Investigación Clínica
versión impresa ISSN 0535-5133
Invest. clín vol.55 no.4 Maracaibo dic. 2014
Longterm melatonin administration alleviates paraquat mediated oxidative stress in Drosophila melanogaster.
Shirley Medina-Leendertz1, Milagros Paz3, Marylú Mora1, Ernesto Bonilla1,2, Yanauri Bravo1, José Luis Arcaya2, Raikelin Terán3 y Virginia Villalobos3.
1 Laboratorio de Neurobiología, Centro de Investigaciones Biomédicas, Instituto Venezolano de Investigaciones Científicas, Hospital Universitario de Maracaibo, estado Zulia, Venezuela.
2 Instituto de Investigaciones Clínicas “Dr. Américo Negrette”, Facultad de Medicina.
3 Departamento de Biología, Facultad Experimental de Ciencias. Universidad del Zulia. Maracaibo, estado Zulia, Venezuela.
Corresponding autor: Marylu Mora. Centro de Investigaciones Biomédicas, Laboratorio de Neurobiología, Instituto Venezolano de Investigaciones Científicas (IVIC). Maracaibo, Venezuela. Tel.: +58 414 667 3394. E-mail: moramarylu@gmail.com
Abstract. We investigated the effect of melatonin (MEL) in the activities of cytosolic superoxide dismutase (SOD) and catalase as well as in the levels of H2O2 and mitochondrial malondialdehyde (MDA) in paraquat-intoxicated Drosophila melanogaster. Paraquat (40 mM) was administrated for 36 h. Three groups of flies intoxicated with paraquat were used: PQ (exposed during 36h to paraquat), PQ-MEL (exposed during 36h to paraquat and then treated with MEL [0.43 mM] for 12 days) and PQ-Control (maintained in standard corn meal for 12 days). Two additional groups without pre-intoxication with PQ were added: Control (maintained in standard corn meal) and MEL (treated with MEL for 12 days). Immediately after PQ intoxication the concentration of MDA (17.240 ± 0.554 nmoles MDA/mg protein) and H2O2 (3.313 ± 0.086 nmol hydrogen peroxide/mg protein) and the activities of SOD and catalase (419.667 ± 0.731 and 0.216 ± 0.009 Units/mg of protein, respectively) in the PQ group were significantly increased with respect to Control. After 12 days of intoxication with PQ, the PQ-Control flies showed increases in H2O2 (4.336 ± 0.108) and MDA levels (8.620 ± 0.156), and in the activities of SOD and catalase (692.570 ± 0.433 and 0.327 ± 0.003, respectively) as compared to PQ-MEL (p<0.001). Treatment with MEL extended the life span of the groups PQ-MEL and MEL when compared to their corresponding controls. Motor activity decreased significantly in PQ-Control and PQ-MEL flies, suggesting that the damage caused by PQ affected the nervous system of flies. Our findings showed that oxidative damage caused by paraquat was observed even after 12 days and that melatonin mitigates this damage.
Keywords: paraquat, melatonin, reactive oxygen species, Drosophila melanogaster.
La administración de melatonina a largo plazo mitiga el estrés oxidativo mediado por paraquat en Drosophila melanogaster.
Resumen. Investigamos el efecto de la melatonina (MEL) en la actividad de la superóxido dismutasa citosólica (SOD) y la catalasa, así como en las concentraciones del H2O2 y del malondialdehido mitocondrial (MDA) en la toxicidad inducida por paraquat (PQ) en Drosophila melanogaster. El paraquat (40 mM) fue administrado durante 36h. Tres grupos de moscas se utilizaron después de la intoxicación con paraquat: PQ (expuestas a paraquat durante 36 h), PQ-MEL (expuestas durante 36 horas a PQ y luego tratadas con MEL [0,43 mM] por 12 días) y PQ-Control (mantenidas en medio estándar por 12 días). Se incluyeron dos grupos adicionales sin pre-intoxicación con PQ: Control (mantenido en medio estándar) y MEL (tratado con MEL por 12 días). Inmediatamente después de la intoxicación con PQ, las concentraciones de MDA (17,240 ± 0,554 nmol de MDA/mg de proteína), H2O2 (3,313 ± 0,086 nmol de H2O2/mg de proteína) y las actividades de la SOD y catalasa (419,667 ± 0,731 y 0,216 ± 0,009 unidades/mg de proteína, respectivamente) se incrementaron significativamente con respecto al Control. Doce días después de la intoxicación con PQ, las moscas PQ-Control mostraron un aumento en la concentración de H2O2 (4,336 ± 0,108), de los niveles de MDA (8,620 ± 0,156) y en las actividades de la SOD y la catalasa (692,570 ± 0,433 y 0,327 ± 0,003, respectivamente) en comparación con el grupo PQ-MEL (p<0,001). El tratamiento con MEL extendió el tiempo de vida de los grupos PQ-MEL y MEL en comparación con sus correspondientes controles. La actividad motora disminuyó significativamente en las moscas de los grupos PQ-Control y PQ-MEL, lo que sugiere que el PQ afectó el sistema nervioso de las moscas. Nuestros hallazgos demostraron que el daño oxidativo causado por paraquat en las moscas fue observado aún después de 12 días de intoxicadas y que la melatonina logró mitigar este daño.
Palabras clave: paraquat, melatonina, especies reactivas de oxígeno, Drosophila melanogaster.
Recibido: 29-1-2014 Aceptado: 3-7-2014
INTRODUCTION
Paraquat (PQ) is a nonselective, contact herbicide, used in agricultural industries. Epidemiological studies link PQ to increased incidence of Parkinson’s disease (PD) in farmers when exposed to this herbicide for long periods of time without adequate protection (1, 2). Paraquat has been used as a model to understand its role in the occurrence and progression of PD due to its structural similarity with the 1-methyl-4-phenylpyridinium ion (MPP+), which is an inducing agent of classical Parkinsonism (3-5). Paraquat has been used to generate superoxide radicals in isolated mitochondria, and in Caenorhabditis elegans, rodents and Drosophila melanogaster (6-8). The complex I respiratory chain has been identified as the major site of mitochondrial superoxide production by this herbicide (9). The toxicity of PQ has been shown to be associated to its intracellular reduction to a free radical form that is reoxidized by oxygen to initiate an oxidative cascade that includes the generation of superoxide radicals (10).
Mitochondria have been implicated as a target of oxidative damage by PQ in many studies. For example, mitochondrial expression of a transgenic catalase is more protective against PQ than cytosolic expression in Drosophila melanogaster (11) and Saccharomyces cerevisiae (12); deficiency of the isoform of superoxide dismutase of the mitochondrial matrix results in hypersensitivity to PQ in mice (7) and in Drosophila (13); mitochondrial swelling is one of the earliest ultrastructural changes on PQ exposure in vivo in cultured human lung cells (14). There is considerable interest in the mechanism of PQ toxicity and its interaction with the mitochondria as an important component of its toxicity.
Melatonin (MEL) is a neurohormone able to cross the cell membrane and it also has access to every subcellular compartment due to its high lipid and water solubility (15). It is also characterized by its high capacity to donate an electron (16). Melatonin interacts with reactive oxygen species (ROS) and reactive nitrogen species (RNS), including the hydroxyl radical (OH·), singlet oxygen (1O2), superoxide anion O2·–, hydrogen peroxide (H2O2), nitric oxide (NO) and peroxynitrite anion (ONOO–) (17, 18, 19). The interaction of MEL with O2– or OH·, generates intermediate metabolites with antioxidant capacity. Its genomic effect in regulating protein expression and the activities of the antioxidant enzymes glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) and catalase (CAT) also contributes to its antioxidant capacity (20).
It has been shown that antioxidants such as MEL, glutathione, serotonin, minocycline, lipoic acid and ascorbic acid, administered before exposure to PQ, offers protection against the damage caused by this herbicide, increasing the survival of the intoxicated flies (8).
The aim of this research was to determine the effect of post-treatment with MEL in oxidative markers, life span and motor activity of Drosophila melanogaster after PQ intoxication for 36 hours.
METHODS AND MATERIAL
Experimental animals stock
Male flies of D. melanogaster (wild-type, Oregon R strain) were used. Flies were maintained in 12h/12h light/dark cycle at 25°C. Standard corn meal contained: agar-agar (0.3 g), corn flour (5 g), yeast (1.5 g), 100% ethanol (1.25 mL), brown sugar solution (5 mL), methyl p-hydroxybenzoate (Sigma Chemistry, USA) (0.065 g) and 43.75 mL of distilled water (8). In this study only males were used because experimental results with female flies tend to be variable, and to avoid this variability we must work only with virgin females, while with males a higher reproducibility in test results.
Paraquat-acute intoxication and antioxidant post-treatment
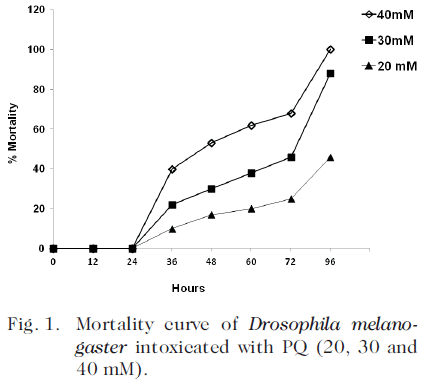
A dose response curve was previously performed with 20, 30 and 40 mM of PQ. The LD 50 was 40 mM in 48 h. Thereafter, the intoxication was performed with 40 mM of PQ, during 36 h, since after 48 h, the flies showed signs of a marked deterioration: the right wing tended to elevate when the fly were resting and the animals showed a marked disorientation.
Two days old flies (300 per group) were exposed to PQ. The flies were starved for 3 h to be sure that no food remained in the digestive tract. Then the flies were transferred to vials containing only filter paper soaked with 40 mM paraquat (SIGMA) in 5% sucrose solution. Three groups of flies were used after the intoxication with paraquat: PQ (exposed during 36h to paraquat), PQ-MEL (exposed during 36h to paraquat and then treated with MEL [0.43 mM; SIGMA] for 12 days) and PQ-Control (maintained in standard corn meal for 12 days). Two additional groups without pre-intoxication with PQ were added: Control (maintained in standard corn meal) and MEL (treated with MEL for 12 days). The MEL concentration was previously standardized by Bonilla et al. (8). The flies were transferred daily to fresh medium during 12 days. Three replicates of each treatment were carried out.
Isolation of mitochondria
Mitochondria from whole body of Drosophila melanogaster were prepared by differential centrifugation. Briefly, a homogenate was prepared with one hundred and fifty flies in 990 µL ice-cold Tris-sucrose buffer (0.32 M sucrose, 1 mM EDTA and 10 mM Tris-HCl at pH 7.4) containing 1% butylated hydroxytoluene (BHT), using a glass-glass grinder (PYREX 2 mL; Thermo Fisher Scientific Inc. USA). All reagents used to prepare this buffer were from Sigma-Aldrich, USA. The homogenate was centrifuged 15000 g for 2 min at 4°C (IEC Centra MP4R International Equipment Co.).The supernatant was removed carefully and the pellet, which contains the mitochondria, was centrifuged 15000 g for 2 minutes at 4°C three times in ice-cold Tris-sucrose buffer (21).
Determination of malondialdehyde
Lipid peroxidation determination was done by measuring MDA-TBA adducts, using the OXISELECT™ TBARS assays kit (MDA quantitation) (Cell Biolabs, Inc.). The supernatant obtained during the isolation of mitochondria was removed and the pellet was suspended in phosphate-buffered saline (PBS), following the protocol described in the kit. The absorbance was read at 532 nm (Synergy HT, Bioteck). The results were expressed in nmoles MDA/mg protein.
Determination of protein
The Bicinchoninic Acid Protein Assay Kit BCA1 (Sigma) was used to determine soluble protein concentrations. The supernatant obtained during the isolation of mitochondria was removed and the pellet was suspended in deionized water, following the protocol described in the kit. The absorbance was measured at 562 nm (Synergy HT, Bioteck). The results were expressed in mg protein/mL.
Determination of oxidative markers in whole body homogenate (cytosolic fraction)
Hydrogen peroxide determination. A homogenate of whole body of Drosophila melanogaster was prepared with 50 flies in 500 µL PBS, using a glass-glass grinder (PYREX 2 mL; Thermo Disher Scientific Inc. USA). The homogenate was centrifuged at 10000 g for 5 min. at 4°C. Ninety µL of the supernatant was removed to determine H2O2. The OXISELECT™ hydrogen peroxide Assay Kit (Cell Biolabs, Inc.) was used to measure the hydrogen peroxide present in the samples. The absorbance was read at 540 nm. The results were expressed in nmol H2O2/mg protein.
Catalase activity assay. A homogenate of whole body of Drosophila melanogaster was prepared with 10 flies in 500 µL ice-cold PBS with 1mM EDTA per gram of tissue, using a glass-glass grinder. The homogenate was centrifuged at 10000 g for 15 min at 4°C. The supernatant was removed to determine catalase activity using the OXISELECT™ Catalase activity Assay Kit colorimetric (Cell Biolabs, Inc.). Twenty µL of the supernatant were removed and the activity of catalase was determinate following the protocol described in the kit. The absorbance was read at 520 nm. The results were expressed in Units/ mg protein.
Superoxide dismutase activity assay. A homogenate of whole body of Drosophila melanogaster was prepared with 10 flies in 400 µL of ice-cold lysis buffer (10mM Tris, pH 7.5, 150 mM NaCl, 0.1 mM EDTA, 0.5% Triton-100), using a glass-glass grinder. The homogenate was centrifuged at 12000 g by 10 min at 4°C. The supernatant was removed to determine superoxide dismutase activity. The OXISELECT™ Superoxide dismutase activity Assay Kit (Cell Biolabs, Inc.) was used to measure the activity present in the samples. Seventy µL of the supernatant were removed to determine superoxide dismutase activity following the protocol described in the kit. The absorbance was read at 490 nm. The results were expressed in Units/mg protein. Cytosolic SOD (Cu/ZnSOD) was determined, since only the supernatant was used.
Longevity
Two days old flies (300 per group) were exposed to PQ (40 mM in 5% sucrose solution) during 36 h as described previously (8). To determine the longevity two groups of PQ-intoxicated flies were used: PQ-MEL (exposed during 36h to paraquat and then treated with MEL [0.43 mM; Sigma] and PQ-Control (maintained in standard corn meal). Two additional groups without pre-intoxication with PQ were added: Control (maintained in standard corn meal) and MEL (treated with MEL). The animals of these groups were counted and transferred to fresh medium daily for their entire life span. Three replicates of each experiment were done.
Motor activity
The number of movements of each fly from the treatment and control groups was determined using DAM2 Drosophila Activity Monitor (TRIKINETIC, USA). Flies were placed within glass capillary tubes (5 mm diameter and 65 mm length) and were supplied with a food source located in one of its extremes. Activity levels of each animal were measured at 15 min time intervals for a 24 hour time period (at 36 hours, 1, 2, 3, 4, and 5 week). Flies were maintained at 25°C during the 12 hour light/dark cycle.
Statistical analysis
Data were expressed as means ± S.E.M. and the significance was determined by one-way ANOVA test, and differences among experimental groups were tested for significance using the test of Tukey for multiple comparisons. Differences were considered statistically significant when p<0.05.
RESULTS
A dose response curve was performed with 20, 30 and 40 mM of PQ. The LD 50 was 40 mM at 48 h. The acute intoxication of D. melanogaster with PQ (40 mM) induced 40% mortality after 36 hours of intoxication (Fig. 1).
Effect of PQ on oxidative markers and antioxidant enzymes
A highly significant increase (p<0.001) in MDA levels (17.240 ± 0.554 nmoles/mg protein) in the mitochondrial fraction of adult male D melanogaster exposed to PQ with respect to controls (5.640 ± 0.173) was observed (Table I).
OXIDATIVE MARKERS AND ENZYMATIC ACTIVITY IN ADULT MALE D. melanogaster EXPOSED TO PQ (40 MM) FOR 36 HOURS
| Oxidative markers | Control | PQ (40 mM) |
| Mitochondria MDA1 | 5.640 ± 0.173 | 17.240 ± 0.554*** |
| Cytosolic fraction | ||
| Hydrogen peroxide2 | 1.070 ± 0.075 | 3.313 ± 0.086*** |
| Enzymatic activity | ||
| Catalase3 | 0.106 ± 0.008 | 0.216 ± 0.009*** |
| SOD4 | 202.755 ± 0.926 | 419.667 ± 0.731*** |
Data were analyzed by one-way ANOVA test and compared with control, ***p<0.001.
1nmolMDA/mg protein; 2nmol H2O2/mg protein; 3 Units/mg protein, 4 Units/mg protein.
We also found an increase (p<0.001) in H2O2 levels in the cytosolic fraction (3.313 ± 0.086 vs 1.070 ± 0.075 nmol H2O2/mg protein) and in the activities of SOD (419.667 ± 0.731 vs 202.755 ± 0.926 Units/mg protein) and catalase (0.216 ± 0.009 vs 0.106 ± 0.008 Units/mg protein) in whole body homogenates of adult male D. melanogaster exposed to PQ with respect to Control (Table I).
In the MEL group (treated with MEL for 12 days) without pre-PQ intoxication, H2O2 levels in the cytosolic fraction were significantly reduced compared to control (p <0.05), probably due to the increase in the enzymatic activity of catalase and SOD (Table II).
OXIDATIVE MARKERS AND ENZYMATIC ACTIVITY IN ADULT MALE D MELANOGASTER WITHOUT PRE-INTOXICATION AND WITH PRE-INTOXICATION WITH PQ (40MM) FOR 36 HOURS AND SUBSEQUENTLY TREATED WITH MEL (0.43mM) FOR 12 DAYS
| Oxidative markers | Without PQ pre-intoxication | With PQ pre-intoxication | ||
| Control | MEL | PQ-Control | PQ-MEL | |
| Mitochondrial MDA1 | 6.640 ± 0.156 | 5.381 ± 0.296 | 8.620 ± 0.156†· | 5.056 ± 0.432* |
| Cytosolic fraction | ||||
| Hydrogen peroxide2 | 1.197 ± 0.070 | 0.716 ± 0.014 | 4.336 ± 0.108†· | 1.303 ± 0.020* |
| Enzymatic activity | ||||
| Catalase3 | 0.143 ± 0.012 | 0.199 ± 0.005 | 0.327 ± 0.003†· | 0.220 ± 0.005* |
| SOD4 | 198.200 ± 1.242 | 277.070 ± 3.091 | 692.570 ± 0.433†· | 333.001 ± 0.381* |
Data were analyzed by one-way ANOVA test and differences among experimental groups were tested for significance using the test of Tukey for multiple comparisons.
PQ- MEL compared with PQ-Control * p<0.001
PQ-Control compared with Control † p<0.001
PQ-Control compared with MEL · p<0.001
1nmol MDA/mg protein; 2nmol H2O2/mg protein; 3 Units/mg proteins, 4 Units/mg protein
Table II also shows the effect of MEL on the oxidative markers and enzymatic activities in flies treated with PQ (40 mM) for 36 h and subsequently treated with MEL (0.43 mM) for 12 days. In the PQ-Control flies a significant increase (p<0.001) in the cytosolic H2O2 levels and in the mitochondrial MDA content as well as in the activities of catalase and SOD in whole body homogenates was detected when compared to PQ-MEL, Control, and MEL groups.
Life span
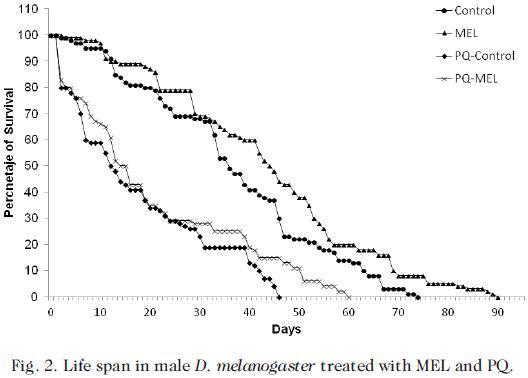
Fig. 2 shows the life span of male D. melanogaster without pre-intoxication and pre-intoxicated with PQ (40 mM) for 36 h and subsequently treated with MEL (0.43 mM) to complete their life cycle. The MEL fed flies had a significant higher (p<0.001) life span than Control, PQ-MEL and PQ-Control flies (90.000 ± 0.296, 74.000 ± 1.000, 60.000 ± 1.000, and 45.670 ± 1.155 days, respectively).
In the PQ-MEL flies a highly significant increase (23.88%) (p<0.001) in the longevity (60.000 ± 1.000 days) with respect to the PQ-Control flies (45.670 ± 1.155 days) was observed as well as a significant decrease (p<0.001) in longevity with respect to the Control flies.
Motor activity
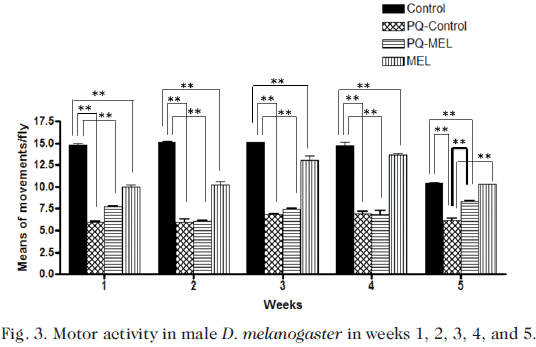
Measurements of motor activity of D. melanogaster were performed in surviving flies. Both intoxicated groups (PQ-Control and PQ-MEL) and not pre-intoxicated groups (Control and MEL) were measured once a week for 24 hours until the completion of the life cycle. In all groups, it was evident a peak of increased activity from 15:00 p.m. to 00:00 a.m. Fig. 3 shows the means of the number of movements per fly, at 36 h and on the 1st, 2nd, 3rd, 4th, and 5th week.
The motor activity in the PQ group (3.288 ± 0.650) measured immediately after 36 h of intoxication was significantly decreased p<0.001 with respect to control (10.759 ± 0.831). No significant differences between the intoxicated groups (PQ-Control and PQ-MEL) in weeks 1, 2, 3, and 4 were observed. In the PQ-Control and PQ-MEL groups a significant decrease (p<0.001) was observed with respect to Control in weeks 1, 2, 3, and 4. In the PQ-Control flies a significant decrease (p<0.001) in the number of movements (6.110 ± 0.165) was detected on the 35th day (5th week) with respect to Control (10.456 ± 0.426), PQ-MEL (8.285 ± 0.128) and MEL (10.290 ± 0.280) flies. No difference was found in the motor activity between the Control and the MEL flies on the 5th week (Fig. 3).
DISCUSSION
In this study, we determined the effect of post-treatment with MEL in oxidative markers (MDA, H2O2, SOD and Catalase), life span and motor activity of Drosophila melanogaster after PQ intoxication for 36 h.
In the MEL group (MEL treated for 12 days) without pre-PQ poisoning, H2O2 levels were significantly reduced compared to control (p <0.05), probably due to the increase in the enzymatic activity of catalase and SOD; MEL could be indirectly acting generating increase in mRNA levels and in the activities of superoxide dismutase (SOD), glutathione peroxidase, glutathione reductase, and catalase (22).
In the surviving flies a significant increase in malondialdehyde levels in mitochondria was detected indicating an oxidative damage to the mitochondrial membrane. Previous studies have shown that PQ is able to induce oxidative stress in D. melanogaster as early as 24 hours after exposure (23, 24). The toxicity of PQ has been shown to be associated to its intracellular reduction to a free radical form that is reoxidized by oxygen to initiate an oxidative cascade that includes the generation of superoxide radicals (2).
Although, the underlying mechanisms of acute PQ toxicity have been studied in diverse model systems, the long term effect of the exposure to this toxicant on the oxidative status of D. melanogaster has not been studied. The oxidative damage induced by PQ in our study was long lasting since 12 days after intoxication oxidative stress parameters remained significantly altered with respect to the control flies. Previous studies (24) reported an increase (45%) of mitochondrial malondialdehyde after 24 h exposure to PQ. Our observation of elevated activities of both SOD and catalase in whole body homogenates of flies after 12 days of intoxication with PQ, suggests that flies were exposed to elevated oxidative stress in vivo, and that the damage persists for several days after intoxication. Our results agree with those of Hosamani and Muralidhara (24), who reported a significant elevation of catalase (21-24%) and SOD (25%) activities 24h after exposure to PQ (40 mM).
Rodriguez-Rocha et al. (25) found that the administration of PQ, MPP+, and rotenone produced significant mitochondrial damage. Their results further demonstrate that PQ is able to act as a mitochondrial toxin to induce oxidative stress. Paraquat is a redox cycling agent. In the toxicity due to PQ, the PQ dication (PQ2+) accepts an electron from a reductant to form the PQ monocation radical (PQ.+), which subsequently reacts rapidly with O2 to produce superoxide (O2.–). The parent compound PQ2+ is thus regenerated and able to catalyze further production of O2.– (10). The continuous generation of PQ2+ would explain why even after 12 days post-intoxication high levels of MDA were still observed in mitochondria.
In our study, we also observed that flies exposed to PQ for 36 h and then posttreated with MEL (PQ-MEL flies) for 12 days, the levels of MDA and H2O2, and the activities of both catalase and SOD were significantly reduced. In a previous work we found that pretreatment for 5 days with MEL (0.43 mM) increased (38.6%) the life span in D. melanogaster exposed to PQ for 48 hours (8). Various studies have shown that MEL can exert its protective effects in several ways including what has been named the cascade reaction when scavenging free radicals (17).
An additional antioxidant capacity of MEL is the ability of this indoleamine to promote the metabolism of toxic reactants to innocuous molecules. A number of antioxidant enzymes, such as CuZnSOD, MnSOD, catalase (CAT), GPx and GRd are important in limiting the oxidative damage (26). Two recent publications have considered the mechanisms involved in the stimulation of antioxidant enzyme activities by MEL. Thus, Ozturk et al. (27) found increased SOD activity in rat liver after administration of 10 mg/kg of MEL for 7 days, while Liu and Ng (28) reported enhancement of SOD activity in rat kidney, liver and brain after a single MEL injection (5 mg/kg).
During the last decade, MEL has been shown to possess genomic actions, regulating the expression of several genes. Mayo et al. (29) provided an insight into the mechanisms by which MEL regulates antioxidant enzyme gene expression using cultured dopaminergic cells. They found that MEL induced synthesis of new protein as a condition for regulation of gene expression of all the three antioxidative enzymes, CuZnSOD, MnSOD and GSH-Px. Melatonin also diminished the half-life of mRNAs coding for both CuZnSOD and GSH-Px, without altering that of MnSOD. Finally, nanomolar concentrations of MEL were adequate to induce antioxidant gene expression with a 1-hr exposure to MEL being adequate to sustain elevated mRNA levels 24 h later.
Kotler et al. (30) found that after chronic administration of MEL (50 and 500 µg/kg) to rats, this neurohormone had a stimulatory effect on antioxidant enzyme gene expression. The work of Barlow-Walden et al. (31) also indicates that antioxidant enzyme activity and expression, respectively, are elevated after the administration of MEL.
Given the antioxidant potential that MEL showed in our studies, it is necessary to elucidate the mechanisms of action of this antioxidant against acute PQ exposure. Recently, Kilic et al. (32) reported that MEL attenuates cisplatin-induced nephrotoxicity in rats by modulating Nrf2/HO-1 signaling. Expressions of NF-kB p65 and AP-1 were increased significantly in the kidneys of rats treated with cisplatin compared with the kidneys from the control, melatonin-only-treated and melatonin co-treated rats. Nrf2/HO-1 signaling pathway up regulates the expression of a number of antioxidant genes in response to a different stimuli, and provides protection to the cell against oxidative stress and inflammation. The results of Kilic et al. (32) seem to establish a possible relationship between Nrf2/HO-1 antioxidant stress signaling and melatonin’s nephroprotective effect.
Other possible mechanisms by which melatonin could exert a protective effect on the animals treated with PQ are its ability to scavenge ROS and RNS (33-38) and to neutralize H2O2 and other oxidants including singlet oxygen (1O2), NO, and the ONOO– (18).The superior antioxidant capacity of MEL is, at least partially, attributed to what is referred to as the cascade reaction when scavenging free radicals (17, 19). Anti-inflammatory effects have also been established since MEL blocked H2O2-induced phosphorylation of PI3K/Akt, p38, ERK, JNK, and MAPK, as well as activation of NF-kB. Melatonin and 5-methoxytryptophan (5-MTP or cytoguardin) possess anti- inflammatory actions through inhibition of COX-2 transcriptional activation. (39). Ramamoorthy et al. (40), demonstrated that MEL pretreatment protects the kidneys of rats from tenofovir-induced damage to proximal tubular mitochondria by attenuating oxidative stress, nitrosative stress, and inflammation.
The decrease of spontaneous motor activity observed in PQ treated flies in the present study, suggests that acute intoxication may have a significant deleterious effect on the nervous system of the flies. In this regard, recent studies have shown that PQ induced neuronal damage across the nervous system of the flies and caused a significant increase of DNA damage. In addition, these neuropathological changes were associated with impairment of motor behavior (41). An inverse relationship between altered motor behavior and the markers of oxidative stress was detected in our study. In fact, the decrease in motor activity coincided with the increase in the oxidative markers.
Animals exposed to PQ showed significant impairment of motor activity, which could not be restored by MEL; however, in the 35th day improved motor activity in the MEL treated animals previously exposed to paraquat was observed, without reaching the levels of motor activity of their respective controls. This may be a reflection of mitigation of oxidative stress by MEL. Paraquat exposure has been linked to increased PD risk in agricultural communities (42) and induces movement disorders in some mammalian models (43, 44). However, in other mammalian studies in which paraquat induced dopaminergic neurodegeneration was observed, neuronal loss had not been sufficiently extensive to emulate characteristic PD movements (45, 46). Other studies has shown that in D. melanogaster antioxidants, such as polyphenols (gallic acid, caffeic acid, ferulic acid, coumaric acid), rescue motor activity in flies exposed to 20 mM paraquat (47).
In conclusion, MEL extended the life span of the PQ-MEL and MEL groups when compared to their corresponding controls. Motor activity decreased significantly in PQ-Control and PQ-MEL flies, suggesting that the damage caused by PQ affected the nervous system of the flies. Our findings demonstrated the antioxidant effects of MEL on PQ treated flies. We have also shown that the effects of the PQ exposure are long lasting and that MEL treatment alters these effects by diminishing the sequel of PQ-intoxication, as shown by the decrease in the activities of SOD, catalase, H2O2 levels and mitochondrial MDA.
REFERENCES
1. Thiruchelvam M, Richfield EK, Goodman BM, Baggs RB, Cory-Slechta DA. Developmental exposure to the pesticides paraquat and maneb and the Parkinson’s disease phenotype. Neurotoxicology 2002; 23(4-5):621-633. [ Links ]
2. Dinis-Oliveira RJ, Remião F, Carmo H, Duarte JA, Navarro AS, Bastos ML, Carvalho F. Paraquat exposure as an etiological factor of Parkinson’s disease. Neurotoxicology 2006; 27(6): 1110-1122. [ Links ]
3. Uversky VN. Neurotoxicant-induced animal models of Parkinson’s disease: understanding the role of rotenone, maneb and PQ in neurodegeneration. Cell Tissue Res 2004; 318(1):225-241. [ Links ]
4. Abdulwahid Arif I, Ahmad Khan H. Environmental toxins and Parkinson’s disease: putative roles of impaired electron transport chain and oxidative stress. Toxicol Ind Health 2010; 26(2):121-128. [ Links ]
5. Nisticò R, Mehdawy B., Piccirilli S., Mercuri N. PQ-and Rotenone-induced models of Parkinson’s disease. Int J Immunopathol Pharmacol 2011; 24(2):313-322. [ Links ]
6. Sampayo JN, Olsen A, Lithgow GJ. Oxidative stress in Caenorhabditis elegans: protective effects of superoxide dismutase/ catalase mimetics. Aging Cell 2003; 2(6):319-326. [ Links ]
7. Van Remmen H, Qi W, Sabia M, Freeman G, Estlack L, Yang H, Mao Guo Z, Huang TT, Strong R, Lee S, Epstein CJ, Richardson A. Multiple deficiencies in antioxidant enzymes in mice result in a compound increase in sensitivity to oxidative stress. Free Radic Biol Med 2004; 36(12):1625-1634. [ Links ]
8. Bonilla E, Medina-Leendertz S, Villalobos V, Molero L, Bohorquez A. PQ-induced oxidative stress in Drosophila melanogaster: effects of melatonin, glutathione, serotonin, minocycline, lipoic acid and ascorbic acid. Neurochem Res 2006; 31(12):1425-1432. [ Links ]
9. Cocheme HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by PQ. J Biol Chem 2008; 283(4):1786-1798. [ Links ]
10. Cochemé HM, Murphy MP. The uptake and interactions of the redox cycler paraquat with mitochondria. Methods Enzymol 2009; 456:395-417. [ Links ]
11. Mockett RJ, Bayne AC, Kwong LK, Orr WC, Sohal RS. Ectopic expression of catalase in Drosophila mitochondria increases stress resistance but not longevity. Free Radic Biol Med 2003; 34:207-217. [ Links ]
12. Tien Nguyen-nhu N, Knoops B. Mitochondrial and cytosolic expression of human peroxiredoxin 5 in Saccharomyces cerevisiae protect yeast cells from oxidative stress induced by PQ. FEBS Letter 2003; 544(1-3):148-152. [ Links ]
13. Kirby K, Hu J, Hilliker AJ, Phillips JP. RNA interference-mediated silencing of Sod2 in Drosophila leads to early adult-onset mortality and elevated endogenous oxidative stress. Proc Natl Acad Sci USA 2002; 99(25):16162-16167. [ Links ]
14. Wang GY, Hirai K, Shimada H. Mitochondrial breakage induced by the herbicide paraquat in cultured human lung cells. J Electron Microsc (Tokyo) 1992; 41(3): 181-184. [ Links ]
15. Martín M, Macías M, Escames G, León J, Acuña-Castroviejo D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide induced mitochondrial oxidative stress. FASEB J 2000; 14(12):1677-1679. [ Links ]
16. Reiter R, Tan D, Manchester L, Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species. Cell Biochem Biophys 2001; 34(2):237-256. [ Links ]
17. Tan D, Manchester L, Reiter RJ, Qi W, Karbownik M, Calvo J. Significance of melatonin in antioxidative defense system: reactions and products. Biol Signals Recept 2000; 9(3-4):137-159. [ Links ]
18. Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res 2011; 51(1):1-16. [ Links ]
19. Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuña-Castroviejo D, Reiter RJ. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J Pineal Res 2013; 54(2):127-138. [ Links ]
20. Tomás-Zapico C, Coto-Montes A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J Pineal Res 2005; 39(2):99-104. [ Links ]
21. Fernández-Vizarra E, Ferrín G, Pérez-Martos A, Fernández-Silva P, Zeviani M, Enríquez JA. Isolation of mitochondria for biogenetical studies: An update. Mitochondrion 2009; 10(3):253-262. [ Links ]
22. Emerit I, Filipe P, Freitas J, Vassy J. Protective effect of superoxide dismutase against hair graying in a mouse model. Photochem. Photobiol 2004; 80:579- 582. [ Links ]
23. Hosamani R, Muralidhara. Prophylactic treatment with Bacopa monnieri leaf powder mitigates paraquat-induced oxidative perturbations and lethality Drosophila melanogaster. Indian J Biochem Biophys 2010; 47(2):75-82. [ Links ]
24. Hosamani R, Muralidhara. Acute exposure of Drosophila melanogaster to paraquat causes oxidative stress and mitochondrial dysfunction. Arch Insect Biochem. Physiol 2013; 83(1):25-40. [ Links ]
25. Rodriguez-Rocha H, Garcia-Garcia A, Pickett C, Li S, Jones J, Chen H, Webb B, Choi J, Zhou Y, Zimmerman MC, Franco R. Compartmentalized oxidative stress in dopaminergic cell death induced by pesticides and complex I inhibitors: Distinct roles of superoxide anion and superoxide dismutases. Free Radic Biol Med 2013; 61C:370-383. [ Links ]
26. Reiter RJ, Paredes SD, Korkmaz, Jou MJ, Tan DX. Melatonin combats molecular terrorism at the mitochondrial level. Interdisc Toxicol 2008; 1:137-149. [ Links ]
27. Ozturk G, Coşkun S, Erbaş D, Hasanoglu E. The effect of melatonin on liver superoxide dismutase activity, serum nitrate and thyroid hormone levels. The Jpn J Physiol 2000; 50(1):149-153. [ Links ]
28. Liu F, Ng TB. Effect of pineal indoles on activities of the antioxidant defense enzymes superoxide dismutase, catalase, and glutathione reductase, and levels of reduced and oxidized glutathione in rat tissues. Biochem Cell Biol 2000; 78(4):447-453. [ Links ]
29. Mayo JC, Sainz RM, Antoli I, Herrera F, Martin V, Rodriguez C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci 2002; 59(10):1706-1713. [ Links ]
30. Kotler M, Rodríguez C, Sáinz RM, Antolín I, Menéndez-Peláez A. Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J Pineal Res 1998; 24(2):83-89. [ Links ]
31. Barlow-Walden LR, Reiter RJ, Abe M, Pablos M, Menendez-Pelaez A, Chen LD, Poeggeler B. Melatonin stimulates brain glutathione peroxidase activity. Neurochem Int 1995; 26(5):497-502. [ Links ]
32. Kilic U, Kilic E, Tuzcu Z, Tuzcu M, Ozercan IH, Yilmaz O, Sahin F, Sahin K. Melatonin suppresses cisplatin-induced nephrotoxicity via activation of Nrf-2/HO-1 pathway. Nutr Metab (Lond) 2013; 10(1):7. [ Links ]
33. Vijayalaxmi Meltz ML, Reiter RJ. Melatonin and protection from whole body irradiation: survival studies in mice. Mutat Res 1999; 425:21-27. [ Links ]
34. Taysi S, Koc M, Buyukokuroglu ME, Altinkaynak K, Sahin YN. Melatonin reduces lipid peroxidation and nitric oxide during irradiation-induced oxidative injury in the rat liver. J Pineal Res 2003; 34:173-177. [ Links ]
35. Zavodnik IB, Domansky AV, Lapshina EA, Bryszewska M, Reiter RJ. Melatonin directly scavenges free radicals generated in red blood cells and a cell-free system: chemiluminescence measurements and theoretical calculations. Life Sci 2006; 79: 391-400. [ Links ]
36. Tan DX, Manchester LC, Reiter RJ, Plummer BF, Hardies LJ, Weintraub ST, Vijayalaxmi Meltz ML, Shepherd AM. A novel melatonin metabolite, cyclic 3- hydroximelatonin: a biomarker of in vivo hydroxyl radical generation. Biochem Biophys Res Commun 1998; 253:614-620. [ Links ]
37. Hardeland R, Tan DX, Reiter RJ. Kynuramines metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J Pineal Res 2009; 47:109-126. [ Links ]
38. Tapias V, Escames G, Lopez LC, Lopez A, Camacho E, Carrion MD, Entrena A, Gallo MA, Espinosa A, Acuña-Castroviejo D. Melatonin and its brain metabolite N-acetyl-5-methoxykynuramine prevent mitochondrial nitric oxide synthase induction in Parkinsonian mice. J Neurosci Res 2009; 87: 3002-3010. [ Links ]
39. Wu KK, Cheng HH, Chang TC. 5-methoxyindole metabolites of L-tryptophan: control of COX-2 expression, inflammation and tumorigenesis. J Biomed Sci 2014; 21:17. [ Links ]
40. Ramamoorthy H, Abraham P, Isaac B. Preclinical efficacy of melatonin in the amelioration of tenofovir nephrotoxicity by the attenuation of oxidative stress, nitrosative stress, and inflammation in rats. J Basic Clin Physiol Pharmacol 2014; 27: 1-13. [ Links ]
41. Mehdi SH, Qamar A. Paraquat-Induced Ultrastructural Changes and DNA Damage in the Nervous System Is Mediated via Oxidative-Stress-Induced Cytotoxicity in Drosophila melanogaster. Toxicol Sci 2013; 134(2):355-365. [ Links ]
42. Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC. Environmental risk factors and Parkinson’s disease: a case-control study in Taiwan. Neurology 1997; 48:1583-1588. [ Links ]
43. Brooks AI, Chadwick CA, Gelbard HA, Cory-Slechta DA, Federoff HJ. Paraquat-elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res 1999; 823:1-10. [ Links ]
44. Chanyachukul T, Yoovathaworn K, Thongsaard W, Chongthammakum S, Navasumrit P, Satayavivad J. Attenuation of paraquat-induced motor behavior and neurochemical disturbances by L-valine in vivo. Toxicol Lett 2004; 150:251-269. [ Links ]
45. McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis 2002; 10:119127. [ Links ]
46. Di Monte DA. The environment and Parkinson’s disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol 2003; 2:531-538. [ Links ]
47. Jimenez-Del-Rio M, Guzman-Martinez C, Velez-Pardo C. The effects of polyphenols on survival and locomotor activity in Drosophila melanogaster exposed to iron and paraquat. Neurochem Res 2010; 35:227-238. [ Links ]












 uBio
uBio