Investigación Clínica
versão impressa ISSN 0535-5133
Invest. clín vol.55 no.2 Maracaibo jun. 2014
In vitro assessment of commercial sunscreens available in Latin America.
Juan Pablo Castanedo-Cázares, Karla Martínez-Rosales, Diana Hernández-Blanco, Guillermo Valdés-Rodríguez y Bertha Torres-Álvarez.
Departamento de Dermatología, Hospital Central Dr. Ignacio Morones Prieto, Universidad Autónoma de San Luis Potosí, San Luis Potosí, México.
Corresponding author: Bertha Torres Álvarez. Departamento de Dermatología, Hospital Central Dr. Ignacio Morones Prieto. Venustiano Carranza No. 2395, Zona Universitaria, 78210, San Luis Potosí, S.L.P. México. Telephone: +52 444 8342795. E-mail: torresmab@yahoo.com.mx
Abstract. In Latin America, people have largely abandoned the practice of wearing hats and traditional clothing that provided skin protection. Sunscreen application has therefore become essential to protect against the increased sun exposure. The physician-prescribed medical-grade sunscreens provide sufficient sun protection but the requirement for regular use puts a financial burden on the patient that is often not sustainable. An appropriate sunscreen should provide a high and broad ultraviolet (UV) protection against UVB and UVA. Several over-the-counter (OTC) sunscreens have been developed for sale at affordable prices and are available for purchase in convenient locations, such as local grocery stores. The aim of this study was to assess the in vitro UV protection of 34 popular OTC sunscreens found in the Latin American market. UV absorbance/transmittance was quantified by diffusion transmission spectroscopy using coarse silica plaques. Photostability was tested by irradiating them with simulated solar light and calculating the sun protection factor (SPF), critical length of absorption (C λ ), UVA/UVB ratio, and the spectral uniformity index (SUI). The results indicated that the in vitro SPFs were significantly lower than the value declared on the labels, particularly for those claiming high SPF values; however, the majority of these sunscreens offered high levels of UV protection. Considering the advantages of low cost and ample accessibility, we concluded that this sample of OTC sunscreens can be beneficial to the general public by providing some level of skin protection from solar radiation, and may be promoted to improve compliance with recommended photoprotection behavior.
Keywords: sunscreens, absorbance, solar radiation, ultraviolet radiation.
Valoración in vitro de protectores solares comerciales existentes en Latinoamérica.
Resumen. En Latinoamérica, la población ha abandonado la costumbre de usar sombrero y ropa tradicional para protegerse del sol. En consecuencia, es básico el uso de protectores solares si se realizan actividades bajo sol. Los protectores solares que se usan en la práctica médica son adecuados, pero su uso frecuente condiciona una carga económica que muchos pacientes no pueden solventar debido a sus costos considerables. Un protector apropiado contiene una amplia y elevada protección ultravioleta (UV) A y B. En las tiendas de conveniencia, existen numerosos protectores solares a precios más accesibles. El objetivo del estudio fue determinar la protección UV in vitro de 34 protectores solares con amplia presencia comercial (de venta sin prescripción médica) en el mercado latinoamericano. La absorbancia/transmitancia de la radiación UV se cuantificó mediante espectroscopía de transmisión difusa. Placas de sílice esmerilado fueron recubiertas con el producto y expuestas a radiación solar simulada para conocer su fotoestabilidad. Se calcularon índices como el factor de protección solar (SPF), longitud crítica de absorción (C λ), relación UVA/UVB y el índice de uniformidad espectral (SUI). Se encontró que el SPF in vitro fue inferior al establecido en las etiquetas, especialmente en aquellos con valores altos. No obstante, la mayoría de los protectores incluidos ofrecen niveles de protección UV elevados. Considerando su amplia accesibilidad y menor costo, concluimos que esta muestra comercial de protectores solares podría utilizarse en el entorno clínico para favorecer su apego junto a las otras medidas de fotoprotección sugeridas.
Palabras clave: filtros solares, absorbancia, radiación solar, radiación ultravioleta.
Received: 06-08-2013. Acepted: 20-04-2014
INTRODUCTION
Mexico, as most Latin American countries, is located in a tropical area of the world; the longer sunlight hours equate to more intense exposure to solar radiation (1, 2). The ultraviolet index in central Mexico is high or extreme most of the year (1, 2) and it is estimated that approximately one-half of the inhabitants are sun-exposed for more than one hour per day and without protective interventions (2, 3). In our region of Mexico, people are generally unaware of the geographic and environmental conditions that lead to their being at greater risk of damage from solar radiation; moreover, the modern-day lifestyle has abandoned the ancient practices of wearing traditional clothing, such as hats and shawls, which provide protection from the sun (1-3). Therefore, there is an urgent need to develop and implement complementary measures of sun protection, such as the use of sunscreens, which are important tools for achieving adequate photoprotection (4-6).
The damaging effects of UVB radiation (290-320 nm) exposure are well recognized and include carcinogenesis (5-8), genetic mutations (7, 8), photoaging (6, 7, 9), immunosupression (6, 7, 10) and ocular cataracts (11). On the other hand, UVA radiation (320-400 nm, which is further divided into the two wave ranges of I: 340-400 nm and II: 320-340 nm), has deleterious synergy with the UVB segment, in addition to being capable, on its own, of inducing immunosupression (8, 10), photoaging (9, 12, 13), phototoxicity and photoallergic reactions (12, 13). As such, a minimal photoprotective measure should at least eliminate these two segments of UV radiation (8-13).
Multiple topical products, known generally as ‘sunscreens’, have been developed to provide a temporary physical barrier against the sun’s UV radiation and are readily available in the global market. Most have been designed to protect against UVB radiation, and their level of protection is represented by the sun protection factor (SPF) (14, 15). The SPF value of a product is calculated as the UV energy required to produce a minimal erythema dose on protected skin divided by the UV energy required to produce a minimal erythema dose on unprotected skin (14, 15). The SPF value is a key marketing feature that drives sales and products with higher values (up to 50 SPF) enjoy greater consumer preference; however, studies have shown that the SPF level of 30 is suitable for absorbing 97% of the UVB and that the SPF index does not correlate with the level of UVA protection met by the product (6, 13, 16, 17). Due to these drawbacks of commercially available (over-the-counter, OTC) sunscreens, dermatologists generally consider OTC products to provide uncertain protection and consequently recommend medical-grade sunscreens, which also include UVA protection (17). A major disadvantage of the medical-grade sunscreens is their remarkably higher cost than the OTC products, which may represent an insurmountable financial burden to some patients. This issue becomes more evident when the consumer considers the requirement of all sunscreens to be reapplied constantly (i.e., every 2 h) and that the effective amount of product to be applied is 2 mg/cm2 (15), which leads to rapid consumption of the product and the need to obtain another prescription.
Recognizing commercial products that offer adequate UV protection is likely to have an important impact on the daily medical practice in dermatology, since dermatologists could recommend accessible and reliable products that reduce the economic burden on the patient and favor compliance with photoprotective behaviors. Thus, the aim of this study was to investigate the photoprotective efficacies, including UV protection, UVB/UVA balance protection and photostability, in a panel of commercially popular sunscreens available in self-service stores in Latin America by determining the internationally accepted indices of photoprotection in an in vitro system.
MATERIALS AND METHODS
Thirty-four commercially available OTC sunscreens were obtained from retail stores, but not drugstores, in the Mexican market in July 2013. The products were selected based on the labeling explicitly stating their presence in more than one Latin American country. The information contained within the label legends was recorded and included the product’s SPF value, manufacturing company, active ingredients (recorded using the United States Adopted Names (USAN) classification), special features (i.e., hypoallergenic, waterproof), and the topical formulation (i.e., cream, lotion, gel). Sunscreen products sold by catalogue or as part of a cosmetic brand were excluded.
After purchase, all products were stored at room temperature without environmental stimuli for no more than 24 hours before assessment. The samples were prepared by one staff member and another researcher conducted the subsequent testing, so that the study was carried out with double-blind experiments. Following the USA Federal Drug Administration (FDA) dose recommendations (15), 2 mg/cm2 of each sunscreen was applied to 10 cm2 quartz plates with an average 18 µm surface roughness and 2 mm thickness, as previously reported by Akrman et al. (18). Briefly, fused quartz plaques were obtained from Edmund Industrial Optics (Barrington, NJ, USA). The plaques were then prepared to simulate the human skin’s surface topography by the Petrography Laboratory of the Geology Institute at The Autonomous University of San Luis Potosí (México) using a process of sand-blasting with compressed air using silica grains, followed by a gradual finer grinding with emery paper (18). The integrity of the surface topography was validated by microscopic observation and roughness analysis. Although this is not a universally accepted method, there are currently no recognized alternatives available. Our group has used this substrate to test sunscreens over the past five years due to its reproducibility and re-use compared to other methods. As Akrman et al. reported, a very close approximation to actual in vivo SPF tests is obtained, though not always to the SPF levels indicated on labels (14, 16-18).
Application of the product was performed by first placing the prepared plaque on an analytical balance scale and setting droplets of the respective sunscreens in a uniform pattern of 5 ´ 5 dots. The emulsions were then spread by circular finger motions under a slight pressure of 1.5 g/cm2 (18) and the samples were fixed by heating at 21°C in 50% relative humidity for 15 min to prepare for subsequent UV spectrophotometer measurements.
All products were evaluated before and after UV radiation exposure with a xenon 16S150 watt Solar Simulator (Solar Light, Inc., Glenside, PA, USA). The dose adjusted to the erythemally weighted radiation was 180 mJ/cm2, which is equivalent to the average environmental conditions in the city of San Luis Potosi, Mexico, during the daytime hours of 12:00 to 2:00 p.m. during the month of May (1, 2). The UV absorption spectrum for each product was obtained using an Evolution 600 UV-Visible Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), which is able to detect wavelengths from 200 to 1,200 nm. The values used for analysis were the average of at least five determinations for each sample. Readings for the spectral segment of 290-400 nm were imported into an Excel worksheet (Microsoft, Redmond, WA, USA) for calculation.
To assess the UVB protection of each of the sunscreens analyzed, the in vitro SPF was calculated using spectrophotometric measurements of transmission. Its determination was based on the background using the SPF as a relative index (19). The SPF was calculated using the following equation:
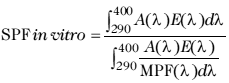
where d(l) is 1 nm, A(l) represents the erythema action spectrum, E(l) represents the sun’s radiation power, MPF is the inverse of the transmission (1/T) at a given wavelength (19) and MPF (l) represents how much radiation is absorbed and the ability of the skin to be damaged. This in vitro testing approach was chosen for the current study since previous comparative analyses of in vivo SPF and in vitro SPF results showed a high degree of correlation (R2=0.94) (14). The in vitro SPF testing method is not considered to be an alternative to the in vivo test, but rather an initial screen to evaluate popular or newer sunscreen products, as in vivo tests are expensive, time-consuming and need the supervision by ethics committees.
There is currently no internationally established standard for testing and measuring UVA protection (20); therefore, three reference indices were used: the critical value of absorption (Cl) determined by the critical wavelength method) (21), the UVA/UVB absorbance ratio (22) and the spectral uniformity index (SUI) (23).
The Cl represents the wavelength where the integral of the spectral absorbance curve attains 90% of the integral between 290 and 400 nm (21). The accepted minimum standard of the Cl is the value that reaches 370 nm in length (15, 21), and this value is obtained using the following equation:
![]()
where A is the absorption and lambda (l) is the wavelength. For each absorption spectrum, the integral (∫) represents the absorbance curve (area under the curve, AUC) estimated by trapezoidal integration.
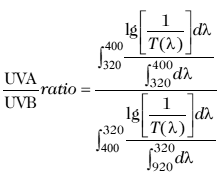
The UVA/UVB absorbance ratio assesses the uniformity of spectral absorbance across the UVB and UVA spectrum. The closer the ratio is towards unity, the higher the UVA rating of the product; therefore, this ratio allowed for the products tested to be classified into five protection levels (described by 0 to 5 stars, respectively): none, 0-0.2; minimal, 0.21-0.4; moderate, 0.41-0.6; good, 0.61-0.8; superior, 0.81-0.9; ultra, >0.91. The absorbance of the product is the sum of the UVB and UVA ranges, and the UVA/UVB absorbance ratio is calculated according to the following equation (22):
The SUI is used to quantify the degree of uniformity present in the UV absorption band and is calculated as the ratio of the sum of absorption from 290 to 380 nm, with its value being the sum of this band minus its average (23). The following equation is used for the calculation:
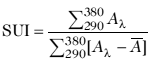
where S is the sum of the 290 to 380 nm wavelength, Al is the spectral absorbance of the sunscreen determined by the in vitro measurement for each nm wavelength (l) by substrate spectrophotometry, and Ā is the average spectral absorbance in the 290 to 380 nm spectral region. The index is interpreted as follows: low, <2; intermediate, 2-4; high, 5-11; very high, >12.
The calculated indices were used in correlation analyses to study the association between the labeled SPF and the number of active ingredients. A t-test was performed to examine differences in values obtained from the before and after irradiation periods. Differences were considered significant if the P value was less than 0.05. All statistical analyses were carried out using the SPSS software package, version 16.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Table I provides a summary of the main features evaluated for all of the products. The most frequent labeled SPF value was 50 (range: 30-110), and 76% (n=26) of the products having an SPF label value of 50 or higher. The samples included 12 active components. The average number of incorporated filters was 4 (range: 3-6). The frequency of each active component identified among the various samples was as follows: avobenzone, 88% (n=30); octocrylene, 82% (n=28); octisalate, 73% (n=25); oxybenzone, 44% (n=15); homosalate, 41% (n=14); bemotrinizol, 38% (n=13); titanium dioxide, 32% (n=11); octinoxate, 17% (n=6); ensulizol, 17% (n=6); iscotrizinol, 5% (n=2); padimate O, 5% (n=2); octyltriazone, 3% (n=1). Considering the total number of filters incorporated in the sample with respect to their absorption spectrum, the proportion of UVB absorbers was 51.7% and of UVA absorbers was 48.3%.
THE SPF, VEHICLE AND INGREDIENTS OF SUNSCREENS EVALUATED IN THIS STUDY, ACCORDING TO INFORMATION PRESENTED ON THE PRODUCT LABEL
| Sample Nº | Product name | Vehicle | SPF | UVB /UVA-II | UVA-I (Broad-band) |
| 1 | Coppertone Water Babies | Lotion | 50 | Hoto, Ocno, OcSto | Avna |
| 2 | Coppertone Sport Oil-Free | Lotion | 30 | Hoto, Ocno, OcSto | Avna |
| 3 | Coppertone Oil-Free | Lotion | 50 | Hoto, Ocno, OcSto | Avna, BZ3 |
| 4 | Hawaiian Tropic Kids Spray | Spray | 50 | Ocno | Avna, BZ3 |
| 5 | Hawaiian Tropic Island Sport Spray | Spray | 30 | Ocno | Avna, BZ3 |
| 6 | Hawaiian Tropic Sheer Touch Spray | Spray | 50 | Ocno | Avna, BZ3 |
| 7 | Hawaiian Tropic Sheer Touch | Lotion | 50 | Ocno | Avna, BZ3 |
| 8 | Hawaiian Tropic Sheer Touch | Lotion | 30 | Ocno | Avna, BZ3 |
| 9 | Hawaiian Tropic Sheer Touch | Lotion | 45 | Ocno | Avna, BZ3 |
| 10 | Hawaiian Tropic Sheer Touch | Lotion | 65 | Ocno | Avna, BZ3 |
| 11 | Hawaiian Tropic Ozono | Lotion | 80 | Ocno | Avna, BZ3 |
| 12 | Hawaiian Tropic Baby Faces | Lotion | 50 | OcSto, Octo | TiO2 |
| 13 | Nivea Sun Invisible Spray | Spray | 30 | Hoto, Ocno, OcSto | Avna |
| 14 | Nivea Sun Sensación Ligera | Lotion | 30 | Hoto, Ocno, OcSto | Avna, BZ3 |
| 15 | Nivea Sun Hidratante | Spray | 30 | Enle, Ocno, OcSto | Avna, Beol |
| 16 | Nivea Sun Hidratante | Lotion | 50 | Enle, Octo | Beol, TiO2 |
| 17 | Nivea Protect & Bronze | Lotion | 60 | Enle, Hoto, Ocno | Avna, Beol |
| 18 | Nivea Sun Pure Sensitive Aloe Vera | Lotion | 50 | Ocno | Avna, Beol, TiO2 |
| 19 | Nivea Sun Kids Hidratante | Lotion | 50 | Enle, PadO, Octo, Isol | Avna, TiO2 |
| 20 | Nivea Sun Kids Swim & Play | Lotion | 60 | PadO, Ocno | Avna, Beol, TiO2 |
| 21 | Nivea Sun Kids Piel Sensible | Spray | 60 | Enle, Hoto, Ocno | Avna, Beol, TiO2 |
| 22 | Nivea Sun Kids Piel Sensible | Lotion | 60 | Ocno | Avna, Beol, TiO2 |
| 23 | Nivea Babies Sun | Lotion | 50 | - | Avna, Beol, TiO2 |
| 24 | Nivea Sun Protect and Refresh | Spray | 50 | Hoto, Ocno, OcSto | Avna |
| 25 | Nivea Sun Pure & Sensitive con Aloe Vera | Lotion | 50 | - | Avna, Beol, TiO2 |
| 26 | Nivea Bloqueador Facial Humectante Contra Arrugas | Lotion | 50 | Enle, Ocna, Octo, Isol | Beol, TiO2 |
| 27 | Banana Boat Kids | Lotion | 50 | Ocno | Avna, TiO2 |
| 28 | Banana Boat Kids Sin Lágrimas | Lotion | 50 | Hoto, Ocno, OcSto, Octo | Beol |
| 29 | Banana Boat Kids Protect and Play | Lotion | 100 | Ocno | Avna, BZ3 |
| 30 | Banana Boat Sport Performance Active Sheer Protect | Lotion | 50 | Hoto, Ocno, OcSto | Avna, BZ3 |
| 31 | Banana Boat Ultradefense Sheer Protect Vit E & Aloe Vera | Lotion | 30 | Hoto, Ocno, OcSto | Avna, BZ3 |
| 32 | Banana Boat Ultradefense Sheer Protect Aloe Vera | Spray | 110 | Hoto, Ocno, OcSto | Avna, BZ3 |
| 33 | Banana Boat Ultradefense Sheer Protect Aloe Vera | Lotion | 50 | Hoto, Ocno, OcSto | Avna, BZ3 |
| 34 | Banana Boat Kids Ultramist | Lotion | 60 | Hoto, Ocno, OcSto, Octo | Avna, Beol |
Abbreviations: Avna, abovenzone; Beol, bemotrizinol; BZ3, oxybenzone; Enle, ensulizole; Hoto, homosalate; Isol, iscotrizinol; Ocna, octyltriazone; Ocno, octocrylene; OcSto, octisalate; Octo, octinoxate; PadO, padimate O; SPF, sun protection factor; TiO2, titanium dioxide.
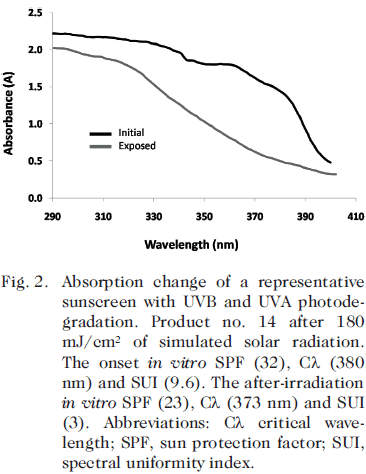
Regarding the UVB protection analysis, the SPF values measured by quartz testing plates before and after exposure to the simulated solar radiation conditions are shown in Table II. Comparison of the manufacturer-declared SPF values on the label with the SPF obtained in vitro showed that the in vitro SPF values were similar or superior to the label values for only 9 of the products (26%). The mean value of the in vitro SPF was significantly lower than the mean SPF label value (47 ± 14 vs. 51 ± 18, P=0.003). When the SPF label value was compared with the in vitro SPF measured after irradiation, the difference was even more pronounced (51 ± 18 vs. 41 ± 13, P<0.001). In addition, the mean in vitro SPFs acquired before and after irradiation also showed a significant difference (47 ± 14 vs. 41 ± 13, P<0.001); the loss of UBV photostability among the samples is shown in Fig. 1.
SUMMARY OF THE INDICES RESULTING FROM IN VITRO SPECTRAL ABSORBANCE ASSESSMENT AT ONSET AND AFTER 180 mJ/cm2 OF SIMULATED UV SOLAR RADIATION
| Sample Nº | In vitro SFP | C l(nm) | UVA/UVB ratio | SUI | ||||
| Initial | Exposed | Initial | Exposed | Initial | Exposed | Initial | Exposed | |
| 1 | 52.6 | 27.5 | 376 | 376 | 0.61 | 0.58 | 4.90 | 4.80 |
| 2 | 18.3 | 15.4 | 382 | 382 | 0.76 | 0.74 | 7.40 | 7.20 |
| 3 | 42.4 | 37.9 | 381 | 379 | 0.92 | 0.74 | 8.30 | 6.30 |
| 4 | 50.9 | 47.9 | 385 | 385 | 0.84 | 0.80 | 10.30 | 9.70 |
| 5 | 26.9 | 26.2 | 381 | 377 | 0.75 | 0.64 | 9.30 | 6.20 |
| 6 | 45.5 | 45.2 | 385 | 385 | 0.88 | 0.86 | 7.20 | 7.40 |
| 7 | 46.8 | 45.7 | 389 | 387 | 0.96 | 0.92 | 38.00 | 28.00 |
| 8 | 29.2 | 24.6 | 384 | 384 | 0.81 | 0.79 | 13.70 | 11.30 |
| 9 | 32.2 | 28.6 | 384 | 383 | 0.93 | 0.91 | 17.30 | 12.10 |
| 10 | 66.3 | 51.9 | 387 | 386 | 0.93 | 0.86 | 32.10 | 18.60 |
| 11 | 62.2 | 59.9 | 386 | 386 | 0.95 | 0.86 | 21.80 | 18.00 |
| 12 | 42.0 | 40.8 | 382 | 378 | 0.52 | 0.40 | 3.40 | 2.30 |
| 13 | 36.1 | 28.5 | 377 | 374 | 0.73 | 0.63 | 11.10 | 6.80 |
| 14 | 32.2 | 23.8 | 380 | 373 | 0.74 | 0.47 | 9.60 | 3.00 |
| 15 | 35.0 | 34.1 | 380 | 380 | 0.77 | 0.72 | 12.70 | 9.80 |
| 16 | 47.6 | 44.5 | 387 | 386 | 0.7 | 0.62 | 5.50 | 5.40 |
| 17 | 62.2 | 47.6 | 384 | 380 | 0.69 | 0.57 | 6.50 | 4.10 |
| 18 | 43.5 | 40.4 | 384 | 384 | 0.89 | 0.87 | 25.70 | 22.40 |
| 19 | 44.5 | 43.1 | 386 | 386 | 0.67 | 0.53 | 5.16 | 5.14 |
| 20 | 63.8 | 58.3 | 387 | 387 | 0.94 | 0.93 | 40.60 | 27.60 |
| 21 | 53.6 | 53.2 | 387 | 386 | 0.77 | 0.63 | 6.25 | 4.90 |
| 22 | 58.8 | 51.2 | 387 | 385 | 0.95 | 0.93 | 35.00 | 18.80 |
| 23 | 51.9 | 42.2 | 386 | 385 | 0.88 | 0.76 | 26.40 | 9.20 |
| 24 | 53.7 | 46.0 | 378 | 377 | 0.72 | 0.67 | 10.80 | 7.70 |
| 25 | 48.6 | 47.3 | 389 | 389 | 0.97 | 0.96 | 33.80 | 32.20 |
| 26 | 51.4 | 45.0 | 388 | 388 | 0.74 | 0.73 | 7.10 | 6.30 |
| 27 | 50.0 | 45.7 | 385 | 380 | 0.84 | 0.65 | 18.50 | 6.50 |
| 28 | 21.3 | 19.1 | 383 | 377 | 0.77 | 0.69 | 12.40 | 9.00 |
| 29 | 74.7 | 62.8 | 385 | 382 | 0.83 | 0.78 | 33.30 | 12.10 |
| 30 | 42.6 | 30.2 | 389 | 387 | 0.97 | 0.88 | 6.81 | 5.79 |
| 31 | 29.6 | 27.8 | 389 | 387 | 0.98 | 0.88 | 6.68 | 5.67 |
| 32 | 81.5 | 75.9 | 385 | 384 | 0.85 | 0.83 | 23.90 | 18.30 |
| 33 | 66.0 | 41.7 | 389 | 384 | 0.97 | 0.95 | 36.90 | 16.40 |
| 34 | 54.8 | 50.5 | 377 | 376 | 0.53 | 0.52 | 4.98 | 3.65 |
Abbreviations: Cl critical wavelength; SPF, sun protection factor; SUI, spectral uniformity index.
Regarding the UVA protection analysis, all products fulfilled the FDA requirement of covering the UV spectrum equivalent to or higher than 370 nm before and after UV radiation. The non-irradiated average Cl value of the samples was 384 nm (range: 376-389 nm). After exposure to the simulated solar radiation condition, the average value was significantly reduced (to 383 nm, range: 373-389 nm; P<0.001). Thus, despite this significant reduction following UV radiation, the absorption capacity in the range of 290 to 370 nm of all 34 sunscreens tested remained >90%.
The mean UVA/UVB ratio of the samples was 0.82 (range: 0.52-0.98). After exposure to the solar simulated irradiation conditions, the mean UVA/UVB ratio decreased to 0.74 (range: 0.40-0.96; P<0.001). The UVA protection that remained after UV exposure was minimal in 3% (n=1) of the products, moderate in 15% (n=5), good in 44% (n=15), superior in 20% (n=7), and ultra in 18% (n=6). The loss of UVA photostability among the samples is shown in Fig. 2.
The mean SUI for the samples before UV radiation was 16.2 (range: 3.4-40.6). After UV radiation, the mean value decreased to 10.9 (range: 2.3-32.2). After the photostability test, medium indices were present in 18% (n=6) of the products, high in 50% (n=17), and very high in 32% (n=11). Data for each of the sunscreens tested are shown in Table II.
A significant correlation was found between the SPF label value of a product and the in vitro SPF value (R2=0.85, P<0.001), but no association was found between the SPF label value and the Cl UVA/UVB ratio, or SUI value either before or after UV irradiation. There was a moderate relationship observed between the in vitro SPF value and the SUI (R=0.4, P=0.01), as well as the Cl (R=0.4, P=0.01). Among the UVA protection indices, the most robust association was found between the SUI and the UVA/UVB ratio (R=0.75, P<0.001), followed by the Cl and the UVA/UVB ratio (R=0.68, P<0.001) and the SUI and Cl ratio (R=0.52, P=0.02). There was no significant relationship found between the number of active ingredients and any of the evaluated indices.
DISCUSSION
Sun exposure is the major cause of photocarcinogenesis (7, 8, 13), photosensitivity (12, 13) and photoaging (9, 12). Therefore, core strategies to prevent the development of skin cancer, solar allergies or aging, rely on behavioral measures to avoid sun exposure by practicing physical protection (2-6, 12, 13). The earliest sunscreens were designed to prevent sunburn resulting from UVB solar radiation (4, 14-16), but research revealed that imperceptible exposure to UVA also exerted harmful effects on the skin, including photosensitizing, hyperpigmentation and aging (5, 10, 12, 13). As a result, it became necessary to improve the UVA/B protection offered by sunscreen products and developers began to consider a multitude of features that may be exploited to optimize effectiveness, such as the application technique, the product’s (and its constituents) thickness, uniformity, absorptive capacity, spectrum and photostability (5-7, 14-18, 20).
In this study, we used an in vitro-based assessment approach to investigate the UV absorption capacity of commercially available sunscreens sold without a medical prescription. The objective was not to prove or correlate the in vivo to in vitro SPF of sunscreens, but to screen their efficacy by using internationally proposed in vitro UV (i.e., 290-400 nm) protection indices before and after solar simulated irradiation (i.e., SPF, critical wavelength, UVA/UVB ratio, SUI). Although the most controversial index is the in vitro SPF, the results of this study are expected to provide insights into the efficacy of lower cost and more conveniently obtainable alternative products that will help to overcome the burdens (high cost and requirement for a doctor’s prescription) of medical-grade sunscreens in Latin American countries. Currently, most of the medically prescribed sunscreens available in the Latin American market are imported and sold by selected retailers (e.g., pharmacies) at costs that are even higher than their already high original price; therefore, they fall into the category of luxury items for the common consumer (17). Physicians may find commercially available sunscreens adequate for recommendation to their patients if the efficacy and scope of UV protection was similar to those available from pharmaceutical manufacturers. The availability of such sunscreens may generate a positive impact in terms of topical compliance due to their widespread availability and lower cost (17).
Although several in vitro techniques have been developed for testing the SPF of a product, no single method has gained widespread acceptance and more research is needed to improve the level of reliability achieved, so that it reaches that of the in vivo method (20). However, the in vitro approach is faster and less expensive as it allows for evaluation of a greater number of products in a shorter period of time and avoids ethical concerns associated with in vivo studies (16-21, 23).
Collectively, the results of this study indicate that the panel of 34 commercially available sunscreens tested in general has a 10% overestimation of the products’ SPF with respect to the value indicated on the label. Moreover, this index was found to be reduced after two hours of tropical-level solar exposure, specifically showing an additional 10% reduction. Thus, under optimal conditions and ideal use of a regular 50 SPF sunscreen, we estimate that the real value attained would be approximately 40.
Considering that the observed mismatch between the in vitro and labeled SPF values may be due to failure or unsuitability of the testing procedure, we cannot make any conclusions regarding other potentially influencing variables, such as exaggerated SPF claims made purposefully by manufacturers to boost sales (as has previously been reported in Mexico (14)). In spite of this potential shortcoming in the study design, the protection afforded by the sunscreens tested is certainly sufficient to support recommendation by local physicians to patients. When taken into account the fact that most of the population in this region of Mexico have dark phototypes (i.e., IV, V) (1-3), a 30 SPF commercial sunscreen is likely to offer convenient photoprotection against the full UV radiation spectrum; lighter phototypes (i.e., II, III) would benefit from applying a 50 or higher SPF among the sunscreens tested (14,16,17).
The current panel of 34 sunscreens tested contains ingredients that protect against UVA radiation, which were lacking from products tested in previous studies (14, 16). Moreover, the current sunscreens showed a UVA absorption ability that was comparable to that of pharmaceutical sunscreens designed for distribution via medical prescription (17). This finding is further supported by the wide protection level offered by all of the tested products, where at least 90% of the UV absorption surpassed the 370 nm wavelength before and after exposure to the simulated solar irradiation and by the good to ultra UVA/ UVB ratio found in 97% of the samples, and moderate to high SUI indices for all these sunscreens. Although the UVA protection indices were statistically reduced after the solar test, they maintained an acceptably high level of protection that was independent of the SPF label value of the product. This means that, in this study, the UVA protection level was similar among products with SPFs lower and higher than 50.
As a sunscreen’s efficacy depends on its constituents, the observed improvement in photostability is mainly due to the integration of UVA filters as ingredients, such as avobenzone, bemotrizinol and titanium dioxide present in the current formulations (17, 24). Therefore, these sunscreens may also be recommended by dermatologists for their patients with photosensitivity and/or pigmentation and photoaging conditions (5, 10, 12, 13). It is important to remember, however, that sunscreens alone are not sufficient to ensure complete protection against the molecular harm induced by UV radiation. This limitation may be addressed by inclusion of stable enzymes in the topical product that can protect or repair UV-induced DNA damage (26, 27). Another concern involves safety of topical products that include oxybenzone as an ingredient; this organic UV broad-band filter has a potential harmful side effect of hormonal disruption and was found to be present in 44% of our sample. Specifically, oxybenzone has been shown to have estrogenic effect, both in in vitro studies and studies involving animal models (28). Although no harmful effects have been reported in humans to date, careful observation is warranted (28).
In conclusion, despite the importance of photoprotection within the clinical practice of dermatologic care and for human health in general (1-3), there are no previous studies from Latin America that have investigated the effectiveness of sunscreen products in relation to their safe prescription and/or recommendation in daily clinical practice. Although regulation agencies in this area of the world consider these products cosmetics and not drugs (25), it was intriguing to observe the great improvement that has occurred in the commercial formulations supporting higher UV protection in these products, to levels comparable to the existing pharmaceutical products (17). Considering the recent advances in sunscreen design worldwide and stricter international regulations, which facilitate improved products becoming available to the public, it is paramount to educate patients on the correct use of these supplementary interventions (15). For instance, instructing individuals on the need for using sunscreen is more important than discussing the superiority of one product over another (14, 16, 17). This communication will emphasize the benefit of these products to general health, as well as provide details about the amount and frequency of product application (5, 6, 15-17). Another point that needs to be addressed by dermatologists in their patient communications is proper explanation of the confusing factors surrounding SPF labeling; this will help to correct a patient’s possible misunderstanding related to influences of advertising, price, or any other commercial attributes associated with sunscreens (5, 6, 14, 15, 17). For instance, the SPF value can be misleading as it does not have a linear relationship with the UVB absorption (5, 6, 16, 17). Thus, a 100 SPF may be confused by the lay public as an indicator of double-SPF protection (compared to SPF 50) when it really only provides 2-3% better protection (5, 6, 24).
REFERENCES
1. Castanedo-Cazares JP, Lepe V, Gordillo-Moscoso A, Moncada B. Dosis de radiación ultravioleta en escolares mexicanos. Salud Pública Mex 2003; 45: 439-444. [ Links ]
2. Castanedo Cázares JP, Torres Álvarez B, Sobrevilla Ondarza S, Ehnis Pérez A, Gordillo Moscoso A. Estimación del tiempo de exposición solar para quemadura en población mexicana. Gac Med Mex 2012; 148: 243-247. [ Links ]
3. Castanedo-Cazares JP, Torres-Álvarez B, Medellin-Pérez ME, Aguilar-Hernández GA, Moncada B. Conocimientos y actitudes de la población mexicana con respecto a la radiación solar. Gac Med Mex 2006; 142: 451-455. [ Links ]
4. Koh HK, Geller AC, Miller DR, Grossbart TA, Lew RA. Prevention and early detection strategies for melanoma and skin cancer. Arch Dermatol 1996; 132:436-443. [ Links ]
5. Wang SQ, Balagula Y, Osterwalder U. Photoprotection: a review of the current and future technologies. Dermatol Ther 2010; 23:31-47. [ Links ]
6. Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet 2007; 370: 528-537. [ Links ]
7. Nishigori C, Yarosh DB, Donawho C, Kripke ML. The immune system in ultraviolet carcinogenesis. J Invest Dermatol Symp Proc 1996; 1: 143-146. [ Links ]
8. Wikonkai NM, Brash DE. Ultraviolet radiation induced signature mutations in photocarcinogenesis. J Invest Dermatol Symp Proc 1999; 46-49. [ Links ]
9. Yaar M, Gilchrest BA. Aging and photoaging: postulated mechanisms and effectors. J Invest Dermatol Symp Proc 1998; 3: 47-51. [ Links ]
10. Serre I, Cano JP, Picot MC, Meynadier J, Meunier L. Immunosuppression induced by acute solar-simulated ultraviolet exposure in humans: prevention by a sunscreen with a sun protection factor of 15 and high UVA protection. J Am Acad Dermatol 1997; 37: 187-194. [ Links ]
11. Sliney DH. Epidemiological studies of sunlight and cataract: the critical factor of ultraviolet exposure geometry. Ophtalmic Epidemiol 1994; 1: 107-119. [ Links ]
12. Krutmann J. Ultraviolet A radiation-induced biological effects in human skin: relevance for photoaging and photodermatosis. J Dermatol Sci. 2000; 23Suppl 1:S22-26. [ Links ]
13. Lowe NJ. An overview of ultraviolet radiation, sunscreens, and photo-induced dermatoses. Dermatol Clin 2006; 24:9-17. [ Links ]
14. Castanedo-Cazares JP, Torres-Alvarez B, Briones-Estevis S, Moncada B. La inconsistencia del factor de protección solar (FPS) en México. El caso de los filtros solares para piel oleosa. Gac Med Mex 2005; 141: 111-114. [ Links ]
15. Department of Health and Human Services FDA, USA.Federal Register, Part IV. 21 CFR Parts 201, 310, and 352. Sunscreen Drug Products for Over-the-Counter Human Use; Final Rules and Proposed Rules. June 17, 2011. [ Links ]
16. Castanedo-Cázares JP, Torres-Álvarez B, Araujo-Andrade C, Castanedo-Tardan MP, Moncada B. Absorción ultravioleta de los protectores solares para prescripción en México. Gac Med Mex 2008; 144: 35-38. [ Links ]
17. Castanedo Cázares JP, Torres Álvarez B, Valdés González G, Ehnis Pérez A. Evaluación in vitro de la protección uva de los bloqueadores solares para prescripción en México. Gac Med Mex 2013; 149:292-298. [ Links ]
18. Akrman J, Kubác L, Bendová H, Jírová D, Kejlová K. Quartz plates for determining sun protection in vitro and testing photostability of commercial sunscreens. Int J Cosmet Sci 2009; 31: 119-129. [ Links ]
19. Diffey BL, Robson JA. A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum. J Soc Cosmet Chem 1989; 40: 127-133. [ Links ]
20. Pelizzo M, Zattra E, Nicolosi P, Peserico A, Garoli D, Alaibac M. In vitro evaluation of sunscreens: an update for the clinicians. ISRN Dermatol 2012; 2012: 352135. [ Links ]
21. Diffey BL, Tanner PR, Matts PJ, Nash JF. In vitro assessment of the broad-spectrum ultraviolet protection of sunscreen products. J Am Acad Dermatol 2000; 43: 1024-1035. [ Links ]
22. Measurement of UVA: UVB Ratio According to the Boots Star Rating System (2008 Revision), Boots, Nottingham, UK, 2008. [ Links ]
23. Diffey B. Spectral uniformity: a new index of broad spectrum (uva) protection. Int J Cosmet Sci 2009; 31: 63-68. [ Links ]
24. Wang SQ, Stanfield JW, Osterwalder U. In vitro assessments of UVA protection by popular sunscreens available in the United States. J Am Acad Dermatol 2008; 59: 934-942. [ Links ]
25. Norma Oficial Mexicana NOM-141-SSA1/ SCFI-2012, Etiquetado para productos cosméticos preenvasados. Etiquetado sanitario y comercial. Secretaría de Salud, México. [ Links ]
26. Basílico G, Roger CA, Seigelchifer M, Kerner N. UV-specific DNA repair recombinant fusion enzyme: a new stable pharmacologically active principle suitable for photoprotection. J Dermatol Sci 2005; 39: 81-88. [ Links ]
27. Stege H, Roza L, Vink AA, Grewe M, Ruzicka T, Grether-Beck S, Krutmann J. Enzyme plus light therapy to repair DNA damage in ultraviolet-B-irradiated human skin. Proc Natl Acad Sci USA 2000; 97: 1790-1795. [ Links ]
28. Krause M, Klit A, Blomberg Jensen M, Søeborg T, Frederiksen H, Schlumpf M, Lichtensteiger W, Skakkebaek NE, Drzewiecki KT. Sunscreens: are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int J Androl 2012; 35: 424-436. [ Links ]












 uBio
uBio