Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos Venezolanos de Farmacología y Terapéutica
versión impresa ISSN 0798-0264
AVFT v.20 n.2 Caracas abr. 2001
Archivos Venezolanos de Farmacología y Terapéutica, Volumen 20 - Número 2, 2001 (143-151)
G-Protein-Dependent Antagonists Binding In M3 mAchR from Tracheal Smooth Muscle
AJ Misle1, G Bruges1, VN de Herrera1, M Alfonzo1, IL de Bécemberg1 y R González de Alfonzo1.
- Sección de Biomembranas. Instituto de Medicina Experimental (IME) y Cátedra de Patología General y Fisiopatología. Escuela Luis Razetti. Facultad de Medicina. Universidad Central de Venezuela (UCV).
RESUMEN
Los subtipos M3 y M2 del mAchR fueron localizados en fracciones de membranas plasmáticas de músculo liso traqueal de bovino (MLTB). Los mAchRs nativos de MLTB fueron estudiados usando enlazamiento de [3H]QNB. La hetereogeneidad del receptor fue expresada para agonistas y antagonistas muscarínicos siendo los estados de alta afinidad sensibles a GTP
gS. Mostramos que los efectos de GTPgS parecen ser mediados por proteínas Gi/o sensibles a PTX. Se detectó la hetereogeneidad del mAchR hacia 4-DAMP (M3) y pirenzepina (M1). Sin embargo, los antagonistas subtipo M2 como methoctramina y AF-DX 116 no mostraron tales respuestas en la presencia o ausencia de GTPgS. A través de la alquilación con 4-DAMP del subtipo M3 del mAchR, prevaleció el subtipo M2 el cual exhibió un enlazamiento dependiente de GTPgS parecido a un agonista mientras el enlazamiento de los antagonistas fue insensible al GTPgS. Por lo anterior, la heterogeneidad a antagonistas del receptor descrita aquí es debida al subtipo M3 del mAchR, el cual muestra dos estados de afinidad siendo uno regulado por proteínas Gi/o.Palabras Clave: Agonistas muscarínicos, Antagonistas muscarínicos, Pertussis toxin, GTP
gS, Músculo liso traqueal.Abstract
The M3 and M2 mAchR subtypes were located in plasma membranes fractions from bovine tracheal smooth muscle (BTSM). Native mAchRs from BTSM were studied by using [3H]QNB binding. Receptor heterogeneity was expressed for muscarinic agonists and antagonists being the high affinity states sensitive to GTP
gS. We showed that GTPgS effects seem to be mediated by PTX sensitive Gi/o proteins. Antagonist mAchR heterogeneity towards 4-DAMP (M3) and pirenzepine (M1) was detected. However, M2 antagonists as methoctramine and AF-DX 116 did not show such responses either in the presence or absence of GTPgS. Through 4-DAMP alkylation of the M3 mAchR subtype, a M2 subtype prevailed, which exhibits GTPgS-dependent agonist binding whereas antagonist binding was GTPgS-insensitive. Thus, antagonists binding receptor heterogeneity here described is due to the M3 mAchR subtype showing two affinity states, one being regulated by Gi/o-proteins.Key Words: Muscarinic agonists, Muscarinic antagonists, Pertussis toxin, GTPgS, Tracheal smooth muscle.
Introduction
Airway smooth muscle activation induced by acetylcholine is mediated through muscarinic receptors (mAchRs). These are members of a family of cell surface receptors that activate intracellular responses by coupling G proteins to specific effectors (Kotenis et al., 1999). Molecular cloning studies have revealed the existence of five mammalian subtypes of muscarinic receptors (m1-m5) (Caufield, 1993). In trachea, smooth muscle expresses mRNAs coding for both m2 and m3 receptors (Maeda et al., 1988). Tracheal muscarinic receptors have been identified as a mixed population of M2 and M3 subtypes (Luchesi et al., 1990; Eglen et al., 1996) using pharmacological ligand binding studies. The agonist binding heterogeneity of muscarinic receptors is usually analyzed assuming several, non-interacting sites, modulated by guanine nucleotides, sodium and magnesium ions (Ehlert et al., 1981; Hulme et al., 1983; Alfonzo et al., 1998), and controlled by post-translational modifications of these receptors (Hosey et al., 1999). The mAchR affinity for antagonists is also modulated by guanine nucleotides, ionic strength and temperature (Barlow et al., 1979; Hosey, 1982; Horn et al., 1991; Hou et al., 1996) and usually is interpreted as the presence of two different receptor subtypes (Felder, 1995). Previous investigators have shown that the binding of the muscarinic antagonist [3H]NMS (Hulme et al., 1981) or [3H]QNB (Mattera et al., 1985) resembles two-sites behavior in the heart (M2 receptors) affected by guanine nucleotide in low ionic strength buffer. These prior studies prompted us to investigate the effect of the guanine nucleotide analog, GTPgS on the most abundant muscarinic receptor in tracheal membranes, which are the M2 subtype (Roffel et al., 1988; Luchesi et al., 1990; Misle et al., 1994). In the present paper, we demonstrate a heterogeneous antagonist binding activity in tracheal smooth muscle mAchRs, which is not due to the presence of two different mAchR subtypes. Instead, this antagonist heterogeneity is associated with the M3 mAchR subtype present in plasma membrane fractions from tracheal smooth muscle.
MATERIALS AND METHODS
Materials
The following compounds were purchased from Sigma Chemical Co. (St. Louis, Mo. USA): Trizma base, sucrose, DTT, NAD+, ATP, GTP, GTPgS, SDS, PMSF (phenylmethylsulfonyl fluoride), thymidine, HEPES, MOPS, carbamylcholine, atropine sulfate and Sephadex G-50 (20-80 mm). Bovine serum albumin (Fraction V) was purchased from Armour (USA). Pirenzepine dihydrochloride (PZ), AF-DX 116, 4-DAMP mustard and 4-DAMP methobromide were obtained from RBI (Natick, MA. USA). Methoctramine was a gift from Dr. G. Lambrecht (Frankfurt, FRG). L-[3H]QNB (45.5 Ci/mmol) was obtained from the Radiochemical Centre, Amersham (England) and Pertussis toxin was purchased from List Laboratories, Inc. (USA). Other chemical reagents were obtained from E. Merck (Germany) and Fisher (USA).
Plasma membrane preparation
The plasma membrane fraction (P1) was prepared from bovine tracheal smooth muscle as previously described (Misle et al., 1995; González et al., 1999). Aliquots of plasma membrane fractions were diluted with 80 volumes of 20 mM Tris-HCl (pH 7.2)-0.5 mM DTT and centrifuged at 150,000g for 30 min. Later, the sediment was washed and suspended in a small volume of low ionic strength incubation buffer (66 mM Tris-HCl, pH 7.6).
Selective M3-receptor alkylation
Alkylation of plasma membranes with 4-DAMP mustard was performed in excess of methoctramine to protect the M2 mAchR subtype as described elsewhere (Reddy et al., 1995) with some modifications. Briefly, the aziridinium ion was generated from incubation of the M3-selective irreversible receptor antagonist 4-DAMP mustard at 37° for 30 min. Then, washed plasma membrane fractions were exposed to freshly prepared 4-DAMP mustard (40 nM) aziridinium ion for 60 min, in the presence of methoctramine (1 mM). Thus, the reaction mixture proceeded for 10 minutes in the presence of dithiotreitol (1
mM) and the membranes recovered after centrifugation at 150,000g for 30 min. Finally, the membrane sediment was washed and suspended in 20 mM Tris-HCl (pH 7.2)- buffer and centrifuged four times at a 150,000g for 30 min.Competitive drugs displacement studies
The [3H]QNB displacement studies were performed as described previously (Misle et al., 1994; Misle et al., 1995). Briefly, [3H]QNB binding assay was started by adding membrane protein (2-5
mg) in low ionic strength buffer as 66 mM Tris-HCl (pH 7.8) and increasing concentration of unlabeled drug plus L-[3H]QNB (0.625 nM) to a final volume of 120 ml. After one hour of incubation at 37°, the incubation mixture was placed onto a pre-centrifuged Sephadex G-50 column (3 ml) equilibrated with 0.25 M sucrose-5 mM Tris-HCl (pH 7.8) and immediately centrifuged at 700g for 1.5 min to remove free [3H]QNB. The column effluent containing 95-98% of the bound [3H]QNB was transferred to vials containing Aquasol®. Radioactivity was measured in a RackBeta liquid scintillation counter LKB, Wallac 1214/1219) and all the samples were counted with approximately the same efficiency. Specific binding was calculated by subtracting the non-specific binding (which was less than 1% of total binding measured with 1 mM atropine sulfate), from the total binding (Fields et al., 1978; Misle et al., 1995). In all binding experiments, no more than 5% of the fixed radioligand concentration was allowed to bind to the membranes. Similar amounts of active receptors were employed in the agonist/antagonist binding displacement experiments.Pertussis toxin studies
Activation of Pertussis toxin and the ADP-ribosylation reaction were performed as described elsewhere (Woolkalis et al., 1986) and modified by us (Lippo de et al., 1995). Briefly, the pre-activation reaction was performed in 50 mM HEPES-NaOH, pH 8.0; containing: 0.1 mg/ml BSA, 20 mM DTT, 0.125 % SDS and 20-25
mg of toxin in a volume of 100 ml at 30°. The ADP-ribosylation reaction was carried out in a volume of 1 ml in the presence of 50 mM MOPS, pH 8.0, 1 mM ATP, 0.1 mM GTP, 5 mM MgCl2, 1 mM thymidine and 1 mM NAD+ for 1 h at 30°. The ADP-ribosylation reaction was started by the addition of washed plasma membranes fraction (1 mg). To stop the reaction and remove the ions and nucleotides from the ADP-ribosylation step, aliquots of the reaction mixture (0.3 ml) was applied to a pre-packed (3 ml) Sephadex G-50 column. The last pre-packed column was equilibrated with 0.3 M sucrose-20 mM Tris-HCl buffer, pH 7.6 and centrifuged for 70 sec to obtain the membrane material at the void volume of the column. Samples membranes were run also without PTX in the ADP ribosylation assay to serve as control (-PTX). All treated membrane material was kept on ice and the protein content was determined before the determination of [3H]QNB activity.Protein measurements
Protein was determined by using bovine serum albumin as standard as described elsewhere (Bensadoun and Weinstein, 1976).
Data analysis
Results from competitive experiments were analyzed following "one or two sites" models by a computer-assisted non-linear regression program (InPlot, Graph Pad® software, San Diego, California, U.S.A.). The calculation of IC50 and pseudo-Hill coefficients (np-H) was performed as reported previously (Misle et al., 1994).
RESULTS
Agonist competitive binding studies on native membranes
The effects of GTP
gS on the mAchRs binding properties, muscarinic (agonists and antagonists) displacement experiments were performed. Table 1 shows that IC50 in the absence of GTPgS, for agonists such as carbamylcholine, metacholine and oxotremorine Furthermore, the pseudo-Hill coefficients (np-H) values for these agonists were lower than 1.0, suggesting the presence of agonist receptor heterogeneity. Nonetheless, GTPgS induced dramatic changes in the np-H, which reached values of 1.0 indicating the disappearance of the agonist dependent receptor heterogeneity (Table 1). Moreover, in the presence of GTPgS, the IC50 values for agonists became similar indicating the existence of "one receptor" agonist population. This agonist-dependent muscarinic receptor heterogeneity was analyzed by applying the "two sites" model as the best fitting for all agonists assayed. Table 1 shows that two agonist-binding sites such as the high affinity (RH) and low affinity (RL) states were found. These two agonist states are in a ratio 1:1 in the presence of 5 mM MgCl2. Interestingly, upon addition of 100 mM GTPgS, all agonist displacement curves were significantly shifted to the right as shown in Figure 1. A more careful analysis (Table 1) showed that, GTPgS induced significant changes in the carbamylcholine, metacholine and oxotremorine binding parameters producing a complete disappearance of the RH states together with an increment in the relative amount of receptors in the RL state to 100%.Table 1: Agonist competition binding parameters of muscarinic receptor in tracheal
smooth muscle plasma membranes
| Agonist | Additions | |
| MgCl2 | MgCl2 + GTP gS | |
| Carbamylcholine | ||
| np-H | 0.6 ± 0.1 | 1.0 ± 0.1 |
| %RH | 61.6 ± 6.3 | n.d* |
| %RL | 38.4 ± 6.1 | 100§ |
| pIC50H | 7.3 ± 0.1 | n.d* |
| pIC50L | 5.3 ± 0.2 | 5.1 ± 0.1† |
| Metacholine | ||
| np-H | 0.7 ± 0.1 | 1.0 ± 0.1 |
| %RH | 52.1 ± 5.7 | n.d* |
| %RL | 47.9 ± 5.6 | 100§ |
| pIC50H | 7.1 ± 0.1 | n.d* |
| pIC50L | 5.1 ± 0.1 | 5.1 ± 0.1† |
| Oxotremorine | ||
| np-H | 0.7 ± 0.1 | 0.9 ± 0.1 |
| %RH | 46.6 ± 5.7 | n.d |
| %RL | 53.4 ± 6.0 | 100§ |
| pIC50H | 7.4 ± 0.2 | n.d* |
| pIC50L | 5.2 ± 0.1 | 5.2 ± 0.1† |
Tracheal smooth muscle plasma membranes were prepared as described in Materials and methods. These membranes were used to perform competitive binding curves, carried out in presence of 5 mM MgCl2 or 100 mM GTP
gS + 5 mM MgCl2, which were analyzed to determine the interactions of the ligand with "one" or "two" independent classes of receptor binding sites. The proportion (% RH, % RL ± S. E., high and low agonist site respectively) and the affinity (pIC50H, pIC50L ± S. E., high and low agonist binding site affinity respectively, n = 4) of the receptor sites were calculated as described in Materials and methods.* Not detected, § one site receptor affinity, † pIC50 of one binding site.
Figure 1: Comparison of agonist-[3H]QNB competitive curves for bovine tracheal smooth muscle plasma membranes in the presence of 5 mM MgCl2 () and 5 mM MgCl2 plus 100 mM GTP
gS (). Specific [3H]QNB binding expressed as percentage of binding in the absence of agonists, carbamylcholine oxotremorine and metacholine. Concentration of [3H]QNB was 625 pM and 2-3 mg of membrane proteins were assayed at 37o. Other details are given in Materials and methods. Each point represents the mean of four different membrane preparations assayed by triplicate. The S. E. of these values was less than 5% of the mean.
Antagonist competitive binding studies on native membranes
Antagonists binding properties were also studied by using competition assays. Compounds such as 4-DAMP (M3 antagonist), AF-DX 116 and methoctramine (M2 antagonist) and pirenzepine (PZ), (M1 antagonist) in the presence of MgCl2 and GTPgS were evaluated. These results are shown in Figure 2 and Table 2. We have previously shown that in the basal condition (Misle et al., 1994), all muscarinic antagonists displacement curves fit the "one site" model with np-H values close to 1.0 indicating that these drugs seem to bind to a homogeneous receptor-binding site. In the present work, methoctramine, and AF-DX 116 did not change their binding isotherms, either in the presence of Mg2+ or GTP
gS showing np-H values close to 1.0 (Figure 2 and Table 2). Surprisingly, the antagonists 4-DAMP and PZ showed competitive displacement curves with pseudo-Hill coefficients (np-H) lower than 1.0, suggesting the presence of antagonist receptor heterogeneity in the mAchR. Analysis of the latter antagonist competitive curves obtained in the presence of MgCl2 by applying the "two sites" model unveiled two binding sites in the mAchRs. These new sites were designated as Rh and Rl. The high antagonist affinity (Rh) sites were unmasked in the presence of Mg2+ showing a pIC50 of 8.1 ± 0.2 for 4-DAMP and 6.5 ± 0.2 for PZ, these novel Rh sites disappeared upon addition of GTPgS as shown in Table 2. 4-DAMP and PZ competitive displacement curves also showed changes induced by GTPgS similar to the agonist curves above described. These latter antagonist curves exhibit np-H values of 1.0 indicating the disappearance of the antagonist dependent receptor heterogeneity.Table 2 : Antagonist competition binding parameters of muscarinic receptor in tracheal smooth muscle plasma membranes
| Antagonists | Additions | |
| MgCl2 | MgCl2 + GTP gS | |
| Methoctramine | ||
| Np-H | 1.0 ± 0.1 | 1.1 ± 0.1 |
| pIC50† | 6.7 ± 0.1 | 6.5 ± 0.1 |
| Np-H | 1.0 ± 0.1 | 1.0 ± 0.1 |
| AF-DX 116 | ||
| pIC50† | 5.7 ± 0.1 | 5.8 ± 0.1 |
| 4-DAMP | ||
| Np-H | 0.7 ± 0.1 | 1.0 ± 0.1 |
| pIC50h | 8.1 ± 0.2 | n.d* |
| pIC50l | 6.8 ± 0.6 | 6.9 ± 0.2 |
| Pirenzepine | ||
| Np-H | 0.7 ± 0.1 | 1.0 ± 0.1 |
| pIC50h | 6.5 ± 0.2 | n.d* |
| pIC50l | 5.3 ± 0.2 | 5.0 ± 0.2† |
Native tracheal smooth muscle plasma membrane were isolated as described in Materials and methods and used in all the antagonist displacement assays. Competitive binding curves were carried out in presence of 5 mM MgCl2 or 100 mM GTP
gS + 5 mM MgCl2 and analyzed to determine the interactions of the ligand with "one" or "two" independent classes of receptor binding sites. The pseudo-Hill coefficients (np-H) and the affinity ((pIC50h, pIC50l ± S. E., high and low antagonist binding site affinity respectively, n = 4) of the receptor sites were calculated as described in Materials and methods. The values for the pIC50 for the high affinity antagonist sites (pIC50h) and low affinity antagonist sites (pIC50l) were established and estimated. †pIC50 of one binding site, *not detected.Figure 2: Comparison of antagonist-[3H]QNB competitive curves for bovine tracheal smooth muscle plasma membranes in the presence of 5 mM MgCl2 () and 5 mM MgCl2 plus 100 mM GTP
gS (). Specific [3H]QNB binding is expressed as percentage of binding in the absence of antagonist, 4-DAMP, methoctramine, pirenzepine and AF-DX 116. Concentration of [3H]QNB was 625 pM and 2-3 µg of membrane proteins were assayed at 37o. Other details are given in Materials and methods. Each point represents the mean of four different membranes preparations assayed by triplicate. The S. E. of these values was less than 5% of the mean.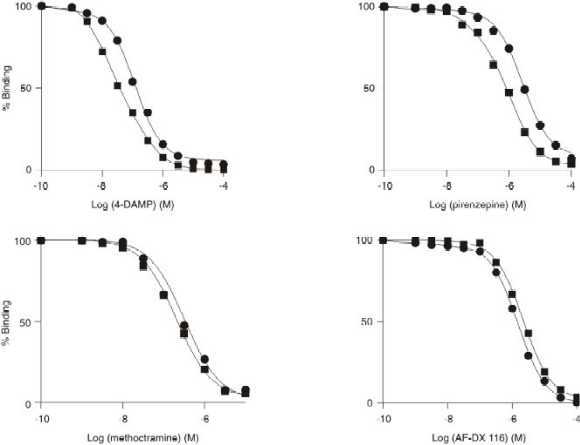
Agonist and antagonist competitive binding studies in alkylated membranes
This antagonist-dependent heterogeneity may be related to a mixture of M2 and M3 mAchR subtypes as above mentioned. In order to explore this possibility, we treated the native plasma membrane fractions with a selective M3-alkylating agent (4-DAMP mustard), and protecting the M2 subtype with methoctramine as described in Methods. As expected, the alkylated membranes (Table 3) showed IC50 values, which correspond to a M2 pharmacological pattern (4-DAMP · methoctramine > AF-DX 116 > PZ). Interestingly, all antagonist-binding isotherms in the alkylated membranes showed np-H values of 1.0 and fitted for "one" homogeneous binding site, which was unaffected in the presence of GTP
gS (Table 3). However, the competition experiments in the presence of the agonist carbamylcholine still showed high (RH) and low sites (RL), (Table 3). Moreover, The competition experiment with this agonist using alkylated membranes were affected by GTPgS in such a way that the high agonist sites (RH) completely disappeared in the presence of this guanine nucleotide analog leaving only low agonist sites (Table 3).Table 3: Drugs competition binding parameters of muscarinic receptor in 4-DAMP alkylated plasma membranes from tracheal smooth muscle
| Muscarinic compounds | Additions | |
| MgCl2 | MgCl2 + GTP gS | |
| Antagonists | ||
| Methoctramine | ||
| np-H | 1.0 ± 0.1 | 1.1 ± 0.1 |
| pIC50† | 6.4 ± 0.1 | 6.4 ± 0.1 |
| np-H | 1.0 ± 0.1 | 1.0 ± 0.1 |
| AF-DX 116 | ||
| pIC50† | 5.3 ± 0.1 | 5.3 ± 0.1 |
| 4-DAMP | ||
| np-H | 1.0 ± 0.1 | 1.0 ± 0.1 |
| pIC50† | 6.4 ± 0.6 | 6.4 ± 0.2 |
| Pirenzepine | ||
| np-H | 1.1 ± 0.1 | 1.0 ± 0.1 |
| pIC50† | 5.1 ± 0.2 | 4.9 ± 0.2 |
| Agonists | ||
| Carbamylcholine | ||
| np-H | 0.6 ± 0.1 | 1.0 ± 0.1 |
| %RH | 37.4 ± 4.5 | n.d* |
| %RL | 63.6 ± 5.0 | 100§ |
| pIC50H | 7.3 ± 0.2 | n.d* |
| pIC50L | 5.1 ± 0.1 | 5.6 ± 0.2† |
4-DAMP mustard-alkylated tracheal smooth muscle plasma membranes were prepared as described in Materials and methods. Similar quantities of active receptors were used in all the muscarinic drug displacement assays. Competitive binding curves were carried out in the presence of 5 mM MgCl2 or 100 mM GTPgS + 5 mM MgCl2 and analyzed to determine the interactions of the ligand with "one" or "two" independent classes of receptor binding sites. The pseudo-Hill coefficients (np-H) and the affinity (pIC50† ± S. E, n = 4) of the receptor sites were calculated as described in Materials and methods. The proportion (% RH, % RL ± S. E., high and low agonist site respectively) and the affinity (pIC50H, pIC50L ± S. E., high and low agonist binding site affinity respectively, n = 4) of the receptor sites were calculated as described in Materials and methods. *Not detected, § one site receptor affinity, † pIC50 of one binding site.
Effect of Pertussis toxin on native membranes
The GTP
gS effects seem to be mediated though G proteins. Thus, additional experiments were performed on native membranes using Pertussis toxin (PTX) to inactivate Gi/o proteins as described in Methods. The PTX effect was only evaluated with the drugs that exhibited the "maximal right-shift" responses in the presence of GTPgS, such as the agonist (oxotremorine) and the antagonist, (PZ). Thus, PTX treatment of native membranes produced a complete decrease of the high agonist affinity binding state (RH) for oxotremorine in the presence of Mg2+ (Table 4). However, GTPgS did not modify the latter response, as shown in Table 4. Similarly, when the antagonist, PZ was assayed on PTX-treated membranes, in the presence of Mg2+, the high affinity antagonist-state (Rh) disappeared. Again, GTPgS did not further affect the PZ displacement curve (Table 4) indicating that PTX abolished the PZ dependent Rh state of these mAchRs.Table 4: Effect of Pertussis toxin and GTP
gS on agonist and antagonist binding parameters of muscarinic receptor in tracheal smooth muscle plasma membranes| Drugs | -PTX | +PTX | ||
| MgCl2 | MgCl2 + GTP gS | MgCl2 | MgCl2 + GTP gS | |
| Oxotremorine | ||||
| np-H | 0.7 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| pIC50H | 7.5 ± 0.1 | n.d* | n.d* | n.d* |
| pIC50L | 5.2 ± 0.5 | 5.2 ± 0.4† | 5.2 ± 0.2† | 5.2 ± 0.2† |
| Pirenzepine | ||||
| np-H | 0.7 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| pIC50h | 6.3 ± 0.1 | n.d* | n.d* | n.d* |
| pIC50l | 5.3 ± 0.2 | 5.0 ± 0.1† | 5.0 ± 0.1† | 5.0 ± 0.1† |
Native membranes treated with pertussis toxin (+PTX) and control membranes (-PTX) were prepared as described in Materials and methods and competition experiments carried out in the presence of 5 mM MgCl2 or 100 mM GTP
gS + 5 mM MgCl2. Competitive binding curves were analyzed to determine one or two independent receptor binding sites. The affinity (pIC50H, pIC50L ± S. E., high and low agonist binding site affinity respectively, pIC50h, pIC50l ± S. E., high and low antagonist binding site affinity respectively, n = 4) of the receptor sites were calculated as described in Materials and methods. *Not detected, † pIC50 of one binding site.DISCUSSION
The [3H]QNB binding to mAchRs from tracheal smooth muscle plasma membranes behaved as a Langmuir isotherm, as previously reported. (Misle et al., 1994; Misle et al., 1995). In heart (McMahon and Hosey, 1985) or in other tissues (Ham and Gilchrist 1996) as in tracheal plasma membranes (Luchessi et al., 1990; Roffel et al., 1998; Misle et al., 1994) muscarinic receptor agonist heterogeneity modulated by guanine nucleotides has been described as a mixture of M3 and M2 mAchRs subtypes.
Our results using classic muscarinic agonists such as carbamylcholine, metacholine and oxotremorine support these previous findings. Muscarinic agonists operate through several affinity states of the receptor (Mitchelson, 1998, Hulme et al., 1990). The high agonist affinity state (RH) is the receptor-coupled G-protein complex (G-R*), while the low agonist affinity state (RL) is the free uncoupled receptor (R) (Hulme et al., 1990). In the present work, mAchR agonist heterogeneity was detected in the presence of Mg2+, and disappeared in the presence of GTP
gS. These GTPgS effects are explained by the irreversible activation of G-proteins by this nucleotide producing the dissociation of the G-R*, disappearing the RH and displaying the free RL (Birnbaumer et al., 1982; Ham and Gilchrist, 1996). The GTPgS and the PTX effects on agonists binding here described support the fact that PTX-sensitive Go/i-proteins are involved in these mAchR responses. Whenever, the ADP-ribosylation of G-proteins (Go/i) is catalyzed by PTX, its biological action is perturbed (Birnbaumer et al., 1982). Interestingly, in such isolated systems as plasma membrane fragments, the relative amount of mAchRs subtypes should not be affected by GTPgS or PTX treatments.Muscarinic receptor antagonist heterogeneity has also been described (Ehlert et al., 1981; Hulme et al., 1981; Hosey, 1982). This antagonist heterogeneity is interpreted as two different receptor subtypes (Felder, 1995). However, other investigators (Hulme et al., 1981; Mattera et al., 1985) showed that this heterogeneity is a function of two-sites behavior modulated by guanine nucleotides.
In the present work the involvement of mAchR subtypes in these GTP
gS effects was investigated using specific muscarinic antagonists. Previous studies have shown that GTP analogs have different opposite effects on receptor affinity for antagonists depending on the system under study. In chick heart, Gpp(NH)p increased receptor affinity (Hosey, 1982), but GTPgS decreased receptor affinity in rat parotid membranes (Horn et al., 1991). Thus, [3H]QNB displacement curves were performed with antagonists related to the M2, M4 family such as methoctramine and AF-DX 116 and the M3, M1, M5 family as 4-DAMP and PZ. M2 antagonists (methoctramine and AF-DX 116) bind to tracheal smooth muscle mAchRs with a similar pharmacological pattern either in the presence or absence of GTPgS. Apparently, these compounds are no able to detect the two-mAchR states (Birdsall et al., 1983) induced by Mg2+ and regulated by GTPgS through G-proteins as suggested by PTX experiments. Therefore, these drugs bind with equal affinity to both "G-R*" and "R" states behaving as "neutral antagonists" as defined elsewhere (Costa et al., 1991).However; M3 and M1 antagonists such as 4-DAMP and PZ, exhibited heterogeneous binding isotherms with two different binding sites in the presence of Mg2+, being sensitive to GTP
gS suggesting that G-proteins may be involved in this biological action. This is an original finding related to 4-DAMP and PZ showing a G-protein dependent differential binding in these mAchRs associated with a plasma membranes fraction isolated from tracheal smooth muscle. The apparent heterogeneity of 4-DAMP and pirenzepine seems to be specific for these drugs because under identical experimental conditions specific M2 antagonists such as methoctramine and AF-DX 116 did not modify the displacement pattern curves. In addition, the competitive curves obtained with the four antagonists here studied using the 4-DAMP mustard alkylated membranes, showed a classic M2 profile.This antagonist heterogeneity showed by 4-DAMP and PZ in these plasma membranes fractions is assumed as two populations of binding sites for muscarinic antagonists as described in rat heart (Hulme et al., 1981), colonic circular smooth muscle (Zhang et al., 1992) and airway smooth muscle (Luchessi et al., 1990; Mak et al., 1992; Roffel et al., 1988). However, there was not direct experimental evidence to support that assumption. In this work, we found that the 4-DAMP alkylated plasma membranes displayed a pharmacological profile that belongs to a M2 subtype as expected after abrogating the M3 subtype. In this sense, our results with 4-DAMP mustard supported the existence of both M2 and M3 mAchR subtypes in these plasma membranes fractions. Also, specific binding affinities for antagonists of the "remaining M2 subtype" were not affected by GTP
gS. Nonetheless, the binding affinity for the agonist, carbamylcholine was affected by GTPgS showing the functionality of the "remaining M2 subtype". The latter finding suggests that, under our experimental conditions, the M3 subtype is the mAchR subtype involved in the GTPgS-dependent heterogeneous binding activity here observed. At this time it is difficult to explain the molecular mechanism that allow the "agonist-like behavior" exhibited by 4-DAMP and PZ.Several studies in airway smooth muscle have shown a M3 subtype coupled via a G-protein which increases PI metabolism (Felder, 1995), while the M2 subtype strongly inhibits adenylyl cyclase through coupling to a Pertussis toxin-sensitive G-protein (Widopp et al., 1993). G-proteins coupled to the M3 mAchRs seem to be diverse. It has been claimed that M3 mAchR is coupled to PTX insensitive G-proteins (Luchessi et al., 1990; Sawaki et al., 1995.) and PTX-sensitive G-proteins (Gi/o proteins) (Schmidt et al., 1996).
Our results using binding assays indicated that the M3 mAchR is coupled to PTX-sensitive Gi/o. A similar signal transducing-cascade is involved in the heterologous potentiation of PLC activity and PLC enhanced substrate supply in human embryonic kidney cells (Schmidt et al., 1996). It is possible that this PLC-Gi/o coupled to M3 mAchR cascade exists in these plasma membranes fractions and exerts an important role in the contraction/relaxation of tracheal smooth muscle as suggested elsewhere (Yang et al., 1993). Although, GTP
gS does not affect M2 mAchR antagonist binding, the agonist binding is indeed affected, suggesting that this subtype is coupled to G-proteins.The [3H]QNB binding affinity here described is an expression of two mAchR subtypes (M2 and M3) as above discussed. Moreover, each one of them may exhibit two antagonist affinity states (high and low). Our results also support the hypothesis (Burgisser et al., 1982; De Lean et al., 1982a; De Lean et al., 1982b), where the high and low affinity states of a receptor can be discriminated by both agonists and antagonists exhibiting a G-protein dependent inter-conversion. It can be postulated that, under our experimental conditions, the M3 mAchR subtype associated with plasma membranes from tracheal smooth muscle, displays the agonist binding affinity states (RH, RL) and the antagonist binding affinity states (Rh, Rl). Apparently, there is an experimental correlation between the agonist (RH) with the antagonist (Rh) states for the M3 mAchR being both regulated by G-proteins. At this time, RL and Rl affinity states are difficult to identify using this binding approach.
In summary, mAchRs associated with tracheal smooth muscle plasma membrane fractions are a mixture of M2 and M3 muscarinic receptor subtypes, which exhibit antagonist receptor heterogeneity for 4-DAMP and PZ mediated through PTX-sensitive G-proteins. This antagonist heterogeneity can be explained in part, by the co-existence of at least two different affinity states for the M3 subtype, being ones regulated by Gi/o proteins.
FOOTNOTES
mAchR, muscarinic acetylcholine receptor; GTP
gS, guanosine 5’-(3-O-thio) triphosphate; DTT, 1,4-dithiothreitol; [3H]QNB, L-[benzilic-4,4'-3H]-quinuclidinyl benzilate; 4-DAMP, 4-diphenylacetoxy-N-methylpiperidine; PTX, Pertussis toxin; AF-DX 116, 11[[2-[(diethyl-amino)methyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H-pyrido-[2,3-b][1,4]benzodiazepine-6-one.
Acknowledgments
This work was supported by grants from CONICIT (S1-97000116) to R.A and partially by CONICIT (S1-2749) and CDCH (09.33.3436/99) to I. L. de B.
References
1. Alfonzo MJ, IL de Bécemberg, SS de Villaroel, VN de Herrera, JA Misle and RG de Alfonzo. Two opposite signal transducing mechanisms regulate a G protein coupled guanylyl cyclase. Arch. Biochem. Biophys. 1998; 350: 19. [ Links ]
2. Barlow RB, NJM Birdsall and EC Hulme. Temperature coefficients of affinity constants for the binding of antagonist to muscarinic receptors in the rat cerebral cortex. Br. J. Pharmacol. 1979; 66: 587. [ Links ]
3. Bensadoun A and D Weinstein. Assay of proteins in the presence of interfering materials. Anal. Biochem. 1976; 70: 241. [ Links ]
4. Birdsall NJM, EC Hulme and JM Stockton. Muscarinic receptor subclasses: Allosteric interactions. In Cold Spring Harbor Symposia on Quantitative Biology, 1983; 48: 53-56. [ Links ]
5. Birnbaumer L, J Abramowitz and AM Brown. Receptor-effector coupling by G proteins. Biochim. Biophys. 1990; 163: 1031. [ Links ]
6. Burgisser E, De Lean A and RJ Lefkowitz. Reciprocal modulation of agonist and antagonist binding to muscarinic cholinergic receptors by guanine nucleotide. Proc. Natl. Acad. Sci. U.S.A. 1992; 79: 1732. [ Links ]
7. Caufield MP. Muscarinic receptors–Characterization, coupling and function. Pharmacol. Ther. 1993; 58: 319. [ Links ]
8. Costa T, Y Ogino, PJ Munson, HO Onaran and D Rodbard. Drug efficacy at guanine nucleotide-binding regulatory protein-linked receptors: thermodynamic interpretation of negative antagonism and receptor activity in the absence of ligand. Mol. Pharmacol. 1991; 41: 549. [ Links ]
9. De Lean A, BF Kilpatick and M Caron. Dopamine receptor of the porcine anterior pituitary gland: evidence for two affinity states discriminated by both agonist and antagonist. Mol. Pharmacol. 1982; 22: 290. [ Links ]
10. De Lean A, JM Stadel and RJ Lefkowitz. A ternary complex-model explains the agonist-specific binding properties of the adenylate cyclase-coupled -adrenergic receptor. J. Biol. Chem. 1982; 255: 7108. [ Links ]
11. Eglen RM, SS Hedge and N Watson. Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 1996; 48: 531. [ Links ]
12. Ehlert F-J, WR Roeske and HI Yamamura. Muscarinic receptor regulation by guanine nucleotides, ion and N-etlhylmaleimide. Fed. Proc. 1981; 40: 153. [ Links ]
13. Felder CC. Muscarinic acetylcholine receptors: signal tranduction through multiple effectors. FASEB J. 1995; 9: 619. [ Links ]
14. Fields JZ, WR Roeske, E Morkin and HI Yamamura. Cardiac muscarinic cholinergic receptors. Biochemical identification and characterization. J. Biol. Chem. 1978; 253: 3251. [ Links ]
15. González LG, A Misle, G Pacheco, VN de Herrera, RG de Alfonzo, IL de Bécemberg and MJ Alfonzo. Effects of 1H-[1, 2, 4]Oxadiazolo[4, 3,] quinoxalin-1-one (ODQ) and Nw(6)-nitro-L-arginine methylester (NAME) on cyclic GMP levels during muscarinic activation of tracheal smooth muscle. Biochem. Pharmacol. 1999; 58: 563. [ Links ]
16. Ham HE and A Gilchrist. Heterotrimeric G proteins. Curr. Opinion Cell. Biol. 1996; 8: 189. [ Links ]
17. Horn VJ, IS Ambudkar and BJ Baum. High affinity quinuclidinyl benzilate binding to rat parotid membranes requires muscarinic receptor G protein interactions. FEBS 1991; 282: 289. [ Links ]
18. Hosey MM. Regulation of antagonist binding to cardiac muscarinic receptors. Biochem. Biophys. Res. Commun. 1982; 107: 314. [ Links ]
19. Hosey MM, R Pals-Ryaarsdam, KB Lee, AG Roseberry, JL Benovic, VV Gurevich and M Büneman. Molecular events associated with the regulation of signaling by M2 muscarinic receptors. Life Sci. 1999; 64: 363. [ Links ]
20. Hou X, JM Wehrle, E Ciccarelli, J Wess, E Mutschler, G Lambrecht, H Timmerman and M Waelbroeck. Influence of monovalent cations on the binding of a charged and an uncharged (‘carbo’-) muscarinic antagonist to muscarinic receptor. Br. J. Pharmacol. 1996; 117: 955. [ Links ]
21. Hulme EC, CP Berrie, NJM Birdsall and ASV Burgen. Two populations for binding sites for muscarinic antagonists in the rat heart. Eur. J. Pharmacol. 1981; 73: 137. [ Links ]
22. Hulme EC, CP Berrie, NJM Birdsall, M Jameson and JM Stockton. Regulation of muscarinic agonist binding by cations and guanine nucleotides. Eur. J. Pharmacol. 1983; 94: 59. [ Links ]
23. Hulme EC, NJM Birdsall and NJ Buckley. Muscarinic receptor subtypes. Annu. Rev. Pharmacol. Toxicol. 1990; 30: 633. [ Links ]
24. Kotenis E, Zeng Fu-Yue and J Wess. Structure-function of muscarinic receptors and their associated proteins. Life Sci. 1999; 64: 335. [ Links ]
25. Lippo de Bécemberg I, MF Correa de Adjounian, E Peña de Aguilar, R González de Alfonzo and M Alfonzo. G-protein-sensitive guanylyl cyclase activity associated with plasma membranes. Arch. Biochem. Biophys. 1995; 324: 209. [ Links ]
26. Luchesi PA, CR Scheid, FD Romano, ME Kargacin, D Mullikin-Kilpatrick, H Yamaguchi and TW Honeyman. Ligand binding and G protein coupling of muscarinic receptors in airway smooth muscle. Am J Physiol 1990; 258: C730. [ Links ]
27. Maeda A, Kubo T, M Mishina and S Numa. Tissue distribution of mRNAs encoding muscarinic Acetylcholine receptor subtypes. FEBS Lett. 1988; 239: 339. [ Links ]
28. Mak JCW, JN Baranouk and PJ Barnes. Localization of muscarinic receptor subtypes in human lung. Am. J. Respir. Cell Mol. Biol. 1992; 7:344. [ Links ]
29. Mattera R, JR Pitts, ML Entman and L Birnbaumer. Guanine nucleotide regulation of a mammalian myocardial muscarinic receptor system. J. Biol. Chem. 1985; 260: 7410. [ Links ]
30. McMahon KK and M Hosey. Agonist interaction with cardiac muscarinic receptors. Mol. Pharmacol. 1985; 28: 400. [ Links ]
31. Misle AJ, Lippo de I Bécemberg, González de R Alfonzo and M Alfonzo. Methoctramine binding sites sensitive to alkylation on muscarinic receptors from tracheal smooth muscle. Biochem. Pharmacol. 1994; 48: 191. [ Links ]
32. Misle JA, Lippo de I Bécemberg, R González de Alfonzo and MJ Alfonzo. Subcellular distribution and pharmacological characterization of receptors from tracheal smooth muscle. Acta Cient Ven. 1995; 46: 1. [ Links ]
33. Mitchelson F. Muscarinic receptor differentiation. Pharmacol. Ther. 1988; 37: 357. [ Links ]
34. Reddy H, N Watson, APDW Ford and RM Eglen. Characterization of the interaction between muscarinic M2 receptor and -adrenoreceptor subtypes in guinea-pig isolated ileum. Br. J. Pharmacol. 1995; 114: 49. [ Links ]
35. Roffel AF, CRS Elzinga, RGM Van Amsterdam, RA De Zeueuw and J Zaagsma. Muscarinic receptors in bovine tracheal smooth muscle: Discrepancies between binding and function. Eur. J. Pharmacol. 1988; 153:73. [ Links ]
36. Sawaki K, BJ Baum and IS Ambudkar. Alpha 1-adrenergic and m3-muscarinic receptor stimulation of phosphatidylinositol 4,5-bisphosphate-specific phospholipase C are independently mediated by G alpha q/11 in rat parotid gland membranes. Arch Biochem Biophys. 1995; 10: 535. [ Links ]
37. Schmidt M, C Nehls, U Rumenapp and KH Jakobs. M3 muscarinic receptor induced and Gi-mediated heterologous potentiation of phospholipase C: Role of phosphoinositide synthesis. Mol. Pharmacol. 1996; 50: 1038. [ Links ]
38. Widopp S, K Daykin and I Hall. Expression of muscarinic M2 receptors in cultured airway smooth cells. Am. J. Respir. Cell. Mol. Biol. 1993; 9: 541. [ Links ]
39. Woolkalis MJ, MT Nakada and DR Manning. Alterations in components of Adenylate cyclase associated with transformation of chicken fibroblasts by Rous sarcoma virus. J. Biol. Chem. 1986; 261: 3408. [ Links ]
40. Yang CM, SP Chou, YY Wang, JT Hsieh and R Ong. Muscarinic regulation of cytosolic free calcium in canine tracheal smooth muscle cells: Ca 2+ requirement for phospholipase C activation. Br. J. Pharmacol. 1993; 110: 1239. [ Links ]
41. Zhang L, B Horowitz and I Buxton. Muscarinic receptors in canine colonic circular smooth muscle I. Coexistence of M2 and M3 subtypes. Mol. Pharmacol. 1992; 40: 943. [ Links ]













