Interciencia
versión impresa ISSN 0378-1844
INCI v.34 n.10 Caracas oct. 2009
Physiological performance, yield, and quality of dry bean seeds under drought stress
Ma. Claudia Castañeda-Saucedo, Leobigildo Córdova-Téllez, Víctor A. González-Hernández, Adriana Delgado-Alvarado, Amalio Santacruz-Varela and Gabino García-de los Santos
María Claudia Castañeda-Saucedo. Agronomist, Universidad Autónoma de Chapingo (UACH), Mexico. M.Sc. and Ph.D., Colegio de Postgraduados (COLPOS), Mexico. Professor, Universidad de Guadalajara (UDG), Mexico. Address: CUSUR-UDG. Av. Colón S/N Km1 carretera Guadalajara-Cd. Guzmán. Cd. Guzmán, Jal. México. C.P. 49,000. e-mail: csaucedo@colpos.mx
Leobigildo Córdova-Téllez. Agronomist, UACH, Mexico. M.Sc., Mississippi State University, USA. Ph.D., Iowa State University (IASTATE), USA. Professor, COLPOS, Mexico.
Víctor A. González-Hernández. Agronomist, UACH, Mexico. M.Sc., COLPOS, Mexico. Ph.D., University of Nebraska-Lincoln, USA. Professor, COLPOS, Mexico.
Adriana Delgado-Alvarado. Agricultural Chemist, Universidad Veracruzana, Mexico. M.Sc., COLPOS, Mexico. Ph.D. University of Sheffield, UK. Professor, COLPOS, Puebla, Mexico.
Amalio Santacruz-Varela. Agronomist, UACH, Mexico. M.Sc., COLPOS, Mexico. Ph.D., IASTATE, USA. Professor, COLPOS, Mexico.
Gabino García-de los Santos. Agronomist, UACH, Mexico. M.Sc., COLPOS, Mexico. Ph.D. Oregon State University, USA. Professor, COLPOS, Mexico.
SUMMARY
Net photosynthesis (A), respiration (RE), stomatal conductance (gs), transpiration rate (E), yield, and its components, as well as physical and physiological quality of seeds were evaluated on dry bean (Phaseolus vulgaris L.) plants cv. Otomí, subjected to drought stress during the stages of flowering (F), pod formation (PF) and seed filling (SF). After 3 days under drought stress, gs, E and A decreased by more than 50% at F, PF and SF, respectively; after 10 days of stress, there was total inhibition of those processes, whereas the maximum reductions showed by RE were 42, 62, and 85% in F, PF and SF, respectively. Drought stress induced seed yield reductions of 10, 57, and 50% at F, PF and SF, respectively. High yield losses at PF and SF were caused by reductions in the number of seeds and pods per plant and seeds per pod. At the SF stage the loss in yield was moderate, because at this stage the plants were able to form new leaves and delay pod formation until water stress was over. The physiological quality was not affected by drought stress, even though the weight of 1000 seeds was reduced by about 10%.
Comportamiento fisiológico, rendimiento y calidad de semilla de frijol sometido a sequía
RESUMEN
Se evalúo la fotosíntesis neta (A), respiración (RE), conductancia estomática (gs), tasa de transpiración (E), rendimiento y sus componentes, así como la calidad física y fisiológica de semillas de plantas de frijol (Phaseolus vulgaris L.) cv. Otomí sometidas a sequía durante las etapas de floración (F), formación de vaina (FV) y llenado de semilla (LLS). Después de 3 días de sequia, gs, E y A disminuyeron en más de 50% en F, FV y LLS, respectivamente; después de 10 días de estrés hubo inhibición total de estos procesos, mientras que las reducciones máximas mostradas por RE fueron de 42, 62 y 85% en F, FV y LLS, respectivamente. La sequía propició reducciones en el rendimiento de semilla de 10, 57 y 50% en F, FV y LLS, respectivamente. Las altas pérdidas de rendimiento en FV y LLS se debieron a las reducciones en número de semillas, de vainas por planta y de semillas por vaina. En F la disminución en rendimiento fue moderada, debido a que en esta etapa las plantas formaron nuevas hojas y retardaron la formación de vainas cuando terminó la sequía. La calidad fisiológica de las semillas no resultó afectada por la sequía, aun cuando el peso de 1000 semillas tuvo una reducción de casi 10%.
Comportamento fisiológico, rendimento e qualidade da semente do feijão submetido à seca
RESUMO
Avaliou-se a fotossíntese neta (A), respiração (RE), condutância estomática (gs), taxa de transpiração (E), rendimento e seus componentes, assim como a qualidade física e fisiológica de sementes de plantas de feijão (Phaseolus vulgaris L.) cv. Otomí submetidas à seca durante as etapas de floração (F), formação de vagens (FV) e enchimento da semente (LLS). Depois de 3 dias de seca, gs, E e A diminuiram em mais de 50% em F, FV e LLS, respectivamente; depois de 10 dias de estresse houve inibição total destes processos, enquanto que as reduções máximas mostradas por RE foram de 42, 62 e 85% em F, FV e LLS, respectivamente. A seca propiciou reduções no rendimento da semente de 10, 57 e 50% em F, FV e LLS, respectivamente. As altas perdas de rendimento em FV e LLS foram devido às reduções em número de sementes, de vagens por planta e de sementes por vagem. Em F a diminuição no rendimento foi moderada, devido a que nesta etapa as plantas formaram novas folhas e retardaram a formação de vagens quando terminou a seca. A qualidade fisiológica das sementes não resultou afetada pela seca, mesmo quando o peso de 1000 sementes teve uma redução de quase 10%.
Keywords / Electrical Conductivity / Germination / Phaseolus vulgaris / Photosynthesis / Respiration / Stomatal Conductance /
Received: 07/06/2009. Modified: 10/09/2009. Accepted: 10/12/2009.
Introduction
In legumes, the reproductive stage is the most sensitive stage to drought stress (Nielsen and Nelson, 1998), whether it takes place during flower formation (Pedroza and Muñoz, 1993), full flowering (Pimentel et al., 1999), pod formation (Castañeda et al., 2006), or grain filling (Nielsen and Nelson, 1998). This is because the water deficit causes falling or abortion of reproductive structures (Acosta and Kohashi, 1989), as it occurs with the pistil in soybean (Glycine max L.; Kokubun et al., 2001) and pollen in dry bean (Shen and Webster, 1986), which results in a low number of pods per plant (Dornbos et al., 1989; Boutra and Sanders, 2001) and seeds per pod (Nielsen and Nelson, 1998).
As a consequence of a low seed production during drought stress the average yield is reduced (Acosta and Kohashi 1989; Acosta et al., 2004; Núñez et al., 2005). In many legume species experimenting water deficit during the flowering and grain filling stages the average yield may show reductions of 40-60% compared with irrigated plants (Acosta and Kohaski, 1989; Nilsen and Nelson, 1998). Yield reduction may be a result of losses in pods per plant, low number of seed per pod and low seed weight (Núñez et al., 2005). Acosta et al. (2004) found an average yield reduction of 53% in eight varieties of dry bean of different origin and growth habit under drought stress, compared with the negative control in which five irrigations levels were applied. Nielsen and Nelson (1998) also reported reductions of 695 and 940kg·ha-1 in black bean plants subjected to drought stress during the flowering and grain filling stages, respectively, in relation to the control plants under irrigation. Similarly, Acosta and Kohashi (1989) found 42 and 50% yield reductions in Bayo Calera and Ojo de Cabra varieties when the drought stress was imposed from the end of the vegetative stage through physiological maturity. Nuñez et al. (2005) registered 60% of yield reduction in dry bean, which was attributed to losses of 63.3% in pods per plant, 28.9% in seeds per pod, and 22.3% in seed weight.
Water is the main limiting factor for dry bean (Phaseolus vulgaris L.) production under rainfed conditions in Mexico, causing significant yield reductions (Pérez et al., 1999). Under drought stress conditions, dry bean presents morphological plasticity characterized by overproduction of reproductive structures (Acosta et al., 2003) and by physiological changes, such as reduction of stomatal conductance (Pattanagul and Madore, 1999). This in turn causes a decrease in transpiration (Vieira et al., 1992) and photosynthesis (Pattanagul and Madore, 1999) and losses of sugars utilized to support growth and development (Pattanagul and Madore, 1999). In México, over 1´106ha are planted with dry bean, mainly at the highland northern plains 1800-2200masl, with annual mean precipitation of 200-400mm (Schneider et al., 1997). In this region, farmers utilize seed from the previous cycle, whose physiological quality is unknown. In dry bean, a stress of 30 days imposed after flowering caused reductions of 24 and 19% in the weight and volume of 100 seeds (Pérez et al., 1999), and Heatherly (1993) reported germination of soybean below 80% after a drought stress imposed during the reproductive stage. In contrast, Vieira et al. (1992) did not detect effects of a similar drought on seed germination and vigor, even though the number of immature, wrinkled, and opaque-coat seed was high. In the present study the physiological responses of dry bean on plant, yield and its components, and on the physical and physiological quality of the seed harvested are evaluated in plants subjected to drought stress during the stages of flowering, pod formation and seed filling.
Materials and Methods
The study was carried out under greenhouse conditions at Montecillo, State of México (19º29N, 98º54W, and 2250masl), using the dry bean (Phaseolus vulgaris L. cv. Otomí) of determinate growth habit, which is recommended for the semiarid highland plains of México (Schneider et al., 1997). Seeds were planted into 6 l plastic containers, using a mixture of loam soil, river sand, peat moss and agrolite (2:2:1:1) as substrate. The field capacity (FC) and the permanent wilting point (PWP) of the substrate were determined through the pressure pot and the pressure membrane, and a moisture retention curve was generated.
Drought stress treatments were applied as follows: 1) at the R6 stage, during flowering (F); 2) at the R7 stage, pod formation (PF); 3) at R8, seed filling (SF), and 4) control, under irrigation (I). For the stress treatments, water supply was suspended until reaching the PWP + 10 days, which is equivalent to 11.5% of the moisture content of the substrate. At the end of the stress, periodic irrigation was resumed. The control was maintained at field capacity (22.5% moisture). Leaf and pod water potentials (Yl and Yp) were determined at each stage using a Scholander pump model A699 (Soil Moisture Equipment Corp., Santa Barbara, CA, USA).
Treatments were distributed under a randomized complete blocks design with three replications, where the experimental unit was a group of 20 pots with a single plant per pot. Each pot was daily weighed during plant development and the amount of consumed water was estimated through the difference of weights from consecutive days. Then, based on the moisture retention curve, the required amount of water was supplied through irrigation for maintaining the substrate at field capacity (22.5%), except during the stress periods. The mean values of temperature and relative humidity inside the greenhouse during the growth season ranged 17-23°C and 57-75%, respectively.
Physiological traits
Net photosynthesis rate (μmol CO2·m-2·s-1), stomatal conductance (mmol H2O·m-2·s-1) and transpiration rate (mmol H2O·m-2·s-1) were measured between 11:00 and 13:00 under illuminated conditions with a portable apparatus LI-6400 (LICOR Inc., Lincoln, NE, USA). Foliar respiration (μmol CO2·m-2·s-1) was also measured with the same instrument after covering the assimilation chamber with a plastic card until total darkness, then allowing a 100s period to reach equilibrium. The five readings were collected from a leaf of the upper stratum and another one from the lower stratum of the plant, in each block, at -2 days (prior to stress) and at 3, 5 and 10 days during stress, plus an additional measurement 8 days after the recovery irrigation. During the measurements across all above mentioned dates, the photosynthetic active radiation varied from 1125 to 1500μmol·m-2·s-1, the vapor pressure deficit ranged 2-5kPa, and the air temperature from 19 to 23oC.
Yield and its components
The harvest of pods was carried out at two periods, on November 24th for treatments I, PF and SF, and one month later for treatment F, due to the fact that plants delayed flowering as a result of stress. The harvested pods were dried at room temperature; then seed yield per plant (g), number of pods per plant, seeds per plant, seeds per pod and weight of pod (g) were determined.
Physical and physiological quality of the seed
The physical quality was quantified through the weight of 1000 seeds (WTS), according to Moreno (1984), except that four replications of 100 seeds were used per treatment. The physiological quality was determined through: 1) Standard germination test (ISTA, 2005), in four replications of 25 seeds, using sand as substrate, and measured in a single count carried out 9 days after the start of the test, considering only normal seedlings; 2) Electrical conductivity test (EC), used as an indicator of membrane damages, performed according to the ISTA protocols (ISTA, 2005) recommended for pea, in four replications of 50 seeds, after being weighed and placed into 250ml of deionized water at 21ºC for 24h; the readings were then taken with an Oakton meter WD-35607-00 (Singapore) and the electrical conductance (Ms·cm-1) was calculated using the equation EC= reading of the target/weight of the seed (g)= Ms·cm-1·g-1; 3) Accelerated aging test, another seed vigor test, was performed according to the protocol of the ISTA (2005) recommended for soybean, in four replications of 25 seeds placed on a screen inside a plastic box that contained 40ml of de-ionized water, and then incubated at 41ºC for 72h; afterwards, an standard germination test was performed, and at the end of the test the average weight per seedling (mg) was obtained after they were dried at 70ºC for 76h.
The data from each date were analyzed with the SAS (Statistical Analysis System) program version 6.12, through analysis of variance of randomized complete blocks design, and treatments compared by a multiple means comparison test (Tukey, p<0.05). It should be noted, however, that the data were not submitted to homogeneity or normality tests.
Results and Discussion
Water potential (Y)
At the end of the stress treatments, leaf water potential (Yl) values were -1.1, -1.1, -1.2 and -0.6MPa for F, PF, SF and I, respectively; and in pods (Ypod) they were -1.2, -1.5 and -0.7MPa for PF, SF and I, respectively. Such results indicate that drought caused large reductions of the Y in both organs, in relation to the control under irrigation, but pods maintained a lower Y than leaves in all treatments, possibly to favor sap flow towards the pods. Acosta and Kohashi (1989) also reported values of Yl of -1.5MPa in dry bean leaf subjected to drought stress for 15 days at the onset of flowering. In chickpea (Cicer arietinum L.) pods, Ma et al. (2001) registered a Yl of -1.4MPa after applying drought stress during 10 days. In maize (Zea mays L), Schussler and Westgate (1991) consider that a moderate drought stress during flowering corresponds to a leaf Yl of -0.7MPa, and a severe one to -1.1MPa. Therefore, the stress applied to dry bean in this study was somewhere between moderate and severe. No reports on Ypod were found.
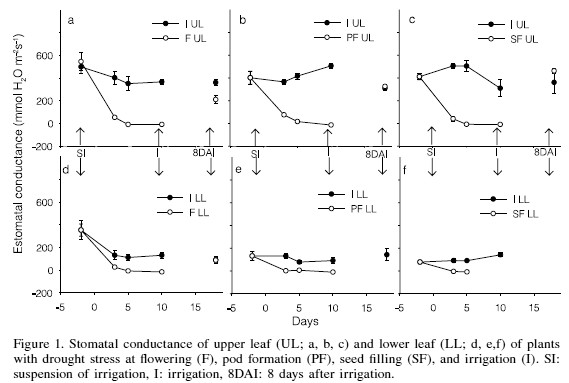
Stomatal conductance
The drought imposed at the three stages of crop development drastically decreased leaf stomatal conductance (gs) in both upper and lower strata of the plant. In the upper stratum, gs decreased after 3 days under stress to 350 (75%), 291 (87%) and 466 (92%) mmol H2O·m-2·s-1 in F, PF and SF, respectively (Figure 1a, b and c). Then, from the 5th day through the end of the stress period, gs reached zero in the stress treatments. In the lower stratum, after 3 days under stress the gs had already decreased 79, 99 and 100%, in F, PF and SF, respectively, while in the rest of the stress period gs was zero (Figure 1d, e and f), implying a more sensitive stomatal closing than in upper leaves. At the 10th day, plants of the SF treatment had already lost their lower leaves, possibly because the leaves were older, and therefore more sensitive to stress, presenting an early senescence (Brevedan and Egli, 2003).
Miyashita et al. (2005) also registered a rapid decrease of gs in kidney bean after 2 days of stress, with values close to zero at the 5th day, and zero at the 7th day of drought stress. Stomatal closing can also occur with a high leaf water potential due to signals from the root, as has been proposed by Miyashita et al. (2005).
The lower recovery rate of gs observed in the upper stratum in F is attributed to the fact that the plants under this treatment began forming new leaves and new flowers, so that the previous leaves possibly became suppliers of water and nutrients for the newly constituted tissues; on the other hand, in the lower stratum of plants in F, gs had completely recovered. As previously indicated, in PF and SF the lower leaves had already fallen down, probably because they were more mature than in F, implying that younger leaves are more resistant to drought, having greater capacity of osmotic adjustment, as pointed out by Turner and Jones (1980).
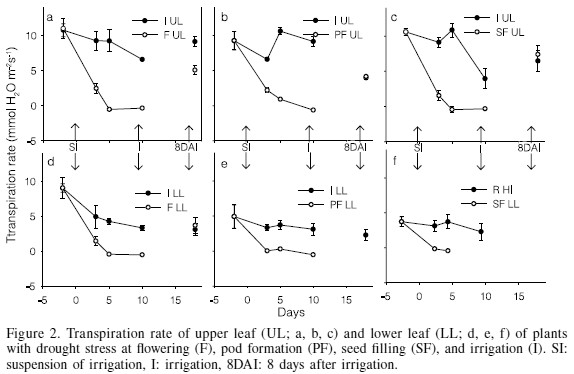
Transpiration rate
Parallel to gs, transpiration (E) decreased as a result of drought in both strata of the plant. In the upper stratum, after 3 days under drought stress the transpiration rate decreased to 6.8 (73%), 4.4 (66%) and 7.6 (83%) mmol H2O·m-2·s-1 at F, PF and SF, respectively (Figure 2a, b, c). From the 5th day through the end of the stress, E became zero in all the stress treatments. Similar effects of drought stress were reported by Miyashita et al. (2005) for kidney bean, with transpiration rates of zero at the 7th day of stress, and by Dornbos et al., (1989) for soybean.
Damages were more severe in the lower stratum, in such a magnitude that after the 3rd day of stress leaf transpiration rate decreased by 71, 99 and 100% at F, PF and SF, respectively; and from the 5th day on it reached zero for all the treatments; remarkably, plants stressed at SF had lost their leaves at the 10th day under stress (Figure 2d, e, f).
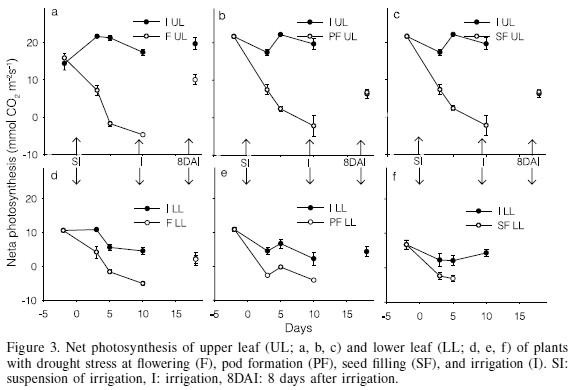
Net photosynthesis
The photosynthesis rate (A) under drought and post-drought recovery varied in a very similar manner as conductance and transpiration. After 3 days of stress, net photosynthesis of the upper stratum decreased by 67, 57 and 75% at F, PF and SF, respectively (Figure 3a, b, c), and after the 5th day photosynthesis was completely inhibited in all the treatments, except for PF, where the inhibition was 89% at the 5th day. Miyashita et al. (2005) also reported a rapid decrease in the photosynthetic rate of kidney bean, just after 2 days of drought stress. According to Brevedan and Egli (2003), drought stress during seed filling causes a rapid reduction of the assimilation rate of carbon, registering 0μmol·m-2·s-1 within 15 days.
Photosynthetic activity in the lower stratum decreased more rapidly than in the upper one. By the third day it had decreased 61, 100 and 100% at F, PF and SF, respectively, and after the fifth day the inhibition was complete in all the treatments (Figure 3d, e, f). Reductions in photosynthesis coincide with those reported by several authors such as Dornbos et al. (1989) and Brevedan and Egli (2003) in soybean, Castañeda et al. (2006) in dry bean subjected to drought stress during seed filling, Pattanagul and Madore (1999) in Coleus blumei subjected to drought stress in 2-month old plants, and by Schussler and Westgate (1991) in maize (Zea mays L.) under moderate (-0.7MPa) and severe (-1.1MPa) drought stress during flowering. Such reductions in photosynthetic assimilation rates, as well as those of transpiration, are strongly related with stomatal closure, as also indicated by Cruz de Carvalho et al. (1998).
After 8 days from the recovery irrigation, gs, E and A of the upper leaves had completely recovered in PF and SF treatments, whereas at F they had only recovered by 58, 55 and 51%, respectively (Figures 1a, 2a, and 3a). The large similitude of responses among gs, E and A is due to the fact that both E and A are gas exchange processes occurring through the stomata pores, so that the smaller the value of gs the smaller would be E and A, and vice versa (Hsiao, 1973). The recovery levels achieved in F are similar to those registered in kidney bean by Miyashita et al. (2005), who reported that the recovery of the physiological processes of bean improves as drought stress is lowered; with a stress of -0.6MPa the recovery is 100% with a stress of -1.2MPa recovery is 80, 60 and 40% for the photosynthetic rate, transpiration rate and stomatal conductance, respectively; with a stress of -1.9MPa recovery is only 50, 35 and 15%. In the lower stratum, the gs, TR and NP completely recovered in F, the only treatment in which plants retained lower leaves after the drought. This is attributed to the ability of these leaves to rehydrate and to prevent damages of the chloroplasts.
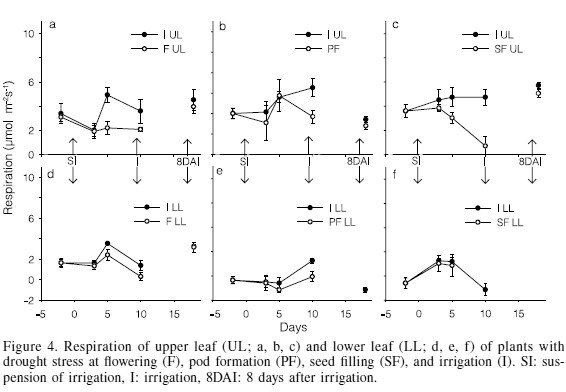
Respiration
Contrary to the previous processes, foliar respiration experienced small changes due to drought stress, probably because respiration is a physiological process indispensable to maintain the cells alive as it provides the chemical energy for metabolic processes, particularly under low or null photosynthesis. In the upper stratum, significant effects did not appear until 5 days of stress, decreasing by 55 and 36% at F and SF, respectively; after 10 days reductions were of 42, 62 and 85% at F, PF and SF, respectively (Figure 4a, b, c). It is thus confirmed that the respiratory process is more resilient to stress than photosynthesis, transpiration, and stomatal conductance (Hsiao, 1973). In the lower stratum, drought stress did not cause significant reductions in the first 5 days, except for F, where respiration decreased 32%; after 10 days reductions were of 77 and 41% at F and PF, respectively, and at SF there were no leaves. Castañeda et al. (2006) also found small changes in respiration due to the effect of drought stress during seed filling in dry bean cv. "Negro Precoz". At the 8th day after the recovery irrigation, respiration of both upper and lower strata completely recovered in all the treatments with remaining leaves (Figure 4d, e, f).
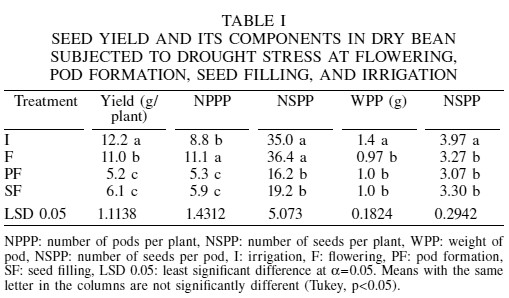
Yield and yield components
Drought stress caused losses in yield of 1.2g (10%), 7.0g (57%) and 6.1g (50%) per plant at F, PF and SF, respectively, with respect to the control under irrigation (Table I). It is thus inferred that the Otomí bean is much more sensitive to drought stress during PF and SF than during F. Castañeda et al. (2006) also reported higher sensitivity of another dry bean variety to drought stress at the pod formation stage. Deproost et al. (2004) observed that a moderate stress imposed during flowering caused yield increases of 30-70% in relation to the control without stress.
At PF, the loss in yield was caused by the reduction of 54, 40 and 23% in the number of seeds per plant, pods per plant and seeds per pod, respectively. At SF, the loss in yield was a result of a reduction of 45% in the number of seeds per plant, as a consequence of a reduction of 33% in the number of pods per plant and 19% in seeds per pod (Table I). The effect of drought stress on the weight of pod was essentially the same in all the three developmental stages, as reductions were 31, 29 and 29% at F, PF and SF, respectively. Acosta and Kohashi (1989), Nielsen and Nelson (1998) and Nuñez et al. (2005) also identified the number of pods per plant as the principal cause of yield losses of bean subjected to drought stress, followed by the number of seeds per pod and seed weight.
The low decrease (10%) in yield at F, despite of 31 and 18% reductions in weight of pod and number of seeds per pod, was due to partial compensation through increases of 26 and 4% in the number of pods and seeds per plant (Table I), implying that during flowering the bean plant still has the opportunity to modify its structure when it sets the new flowers and pods generated in post-drought in the upper part of the plant, provided that only the lower leaves are affected. Núñez et al. (2005) also observed that the most severe effect of the drought from the end of the vegetative stage through physiological maturity was located in the branches of lower nodes, where abortion occurred over 100% of pods, while in the upper nodes (nodes 5 to 10) only 15% of abortion occurred.
It should be taken into account that the water deficit imposed at F caused flowering delaying by one month, suggesting a mechanism of ontogenetic resistance to drought, as pointed out by Pedroza and Muñoz (1993). Boutra and Sanders (2001) also reported that drought stress during flowering retards the development of ovules in bean and detains growth.
It was observed that reduction in seed yield is closely associated to the inhibition of net photosynthesis and, consequently, to the production of photoassimilates in the treatments with no formation of new leaves (i.e. PF and SF). Such an inhibition might have reduced the supply of nutrients toward reproductive organs, as pointed out by Raper and Kramer (1987).
The results allow to infer that the highest tolerance to drought of dry bean is ontogenic, because the severe drought stress imposed at flowering causes much less damage in yield than that at later stages, as the plant has the opportunity to continue developing after the drought, even though the effects of the drought on A, E, and gs are equally severe in all three studied phenological stages.
Seed quality
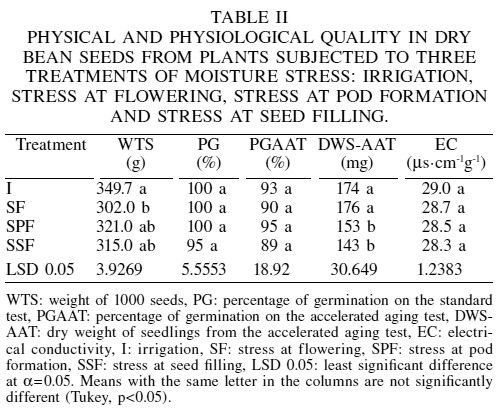
Regarding physical quality, drought caused reductions of 14, 8 and 10% in the weight of 1000 seeds during F, PF and SF, with respect to the control (Table II). França Neto et al. (1993) in soybean and Pérez et al. (1999) in dry bean reported a similar effect of drought stress applied during seed filling. The physiological quality of the seed, measured as percentage of normal seedlings obtained through the standard germination test and accelerated aging test, was not significantly affected by drought stress (Table II). Seed vigor measured through the dry weight of the seedling was reduced by 12 and 18% after imposing the accelerated aging test, but only in plants to which drought had been imposed at PF and SF. This result shows again that F in bean is the developmental stage most tolerant to drought. Similar effects of drought on seed physiological quality have been reported by Castañeda et al. (2006) in bean, Fougereux et al. (1997) in pea, Ghassemi-Golezani et al. (1997) in maize and sorghum, and Zalewski et al. (2001) in lupin (Lupinus angustifolius L.) and triticale (Triticum ´ Secale).
Electrical conductivity of the seeds showed no significant effects from drought stress imposed during F, PF and SF (Table II), thus indicating that these treatments did not affect the membrane permeability of the seeds. In seeds of maize and sorghum (Sorghum bicolor L. Moench) harvested from plants previously subjected to drought stress, there were also no significant effects in electrical conductance (Ghassemi-Golezani et al., 1997). These results suggest that the drought treatments did not cause significant damages in cell membranes of the bean seed. In contrast, in soybean Dornbos et al. (1989) reported increases of 19% in electrical conductance of seeds from plants subjected to drought stress during seed filling.
Given that the physiological quality of seeds was not affected by drought stress, even though their size was reduced, it is possible to infer that drought caused losses in reserves rather than cellular damage in the embryonic axis. In contrast, Dornbos et al. (1989) found reductions of 12% in germination and of 5% in the vigor of seed harvested from soybean plants subjected to severe drought stress in the stage of reserve accumulation; and Lin and Markhart (1996) also detected reductions of 11% in the germination of seeds of two species of bean (P. vulgaris and P. acutifolius) grown under conditions of drought and high temperature stresses.
Conclusions
The drought stress applied to dry bean plants of the Otomí variety reduces water potential of leaves and pods by almost half in both upper and lower strata of the plant and at the three studied phenological stages. The reduction of foliar Yl completely inhibits stomatal conductance and, consequently, transpiration and photosynthesis. Respiration is more tolerant to stress than the other physiological processes evaluated. Drought reduced seed yield, with 5-6 fold losses when it occurred at PF and SF than at F, so that the F stage is more tolerant to drought stress than the PF and SF stages. Reductions in yield are caused by reductions in number of pods and number of seeds per plant, weight and number of seeds per pod, and weight of seeds, except for F, where a 26% increment in the number of pods per plant was observed. Reductions in yield are closely associated to photosynthetic inhibition, except for F. Ontogenetic tolerance to drought in bean presented at flowering is attributed to the fact that leaves were younger and that flowering was delayed by a month and resumed when there was no drought.
The drought stress applied decreased the amount of accumulated reserves between 8 and 12% in the seed, without affecting either the germinative capacity of the embryo or the integrity of its cellular membranes.
ACKNOWLEDGEMENTS
The authors thank Efraín Acosta Díaz, Experimental Station of Calera, Zacatecas, Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) for the donation of the bean seeds cv. Otomí.
REFERENCES
1. Acosta DE, Acosta GJA, Padilla RJS (2004) Relación raíz-vástago en frijol bajo dos condiciones de humedad. Agric. Técn. Méx. 30: 63-73. [ Links ]
2. Acosta DE, Amador RMD, Acosta GJA (2003) Abscisión de estructuras reproductoras en frijol común bajo condiciones de secano. Agric. Técn. Méx. 29: 155-168. [ Links ]
3. Acosta GJA, Kohashi SJ (1989) Effect of water stress on growth and yield of indeterminate dry-bean (Phaseolus vulgaris) cultivars. Field Crops Res. 20: 81-93. [ Links ]
4. Boutra T, Sanders FE (2001) Influence of water stress on grain yield and vegetative growth of two cultivars of bean (Phaseolus vulgaris L.). J. Agron. Crop Sci. 187: 251-257. [ Links ]
5. Brevedan RE, Egli DB (2003) Short periods of water stress during seed filling, leaf senescence, and yield of soybean. Crop Science. 43: 2083-2088. [ Links ]
6. Castañeda SMC, Cordova TL, González HVA, Delgado AA, Santacruz VA, García de los SG (2006) Respuestas fisiológicas, rendimiento y calidad de semilla en frijol sometido a estrés hídrico. Interciencia 31: 461-466. [ Links ]
7. Cruz de Carvalho M, Laffray D and Louguet P (1998) Comparison of the physiological responses of Phaseolus vulgaris and Vigna unguiculata cultivars when submitted to drought conditions. Env. Exp. Bot. 40: 197-207. [ Links ]
8. Deproost P, Elsen F, Geypens M (2004) High yields of mechanically harvested snap beans as induced by moderate water stress during flowering. Acta Hort. 664: 205-212. [ Links ]
9. Dornbos DL, Mullen RE, Shibles RM (1989) Drought stress effects during seed fill on soybean seed germination and vigor. Crop Sci. 29: 476-480. [ Links ]
10. Fougereux JA, Doré T, Ladonne F, Fleury A (1997) Water stress during reproductive stages affects seed quality and yield of pea (Pisum sativum L.). Crop Sci. 37: 1247-1252. [ Links ]
11. França Neto JB, Krzyzanowski FC, Henning AA, West SH, Miranda LC (1993) Soybean seed quality as affected by shriveling due to heat and drought stresses during seed filling. Seed Sci. Technol. 21: 107-116. [ Links ]
12. Ghassemi-Golezani K, Soltani A, Atashi S (1997) The effect of water limitation in the field on seed quality of maize and sorghum. Seed Sci. Technol. 25: 321-323. [ Links ]
13. Heatherly LG (1993) Drought stress and irrigation effects on germination of harvested soybean seed. Crop Sci. 33: 777-781. [ Links ]
14. Hsiao TC (1973) Plant responses to water stress. Annu. Rev. Plant Physiol. 24: 519-570. [ Links ]
15. ISTA (2005) International Rules for Seed Testing. International Seed Testing Association. Bassersdorf, Switzerland. 243 pp. [ Links ]
16. Kokubun M, Shimada S, Takahashi M (2001) Flower abortion caused by preanthesis water deficit is not attributed to impairment of pollen soybean. Crop Sci. 41: 1517-1521. [ Links ]
17. Lin TY, Markhart AH (1996) Phaseolus acutifolius A: Gray is more heat tolerant than P. vulgaris L. in the absence of water stress. Crop Sci. 36: 110-114. [ Links ]
18. Ma Q, Behboudian MH, Turner NC, Palta JA (2001) Gas exchange by pods and subtending leaves and internal recycling of CO2 by pods of chickpea (Cicer arietinum L) subjected to water deficits. J. Exp. Bot. 52: 123-131. [ Links ]
19. Miyashita K, Tanakamaru S, Maitani T, Kimura K (2005) Recovery responses of photosynthesis, transpiration, and stomatal conductance in kidney bean following drought stress. Env. Exp. Bot. 53: 205-214. [ Links ]
20. Moreno ME (1984) Análisis Físico y Biológico de Semillas Agrícolas. Universidad Nacional Autónoma de México. 382 pp. [ Links ]
21. Nielsen DC, Nelson NO (1998) Black bean sensitivity to water stress at various growth stages. Crop Sci. 38: 422-427. [ Links ]
22. Núñez BA, Hoogenboom G, Nesmith DS (2005) Drought stress and distribution of vegetative and reproductive traits of a bean cultivar. Sci. Agric. 62: 18-22. [ Links ]
23. Pattanagul W, Madore MA (1999) Water deficit effects on raffinose family oligosaccharide metabolism in Coleus. Plant Physiol. 121: 987-993. [ Links ]
24. Pedroza FJA, Muñoz OA (1993) Resistencia ontogénica y filogenética a sequía en Phaseolus vulgaris L. I. Caracteres vegetativos. Agrociencia. Ser. Fitociencia 4: 19-33. [ Links ]
25. Pérez HP, Acosta DE, Padilla RS, Acosta GJ (1999) Effect of drought on seed quality of common bean (Phaseolus vulgaris L.). Agric. Técn. Méx. 25: 107-114. [ Links ]
26. Pimentel C, Hebert G, Silva JVda (1999) Effects of drought on O2 evolution and stomatal conductance of beans at the pollination stage. Env. Exp. Bot. 42: 155-162. [ Links ]
27. Raper CD, Kramer PJ (1987) Stress physiology. In Wilcox JR (Ed.) Soybeans: Improvement, Production, and Uses. 2nd ed. ASA/CSSA/SSSA, Madison, WI, USA. 589-641 pp. [ Links ]
28. Schneider KA, Rosales-Serna R, Ibarra-Pérez FJ, Cazares-Enríquez B, Acosta-Gallegos JA, Ramírez-Vallejo P, Wassimi N, Kelly JD (1997) Improving common bean performance under drought stress. Crop Sci. 37: 43-50. [ Links ]
29. Schussler JR, Westgate ME (1991) Maize kernel set at low potential. I. Sensitivity to reduced assimilates during early kernel growth. Crop Sci. 31: 1189-1195. [ Links ]
30. Shen XY, Webster DB (1986) Effect of water stress on pollen of Phaseolus vulgaris L. J. Am. Soc. Hort. Sci. 111: 807-810. [ Links ]
31. Turner NC, Jones MM (1980) Turgor maintenance by osmotic adjustment: a review and evaluation. In Turner NC, Kramer PJ (Eds.) Adaptation of Plants to Water and High Temperature Stress. Wiley. New York. 87-104 Pp. [ Links ]
32. Vieira RD, TeKrony DM, Egli DB (1992) Effect of drought and defoliation stress in the field on soybean seed germination and vigor. Crop Sci. 32: 471-475. [ Links ]
33. Zalewski K, Lahuta LB, Horbowicz M (2001) The effect of soil drought on the composition of carbohydrates in yellow lupin seeds and triticale kernels. Acta Physiol. Plant. 23: 73-78. [ Links ]












 uBio
uBio