Interciencia
versión impresa ISSN 0378-1844
INCI v.34 n.10 Caracas oct. 2009
In vitro effects of garlic (allium sativum l.) And african basil (ocimum gratissimum l.) On pathogens isolated from rotted cassava roots
Raphael N. Okigbo, Rachel E. Okorie and Ramesh R. Putheti
Raphael Nnajiofor Okigbo. B. Sc. M. Sc. and Ph.D., Unversity of Benin, Nigeria. Professor, Nnamdi Azikiwe University, Nigeria. Address: Department of Botany, Nnamdi Azikiwe University, Awka, PMB 5025, Anambra State, Nigeria. e-mail: okigborn17@yahoo.com
Rachel Ezinwanne Okorie. B. Sc., Nnamdi Azikiwe University, Nigeria. Graduate Student, Department of Botany, Nnamdi Azikiwe University, Nigeria.
Ramesh. R. Putheti. Ph.D. Member, American Chemical Society, USA.
SUMMARY
Six fungal pathogens causing rot in post-harvest cassava tuberous roots were investigated in vitro for the study of the fungi toxic effects of Allium sativum (L) and Ocimum gratissimum (L.) using aqueous extraction methods. Fungi were isolated by cutting rotted tissue at the interface between healthy and infected portions of the root. Pathogenicity tests revealed that Fusarium oxysporum, F. solani, Botryodiplodia theobromae, Macrophomina phaseolina, Penicillium oxalicum and Aspergillus niger induced rot in healthy cassava tubers after 8 days of re-inoculation, with P. oxalicum as the most virulent. Four different extract concentrations were obtained from each plant part by blending 25, 50, 75 and 100g in 100ml of sterile distilled water (SDW).The fungi toxic effect of the extracts showed that A. sativum had effective inhibition (25.2-86.9%) on mycelial growth of all tested fungi, while extracts of O. gratissimum showed slight to moderately effective inhibition (0.9 to 64.5%) on mycelial growth of all fungi, with the exception of B. theobromae and M. phaseolina, which showed the lowest percentage of inhibition with both plant extracts. The most toxic effect of the extracts was observed with A. sativum at 10%, with significant (P<0.01) inhibition on all fungi tested. The study showed the potential of crude extracts of A. sativum and O. gratissimum on fungal pathogens affecting cassava. This potential of the crude extract of these plants provides an alternative to farmers to reduce and control cassava rot, since they are inexpensive, non-phytotoxic and easy to prepare.
Efectos in vitro del ajo (Allium Sativum L.) Y Albahaca africana (Ocimum Gratissimum L.) Sobre patógenos aislados de raíces de yuca podrida
RESUMEN
Seis hongos patógenos causantes de pudrición post-cosecha en raíces de yuca fueron investigados in vitro para estudiar los efectos funguitóxicos de Allium sativum (L) y Ocimum gratissimum (L) utilizando métodos de extracción acuosa. Los hongos fueron aislados cortando tejido enfermo en la interfase entre las partes sana e infectada de la raíz. Pruebas de patogenicidad mostraron que Fusarium oxysporum, F. solani, Botryodiplodia theobromae, Macrophomina phaseolina, Penicillium oxalicum y Aspergillus niger inducen pudrición en tubérculos sanos de yuca tras 8 días de reinoculación, siendo P. oxalicum el más virulento. Cuatro concentraciones de extracto fueron obtenidas de cada planta mezclando 25, 50, 75 o 100g con 100ml de agua destilada estéril. El efecto funguitóxico de los extractos mostró que A. sativum produjo una inhibición efectiva (25,2-56,9%) del crecimiento micelial de todos los hongos ensayados, mientras que los extractos de O. gratissimum mostraron débil a moderadamente efectiva inhibición (0,9 a 64,5%) del crecimiento micelial de todos los hongos con excepción de B. theobromae y M. phaseolina, que mostraron el menor porcentaje de inhibición con ambos extractos. El efecto más tóxico de los extractos fue observado con 10% A. sativum, con inhibición significativa (P<0,01) sobre todos los hongos estudiados. El ensayo mostró el potencial de extractos crudos de A. sativum y O. gratissimum sobre hongos patógenos que afectan la yuca. Este potencial del extracto crudo de estas plantas provee una alternativa a los campesinos para el control y reducción de la pudrición de la yuca, por ser económicos, no fitotóxicos y fácil de preparar.
Efeitos in vitro do alho (Allium Sativum l.) E Alfavaca-Cravo (Ocimum Gratissimum L.) Sobre patógenos isolados de raízes de mandioca podre
RESUMO
Seis fungos patógenos causantes de apodrecimento pos-colheita em raízes de mandioca foram investigados in vitro para estudar os efeitos fungitóxicos de Allium sativum (L) e Ocimum gratissimum (L) utilizando métodos de extração aquosa. Os fungos foram isolados cortando tecido doente na interfase entre as partes sadias e infetadas da raíz. Provas de patogenicidade mostraram que Fusarium oxysporum, F. solani, Botryodiplodia theobromae, Macrophomina phaseolina, Penicillium oxalicum e Aspergillus niger induzem apodrecimento em tubérculos sadios de mandioca depois de 8 dias de reinoculação, sendo P. oxalicum o mais virulento. Quatro concentrações de extrato foram obtidas de cada planta misturando 25, 50, 75 ou 100g con 100ml de água destilada estéril. O efeito fungitóxico dos extratos mostrou que A. sativum produziu uma inibição efetiva (25,2-56,9%) do crescimento micelial de todos os fungos ensaiados, enquanto que os extratos de O. gratissimum mostraram fraca a moderadamente efetiva inibição (0,9 a 64,5%) do crescimento micelial de todos os fungos com exceção de B. theobromae e M. phaseolina, que mostraram a menor porcentagem de inibição com ambos extratos. O efeito mais tóxico dos extratos foi observado com 10% A. sativum, com inibição significativa (P<0,01) sobre todos os fungos estudados. O ensaio mostrou o potencial de extratos crus de A. sativum e O. gratissimum sobre fungos patógenos que afetam a mandioca. Este potencial do extrato cru destas plantas provê uma alternativa aos camponeses para o controle e redução do apodrecimento da mandioca, por ser econômicos, não fitotóxicos e fácil de preparar.
KEYWORDS / Cassava / Fungal Pathogens / Fungi Toxic Effect / Plant Extracts / Rot /
Received: 08/06/2008. Modified: 10/02/2009. Accepted: 10/05/2009.
Introduction
Cassava (Manihot esculenta Crantz ) is a dicotyledonous root crop belonging to the Euphorbiaceae family. It is a major food crop in the tropics, particularly in the developing countries of the Sub- Saharan region of Africa (Hahn et al., 1979). Cassava is a root crop grown on an estimated land area of 10.8´106ha in some African countries (FAO, 1999). In most of these countries, cassava is grown mainly for its starchy roots, which are valuable sources of cheap calories, particularly for the low-income earners (IITA, 1990). The mode of cassava utilization varies from one place to another. Among the common products of cassava are gari, fufu, starch, cassava flour, etc. Also, leaves and tender shoots are consumed as vegetable in some countries (Dahniya, 1994). In addition to human consumption, the crop is used for the production of ethanol, animal feed and starch for various industrial, uses particularly in Thailand and Viet Nam (IITA, 1990; FAO, 2000).
Several workers have reported the isolation of many different types of fungi from rotted cassava roots in storage. Some of the fungi found to be pathogenic on cassava roots after re-inoculation include Phytophthora drechsleri Tucker (Booth, 1978; Theberge, 1985); Sclerotium rolfsii (IITA, 1990); Rosellinia necatrix Prill (Lozano and Booth, 1976; Booth, 1978); Fusarium oxysporum Schlecht, Botryodiplodia theobromae Pat, Aspergillus niger Van Tieghem, Aspergillus flavus Link, Rhizopus spp; Fusarium solani (Mart) Sacc., and Macrophomina phaseolina (Tassi) Goidanich (Booth, 1978, Okigbo et al., 2009a).
Different control measures so far suggested for post-harvest cassava root rot diseases include reduced temperature, pre-harvest practices, storage techniques, natural resistance, and use of chemicals (Booth, 1976). However, the use of synthetic fungicides (Majumder, 1955), apart from their potential danger to both farmers and environment are unaffordable by most of them (Obagwu et al., 1997; Amienyo and Ataga, 2007). Recent studies on the use of plant extracts have opened a new opportunity for the control of plant diseases. Plant extracts have been reported to be safe, non-toxic to man, but effective against plant pathogens (Shivpuri et al., 1997; Okigbo et al., 2009b). In Nigeria, plant extracts have been used to control fungal diseases of plants such as cowpea (Amadioha and Obi, 1999), banana (Okigbo and Emoghene, 2004), yam (Onifade, 2000; Okigbo and Nmeka, 2005), sweet potato (Amienyo and Ataga, 2007) and maize (Awuah, 1989), but have been sparsely used in the control of cassava diseases.
Allium sativum L. (garlic; Alliaceae), is used as spice and for treatment of cough and chest pain (Harnel, 1975). Groppo et al. (2007) reported that fresh garlic shows good antimicrobial activity on oral streptococci. Ocimum gratissimum L.(african basil; Lamiaceae) is grown in gardens and its leaves are used as a tea for fever relief (Okigbo and Ogbonnaya, 2006); it is commonly used in folk medicine to treat diseases such as upper respiratory tract infections, diarrhea, skin diseases, pneumonia, cough and conjunctivitis (Onajobi, 1986).
The objective of this study, therefore, was to identify the different root rot pathogens affecting cassava, and use extracts of A. sativum and O. gratissimum to control root rot pathogens of cassava.
Materials and Methods
Sources of material
Samples of cassava with root rot diseases were randomly selected from recently harvested cassava from a farm at Ibadan, Nigeria. The two local plants: Allium sativum L. (bulbs) and Occimum gratissimum L. (leaves) used in this study as botanicals were collected from Bodija market, Ibadan, and from home gardens, respectively. The plant identities were verified and authenticated by C.U Okeke, Botany Department, Nnamdi Azikiwe University, Awka, Nigeria.
Isolation and identification of fungi associated with rotted cassava roots
Each root sample was washed in cleaned running tap water and sections of ~2mm² were cut from the tissue, using a sterile scalpel, at the interface between healthy and infected portions of the tuber. The pieces of tissue were surface-sterilized with 1% sodium hypochlorite for 2min, and rinsed in five changes of sterile distilled water. The pieces were tapped dry with a sterile paper towel. Five sections of the cleaned??? root were plated out on potato dextrose agar (PDA). The inoculated Petri dishes were sealed with parafilm to prevent contamination and then incubated in a Gallenkamp incubator at 28 ±2oC for 7 days and observed daily for fungal development. The various fungal isolates from each of the samples were sub-cultured by transferring hyphal tips from the colony edges to fresh PDA plates using a flame sterilized mounted needle to obtain a pure culture, and incubated at 28oC. Cultures were identified using a compound microscope and identification keys described by Sutton (1973) and Nelson et al. (1983).
Pathogenecity tests of the isolated fungi
Fresh, healthy roots from 8-12 month old cassava plants were washed in running tap water to remove soil and other debris from the root surface. The tubers were surface sterilized in a 1% solution of sodium hypochlorite by immersing them for 2min after which they were rinsed with sterile distilled water and left to dry under a laminar flow hood for 30min. Each root was bored at two edges (upper and lower parts) to a depth of 1cm, using a flame-sterilized 8mm diameter cork borer. A disc of 7 days old PDA culture of the test isolated was washed into sterile beakers with sterile distilled water and 1ml of each isolate was inoculated into the hole and sealed with the root piece removed from the hole. The points of inoculation were sealed with parafilm to prevent entry of external contaminants. The same procedure was used for the control except that 1ml of sterile distilled water was inoculated inside of each hole made in the roots. Each inoculated root was placed at air environment for 8 days and examined for rot development.
Preparation of crude extracts of the plant part
Fresh bulbs of garlic (Allium satvium ) and fresh fully expanded leaves of african basil (Occimum gratissmum) were washed thoroughly under running tap water and soaked in a 1% solution of sodium hypochlorite for 2 min, rinsed severely with sterile distilled water and air dried at room temperature for 2h. Four different water extract concentrations were prepared by weighting 25, 50, 75 and 100g of each plant part on a Mettler-Toledo AG balance, BB2400, Switzerland, and were blended in an Eurosonic, ES210, Great Star, Asia, blender, and 100ml of sterile distilled water added. Extract concentrations of 25, 50, 75, and 100% were thus obtained. The extracts were sieved through four layers of sterile cheese-cloth. One ml of each extract concentration was dispensed per Petri dish and 9ml of molten PDA was added to prepare a PDA-extract mixture with corresponding 2.5 5.0, 7.5 and 10% extract concentrations (Sangoyomi, 2004). The plates were gently rotated to ensure even dispersion of the extracts. The agar-extract mixture was allowed to solidify and used for the inhibition of mycelia growth of the tested fungi.
Effect of the extract on fungal growth
The method of Sangoyomi (2004) was used to determine the effect of the extracts on fungal growth. The experiment was conducted in the Gernplasm Health Laboratory, International Institute of Tropical Agriculture, Ibadan, using a randomized complete block design with three replicates. This was done by inoculating at the centre of the Petri dishes a 4mm diameter mycelia disc obtained from the colony edge of 7 days old culture of each of the six test fungi. The negative controls were set up using blank agar plates (no extracts) and the positive control consisted of the fungicide mancozeb (ethylene bisdithiocarbamate) was prepared according to manufacturer directions by mixing 0.5g in 100ml of sterile distilled water. Mancozeb is a multipurpose, preventive, contact, broad spectrum fungicide. Three replicates plates of PDA-extract per isolate were incubated at 27oC and radial growth was measured daily for 7 days. Colony diameter was taken as the means along two directions on two perpendicular lines drawn on the reverse of the plates.
The percentage of inhibition was calculated according to the method described by Whipps (1987) as
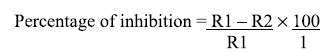
where R1: radial distance of pathogen in control plates, and R2: radial distance of pathogen with treatment.
The percentage of inhibition was determined as a guide for selecting the minimum inhibitory concentration that will be effective in controlling rot-causing fungi. Extracts were rated for their inhibitory effects using the scale described by Sagoyomi (2004); £0% inhibition: not effective, >0-20%: slightly effective, >20-50%: moderately effective, >50-<100%: effective, and 100% inhibition: highly effective..
Statistical analysis
The experimental design used was a randomized complete block design (RCBD) with three replicates. The treatments were subjected to variance analysis (Anova) via a statistical analysis system (SAS Institute, Version 91, 2002, USA) and Gomez and Gomez (1974). Mean separations were carried out using Duncans Multiple Range Tests (DMRT) at p<0.01
Results
Isolation and identification of fungi causing rot on cassava roots
The fungal pathogens isolated from the sample of rotted cassava root collected from recently harvested cassava were Botryodiplodia theobromae Pat, Macrophomina phaseolina (Tassi) Goidanich., Aspergillus niger Van Tieghem, Fusarium solani (Mart.) Sacc., Fusarium oxysporum Schlechtend.:Fr., Penicillium oxalicum Currie & Thom, and Rhizopus stolonifer (Ehrenb.:Fr.) Vuill.
The frequency of isolation varied with the different fungi associated with the rotted root. B. theobromae had the highest frequency of isolation (64.5%) followed by R. stolonifer (28.2%) and Fusarium spp. (17.6%). The other fungi had lower frequencies of isolation ranging from 4.2 to15.0% (Table I).
Screening of A. sativum, O. gratissimum and mancozeb effects
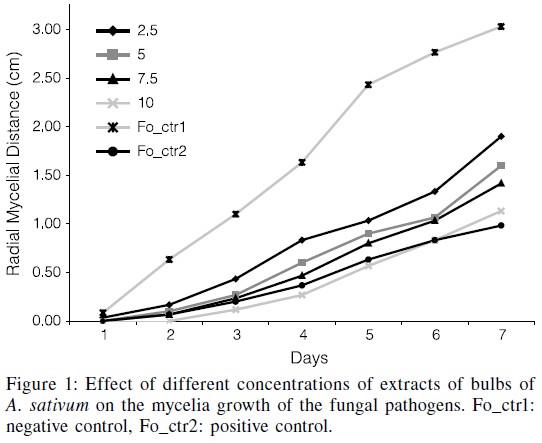
The inhibitory effect of A. sativum, O. gratissimum and mancozeb on mycelial growth of F. oxysporum showed that there was a general increase in percentage of inhibition, which increased with the concentration of the extracts (Figures 1, 2). At 2.5% extract concentration, A. sativum showed a moderately effective inhibition of 41.1% and O. gratissimum also showed a moderately effective inhibition of 27.1% (Table IV). At 5.0% extract concentration, A .sativum showed an effective inhibition (53.3%) while O. gratissimum still had a moderately effective inhibition of 42.1%. At 7.5 and 10% extract concentrations, A. sativum still proved effective having 66.4 and 76.6% of inhibition respectively, while O. gratissimum showed effective inhibition of 50.5 and 58.9%. Mancozeb inhibited mycelial growth with 60.7 percentage inhibition of F. oxysporum (Figures 1, 2).
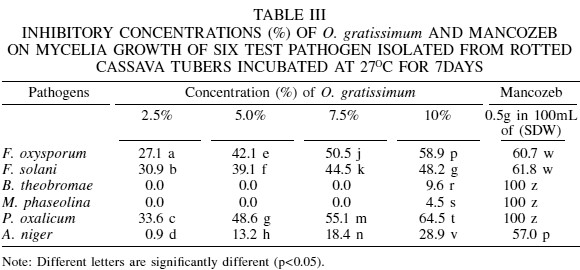
The inhibitory effect of A. sativum, O. gratissimum and mancozeb on mycelial growth of F. solani showed that A. sativum at 2.5% concentration still had a moderately effective inhibition (47.3%), similar to O. gratissimum (39.1%). At 7.5 and 10%, A. sativum proved effective (58.2 and 68.2% inhibition), while moderately effective inhibitions were shown by O. gratissimum (44.5 and 48.2%, respectively). Mancozeb caused effective inhibition of 61.8% of mycelial growth of F. solani (Tables II, III).
At 2.5, 5.0, 7.5 and 10% concentrations of A. sativum, there were slightly effective inhibitory effects of 0.9, 7.0, 12.3 and 15.8%, respectively, on the mycelial growth of B. theobromae (Table III). However, O. gratissimum at 2.5, 5.0 and 7.5% extract concentration had no inhibitory effect, whereas at 10% a slightly effective inhibition of 9.6% was observed (Table III). A highly effective inhibition (100%) on the mycelial growth of B. theobromae was shown by mancozed. A. sativum at 2.5% concentration had no effect on the mycelial growth of M. phaseolina, but at 5.0, 7.5 and 10%, slightly inhibitory effects were observed, with 0.9, 2.6 and 6.1% inhibitions, respectively. No inhibitory effect was observed at 2.5, 5.0 and 7.5% extracts concentrations of 0. gratissimum, whereas a slightly effective inhibition of 4.5% occurred at 10% (Table III). Mancozeb showed a highly effective inhibition (100%) on the mycelial growth of M. phaseolina.
A. sativum at all the extract concentrations tested (2.5, 5.0, 7.5 and 10%) showed effective inhibitions of 63.6, 69.2, 75.7 and 86.9% of mycelial growth of P. oxalicum (Table II). At 2.5 and 5.0% concentrations O. gratissimum had moderately effective inhibitions of 33.6% and 48.6%, while at 7.5 and 10% effective inhibitions of 55.1 and 64.5% were observed. Mancozeb completely inhibited the mycelial growth of P. oxalicum (Table III).
At 2.5 and 5.0% extract concentration, A. sativum had moderately effective inhibition of 25.4 and 32.5%, respectively, on the mycelial growth of A. niger while at 7.5 and 10% concentrations of A. sativum, there were effective in inhibitions of 50.0 and 60.0%, respectively (Table II). However, O. gratissimum at 2.5, 5.0 and 7.5% extract concentration had slightly effective inhibitory effects with corresponding 0.9, 13.3 and 18.4% of inhibition, respectively, whereas at 10% there was a moderately effective inhibition of 28.9% (Table III). Mancozeb caused an effective inhibition of 57.9% on the mycelial growth of A. niger.
Discussion
The organisms associated with post- harvest rot of cassava roots in this study were Botryodplodia theobromae, Fusarium oxysporum, Fusarium solani, Macrophomina phaseolina, Penicillium oxalicum and Aspergillus niger. These were frequently isolated from rotten cassava and their involvements in pathogenesis were also confirmed. Several workers have also reported the isolation of these fungi from post- harvest cassava root (Booth, 1976; Rickard and Coursey, 1981). The pathogenicity tests revealed that all of the six tested fungi induced rot in cassava root, with P. oxalicum being the most virulent one. This agrees with the reports of Sangoyomi (2004), Okigbo and Nmeka (2005), Amienyo and Ataga, (2007), and Okigbo and Ogbonnaya. (2006).
In most cases, fungi gain entrance into cassava roots through natural openings and wounds created during harvesting, transporting, handling and marketing (Amienyo and Ataga, 2007). However, Okigbo and Nmeka (2005) noted that at time of harvest roots may already be infected by pathogens derived from foliage diseases or mother roots.
The fungicidal effects of plant extracts on the inhibition of different pathogens of crop plants have been widely reported by several workers (Amadioha and Obi, 1999; Olufolaji, 1999; Onifade, 2000; Udo et al., 2001; Okigbo and Emoghene, 2004; Okigbo and Ogbonnaya, 2005; Okigbo et al., 2009b). Also, some biological control agents such as Bacillus subtilis and Trichoderma viride have been used to control white yam rot caused by fungi, (Okigbo and Ikediugwu, 2001; Okigbo, 2002).
In this study, crude extracts of A. sativum and O. gratissimum were used in order to develop a cheap and simple method of controlling post-harvest cassava root rot for farmers use. The advantage of water extraction over ethanol or acetone extraction include easy preparation and application, and lower cost, bearing in mind that the ultimate beneficiary is the farmer (Sangoyomi, 2004).
The fungitoxic effects of the plant extracts against mycelial growth varied with plant species, concentrations, and with each fungus tested. The present observations showed that A. sativum and O. gratissimum are effective against mycelial growth of most of the tested fungi, the exception being B. theobromae and M. phaseolina. In relation to F. oxysporum it was shown that A. sativum was effective at all the extract concentrations tested, with inhibitions ranging from 41.1 to 76.6%, while extracts of O. gratissimum ranged from moderately to effective inhibition (27.1-58.9%). This is similar to the results obtained by Amienyo and Ataga (2007) and Okigbo and Ogbonnaya (2006) who reported a moderately effective inhibition of the two plants extracts on this fungus, but differs from Sangoyomi (2004), who reported a highly effective inhibition with A. sativum.
With the positive control mancozeb, a broad- spectrum chemical fungicide, very significant inhibitions were obtained on the radial mycelial growth of all the tested fungal pathogens (57.0-100%), with the highest percentages of inhibition associated with B. theobromae, M. phaseolina and P. oxalicum.
The most fungitoxic extract on F. oxysporum was obtained with O. sativum at 7.5 and 10%, while the 10% concentration proved the most fungitoxic effect on B. theobromae and M. phaseolina. The largest inhibitory effect on F. solani was observed at the 10% extract of A. sativum, while the 5.0 and 7.5% concentrations of O. gratissimum and all those of A. sativum showed highly fungitoxic effect on P. oxalicum. The most fungitoxic extracts on A. niger were also the 7.5 and 10% concentrations of A. sativum. The most fungitoxic effect of the two plant extracts tested in in vitro was observed with the 10% concentration of A. sativum, which completely inhibited mycelia growth of all the six tested pathogens.
This study has shown that both A. sativum and O. gratissimum have the potential to control post harvest rot of cassava, indicating that they could be an alternative way of reducing and controlling rot by farmers, since they are less expensive, environmentally safe, non phytotoxic, and easy to prepare.
Conclusion and Recommendation
Among the two plants extracts investigated in this study, extracts of A. sativum showed the highest inhibition on the radial mycelial growth of all the fungi tested with exception of B. thebromae and M. phaseolina. The two plant extracts used are from plants that are commonly grown in Nigeria, are used for medicinal purposes and have been widely studied. Therefore, it is recommended that further research, involving in vivo assays, should be carried out with the aim of using these plant extracts for the inhibition of fungi growth and development in inoculated cassava tubers.
This would reestablish tracts in inhibiting their growth and development on cassava roots inoculated with these fungi. It would also enforce its fungitoxic potential in preserving healthy cassava roots in storage, instead of the use of chemical fungicides which pose dangers to humans and crop plants involved. Also, research should involve the mixture of the two plant extracts in various proportions for possible synergistic effects. The studies could also involve different dilutions and proportions of the extracts. The extracts should also be tested on other rots caused on cassava by different fungi, not included in the present study.
REFERENCES
1. Amadioha AC, Obi VI (1999) Control of anthracnose disease of cowpea by Cymbopogan citratus and Ocimum gratissimum. Acta Phytopathol. Entomol. Hung. 34: 85-89. [ Links ]
2. Amienyo CA, Ataga AE (2007) Use of indigenous plant extracts for the protection of mechanically injured sweet potato [Ipomoea batatas (L) Lam] tubers. Acad. J. Sci. Res. Essay 2: 167-170. [ Links ]
3. Averre CW (1976) Vascular streaking of stored cassava roots. In Proc. 1st Int. Symp. Tropical Root Crops. Trinidad. Vol. 2, pp. 31-35. [ Links ]
4. Awuah RT (1989) Fungitoxic effects of extracts from some West Africa plants. Ann. Appl. Biol. 115: 451-453. [ Links ]
5. Booth RH (1976) Storage of fresh cassava (Manihot esculenta): I. Post-harvest deterioration and its control. Exp. Agric. 12: 103-111. [ Links ]
6. Booth RH (1978) A review of root diseases in cassava. In Brekelbaum T, Belloti A, Lozano JC (Eds.) Cassava Protection Workshop. CIAT. Colombia. 121-133 pp. [ Links ]
7. Dahniya MT (1994) An overview of cassava in Africa. Afr. Crop Sci. J. 2: 337-343. [ Links ]
8. Dalziel IM (1937) Pipercease: Useful Plants of West Tropical Africa Handbook. African Press. Ibadan, Nigeria. 16-17 pp. [ Links ]
9. FAO (1999) Cassava. In Food Outlook. United Nations Food and Agriculture Organization. N° 5. Rome, Italy. 10 pp. [ Links ]
10. FAO (2000) Championing the cause of cassava. News and Highlights. United Nations Food and Agriculture Organization, April 2000. www.fao.org/news/2000 [ Links ]
11. Gómez KA, Gómez AA (1984) Statistical Procedures for Agricultural Research. 2nd ed. Wiley. New York, USA. 680 pp. [ Links ]
12. Groppo F, Ramcciato J, Motta R, Ferravesi P, Sartoratto A (2007) Antimicrobial activity of garlic against oral Streptococci. Int. J. Dent. Hyg. 5: 109-115. [ Links ]
13. Hahn SK, Terry ER, Leduschner K, Akobundu I.O, Okali C, Lal R (1979) Cassava improvement in Africa. Field Crop Res. 2: 193-226. [ Links ]
14. Harnel PB, Mary UC (1975) Cherokee Plants and their Uses- A 400 Years History. N.C. Herald. Sylvia, North Carolina, USA. 35 pp. [ Links ]
15. IITA (1990) Cassava in Tropical Africa: A Reference Manual. International Institute of Tropical Agriculture. Ibadan, Nigeria. 176 pp. [ Links ]
16. IITA (2000) Disease Control in Cassava Farms. IPM Field Guide for Extension Agents. International Institute of Tropical Agriculture. Ibadan, Nigeria. 26 pp. [ Links ]
17. Lozano JC, Booth RH (1976) Diseases of cassava (Manihot esculenta Crantz). CIAT. Series DE-5. Colombia. [ Links ]
18. Lozano JC, Cock JM, Castaño J (1978) New development in cassava storage. Cassava Protection Workshop. 7-12 November, 1977. CIAT. Colombia. [ Links ]
19. Lozano J C, Belloti A, Rayers JA, Howeler R, Leihner D, Doll J (1981) Field Problems of Cassava. CIAT. Colombia. 208 pp. [ Links ]
20. Nelson PE, Toussoun TA, Marasas WFO (1983) Fusarium Species: An Illustrated Manual for Identification. Pennsylvania State University. University Park, PA, USA. 193 pp. [ Links ]
21. Nilmanee S (1986) Diseases of cassava. In Raychaudhuri SP, Verma JP (Eds.) Rev. Trop. Plant Pathol. 3: 213-247. [ Links ]
22. Obagwu J, Emechebe AM, Adeoti AA (1997) Effects of extracts of garlic Allium sativum L bulb and neem Azadiracha indica Juss seed on mycelial growth and sporulation of Collectorichum capsici Syd. Butter and Bixby. J. Agric. Technol. 5: 51-55. [ Links ]
23. Olufolaji DB (1999) Control of wet rot disease of Amaranthus sp. caused by Choanephora cucurbiktarum with extracts of Azadriachita indic. J. Sust. Agric. Env. 1: 183-190. [ Links ]
24. Okigbo RN (2002) Mycoflora of tuber surface of white yam (Dioscorea rotundata) and post harvest control of pathogens with Bacillus subtillis. Mycopathogia 156: 81-85. [ Links ]
25. Okigbo RN, Edmoghene AO (2004) Antifungal activity of leaf extracts of some plants species on Mycopharerella fijiensis Morelet, the casual organism of black Sigatoka disease in banana (Musa acuminata). KMITL. Sci. J. 4: 20-31. [ Links ]
26. Okigbo RN, Ikediugwu FEO (2001) Biological control of tuber surface mycoflora of yams (Dioscorea rotundata). Trop. Sc. 41: 85-89. [ Links ]
27. Okigbo RN, Nmeka IA (2005) Control of yam tuber rot with leaf extracts of Xylopia aethiopica and Zingiber officinale. Afr. J. Biotechnol. 4: 804-807. [ Links ]
28. Okigbo RN, Ogbonaya OU (2006) Antifungal effects of two tropical plant extracts (Ocimum gratissium and Afromaomum melegueta) on postharvest yam (Dioscorea spp.) rot. Afr. J. Biotechnol. 5: 727-731. [ Links ]
29. Okigbo RN, Putheti RR, Achusi CT (2009a) Post-harvest deterioration of cassava and its control using extracts of Azadirachta indica and Aframomum melegueta. E-J. Chem. 6: 1274-1280. [ Links ]
30. Okigbo RN, Anuagasi CL, Amadi JE, Ukpabi UJ (2009b) Potential inhibitory effects of some African tuberous plant extracts on Escherichia coli, Staphylococcus aureus and Candida albicans. Int. J. Integr. Biol. 6: 91-98. [ Links ]
31. Onajobi FD (1986) Smooth muscle contraction lipidic-soluble principles in chromatography fractions of Ocimum gratissmum. J. Ethnopharmacol. 18: 3-11. [ Links ]
32. Onifade AK (2000) Anifungal effect of Azadirachta indica A. Juss extracts on Colletotricum lindemathianum. Glob. J. Pure Appl. Sci. 6: 423- 428. [ Links ]
33. Rickard JE, Coursey DG (1981) Cassava storage part 1: Storage of fresh cassava. Trop. Sci. 23: 1-32. [ Links ]
34. Sangoyoni TE (2004) Post- Harvest Fungal Deterioration of Yam (Dioscorea rotundata Poir) and its control. Thesis. International Institute of Tropical Agriculture. Ibadan, Nigeria. 179 pp. [ Links ]
35. Sutton BC (1973) Coelomycetes. In Ainsworth GC, Sparrow FK, Sussman AS (Eds.) The Fungi: An Advanced Treatise. Academic Press. New York, USA. 513-574 pp. [ Links ]
36. Udo SE, Madunagu BT, Isemin CD (2001) Inhibition of growth and sporulation of fungal pathogens on sweet potato and yam by garlic extract. Nig. J. Bot. 14: 35-39. [ Links ]












 uBio
uBio