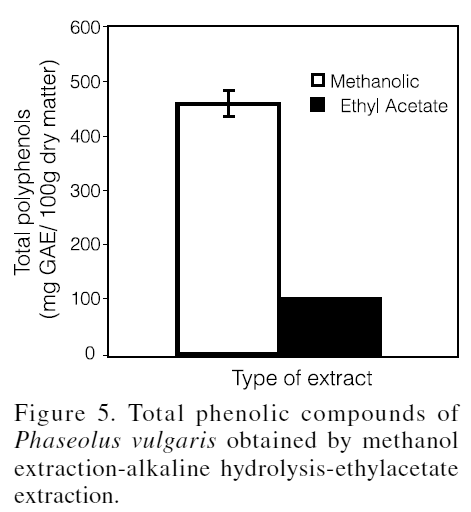
The results obtained with the method of methanol extraction-alkaline hydrolysis-ethylacetate extraction are shown in Figure 5. The total content of phenolic compounds was 564mg GAE/100g, out of which 102mg GAE/100g correspond to those released by alkaline hydrolysis and separated with ethylacetate. When this last value is compared to those found in the alkaline extracts by the method of Singleton and Rossi (1965), which varied from 586 to 926mg GAE/100g, it can be inferred that in the latter method there is an overestimation of the phenolic content when the alkaline extract is used directly in the quantification with the Folin-Ciocalteu reagent, due to the interference of the non-phenolic compounds with reducing power (Vermerris and Nicholson, 2006).
The alkaline extraction in the analysis of P. vulgaris is important since it has been demonstrated that some hydroxycinnamic acids and their esters, mainly those from ferulic acid, can be found conjugated to cell wall polysaccharides (Srisuma et al., 1989; García et al., 1998). However, it is necessary to perform a re-extraction with an organic solvent like ethylacetate to enable the isolation of the phenolic compounds and the decrease of the interferences of hemicelluloses and other compounds with reducing power. Hemicelluloses are soluble in strong alkaline solutions and posses a reducing end group that could react with the phosphomolybdic acid of the Folin-Ciocalteu reagent.
In the procedure of methanol extraction-alkaline hydrolysis-ethylacetate extraction, methanol-water 80:20 v/v with 0,1% HCl as the first solvent was used, aiming to extract most of the phenolic compounds (tannins, flavonoids and phenolic acids) present in their free state or not conjugated, and later, the alkaline hydrolysis was applied to break the ester-type bonds between the ferulic acid, or another hydroxycinnamic acid, and the cell wall polysaccharides. The released Na ferulate was separated from the Na+ by HCl acidification, and after the ferulic acid formed it was extracted with an immiscible solvent, in this case, ethylacetate. Ferulic acid is the main phenolic acid of P. vulgaris and is highly soluble in ethylacetate.
The solubility of phenolic compounds depends on their degree of polymerization, on the interactions with other components, and on the polarity of the solvent. Therefore, it is very difficult to develop an efficient method for the extraction of all the phenolic compounds with only one solvent. Other solvents used are ethanol, acetone, water, ethylacetate and their combinations.
Conclusions
The extraction of phenolic compounds depends largely on the analytical technique, type of solvent used and extraction time, with results that can vary even by one order of magnitude when one or another procedure is used for the same starting sample. Consequently, it is necessary to adjust the methods reported in previous studies to the foodstuff of interest, so as to achieve a higher accuracy in the results.
Among the methods tested to extract the total phenolic compounds of a black variety of Phaseolus vulgaris, the methanol extraction-alkaline hydrolysis-ethylacetate extraction is recommended for being the most suitable method for this type of sample regarding the distribution of its phenolic compounds, and because it includes a re-extraction with ethylacetate to avoid the overestimation produced when the product of the alkaline hydrolysis is used directly in the quantification with the Folin-Ciocalteu reagent.
References
1. Akowuah G, Ismail Z, Norhayati I, Sadikun A (2005) The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical- scavenging activity. Food Chem. 93: 311-317.
2. Anderson J, Story L, Sieling B, Chen W, Petro M, Story J (1984) Hypocholestrolemic effects of oat-bran or bean intake for hypercholestrolemic men. Am. J. Clin. Nutr. 40: 1146-1155.
3. Anton A, Ross K, Beta T, Fulcher G, Arntfield S (2007) Effect of pre-dehulling treatments on some nutritional and physical properties of navy and pinto beans (Phaseolus vulgaris L.). LWT - Food Sci. Technol. 41: 771-778
4. Aparicio-Fernández X, Yousef G, Loarca-Piña G, De Mejia E, Lila M (2005) Characterization of polyphenolics in the seed coat of black Jamapa bean (Phaseolus Vulgaris L.). J. Agric. Food Chem. 53: 4615-4622.
5. Auger C, Al-Awwadi N, Bornet A, Rouanet J, Gasc F, Cros G, Teissedre P (2004) Catechins and procyanidins in Mediterranean diets. Food Res. Int. 37: 233-245.
6. Beninger C, Hosfield G (2003) Antioxidant activity of extracts, condensed tannin fractions and pure flavonoids from Phaseolus vulgaris L. seed coat color genotypes. J. Agric. Food Chem. 51: 7879-7883.
7. Boateng J, Verghese M, Walker L, Ogutu S (2008) Effect of processing on antioxidant contents in selected dry beans (Phaseolus spp. L.). LWT Food Sci. Technol. 41: 1541-1547.
8. Deshpande S, Sathe S, Salunkhe D, Cornforth D (1982) Effects of dehulling on phytic acid, polyphenols, and enzyme inhibitors of dry beans (Phaseolus vulgaris L.). J. Food Sci. 47: 1846-1850.
9. Espinosa-Alonso L, Lygin A, Widholm J, Valverde M, Paredes-Lopez O (2006) Polyphenols in wild and weedy Mexican common beans (Phaseolus Vulgaris L.). J. Agric. Food Chem. 54: 4436-4444.
10. García E, Filisetti T, Udaeta J, Lajolo F (1998) Hard to cook beans: involvement of phenolic compounds and pectates. J. Agric. Food Chem. 46: 2110-2116.
11. Geil P, Anderson J (1994) Nutrition and health implications of dry beans: A review. J. Am. Coll. Nutr. 13: 549-558.
12. Granito M, Paolini M, Pérez S (2007) Polyphenols and antioxidant capacity of Phaseolus vulgaris stored under extreme conditions and processed. LWT Food Sci. Technol. 41: 994-999.
13. Heimler D, Vignolini P, Dini M, RomaniA (2005) Rapid tests to assess the antioxidant activity of Phaseolus vulgaris L. dry beans. J. Agric. Food Chem. 53: 3053-3056.
14. Iriti M, Faoro F (2006) Grape phytochemicals: A bouquet of old and new nutraceuticals for human health. Med. Hypoth. 67: 833-838.
15. Kumar J, Surh Y (2008) Cancer chemopreventive and therapeutic potential of resveratrol: Mechanistic perspectives. Cancer Lett. 269: 243-246.
16. Lapornik B, Proek M, Wondra A (2005) Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 71: 214-222.
17. Luthria D (2006) Significance of sample preparation in developing analytical methodologies for accurate estimation of bioactive compounds in functional foods. J. Sci. Food Agric. 86: 2266-2272.
18. Luthria D, Pastor-Corrales M (2006) Phenolic acids content of fifteen dry edible bean (Phaseolus Vulgaris L.) varieties. J. Food Comp. Anal. 19: 205-211.
19. Nackz M, Shahidi F (2004) Extraction and analysis of phenolics in food. J. Food Chromatogr. A 1054: 95-111.
20. Parkar S, Stevenson D, Skinner M (2008) The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 124: 295-298.
21. Ranilla L, Genovese M, Lajolo F (2007) polyphenols and antioxidant capacity of seed coat and cotyledon from Brazilian and Peruvian bean cultivars (Phaseolus vulgaris L.). J. Agric. Food Chem. 55: 90-98.
22. Robbins RJ (2003) Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 51: 2866-2887.
23. Shahidi F, Nackz M (2004) Phenolics in Food and Nutraceuticals. CRC Pres. Washington DC; EEUU. 483-490.
24. Singleton V, Rossi A (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 16: 144-158.
25. Srisuma, N Hammerschmidt, R Uebersax, S Ruengsakulrach, S Bennink, M & Hosfield G. (1989). Storage induced changes of phenolic acids and the development of Hard-to-Cook in dry beans (Phaseolus vulgaris, var. Seafarer). Journal of Food Science 54: 311-314.
26. Turkmen N, Velioglu S, Sari F, Polat G (2007) Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules 12: 484-496.
27. Vermerris W, Nicholson R (2006) Phenolic Compound Biochemistry. USA: Springer. Nueva York, EEUU. 3-16, 151-153. pp. [ Links ]












 uBio
uBio