Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos Venezolanos de Farmacología y Terapéutica
versión impresa ISSN 0798-0264
AVFT vol.31 no.4 Caracas dic. 2012
Selective Mastoparan inhibition of muscarinic activation of bovine tracheal smooth muscle
1Walid Hassan-Soto, 2Lérida Guerra de González, 3Ramona González de Alfonzo, 4Itala Lippo de Becemberg and 5Marcelo J. Alfonzo*
1 Magister Scientiarum en Bioquímica.
2 Doctora en Bioquímica,
3 Doctora en Bioquímica, Biología Celular y Molecular,
4 Doctora en Medicina
5 Doctor en Bioquímica, Biología Celular y Molecular
Corresponding Author*: Dr. Marcelo J. Alfonzo. Sección de Biomembranas. Instituto de Medicina Experimental. Facultad de Medicina Universidad Central de Venezuela. Ciudad Universitaria. Caracas. Venezuela. Teléfono: 0212-605-3654. Email: hmag5@hotmail.com
Abstract
Muscarinic activation of bovine tracheal smooth muscle (BTSM) leading to smooth muscle contraction involves the generation of two cGMP signals (20 and 60 s), being 20s peak associated with soluble (sGC) and the second (60s) to membrane-bound Natriuretic Peptide- receptor-Guanylylcyclases (NPR-GC). In this study, we showed that pre-incubation of isolated BTSM strips with mastoparan and superactive mastoparan (mastoparan 7) decreased significantly the muscarinic dependent contractile smooth muscle responses in dose-dependent and non-competitive manner. Moreover, mastoparan (50 nM) inhibited completely the BTSM-muscarinic contractile responses and affected dramatically the carbachol-dependent cGMP signals being the first cGMP signal inhibited in a 63 ± 5%, whereas the second signal disappeared. Mastoparan inhibition of muscarinic activation is specific since other spasmogens as serotonin and histamine fully contracted these BTSM strips under mastoparan treatment. Cyclic GMP levels were evaluated by exposing BTSM strips to activators of NO-sensitive sGC as Sodium Nitroprussiate (SNP) and Natriuretic Peptides as CNP-53 for membrane-bound NPR-GC. Thus, SNP and CNP increased in a binary way, in more than 20 fold cGMP levels at 30-40 s being both increments inhibited by mastoparan. Furthermore, the Gi/o-protein involvement on mastoparan inhibition of cGMP elevations induced by CNP and SNP is suggested by Pertussis toxin pre-treatment, which reversed mastoparan effects. These results indicate that muscarinic signal transduction cascades leading to airway smooth muscle contractions involved two different guanylyl cyclases being both regulated by mastoparan-sensitive G-proteins.
Abbreviations: ANP, Natriuretic Peptide type A; ASM, Airway Smooth Muscle; BTSM, Bovine Tracheal Smooth Muscle; CNP-53, Natriuretic Peptide type C-53; GPCR, G-Protein Coupled Receptor; Gq16, Heterotrimeric G protein subtype 16; Gi/o, Heterotrimeric G protein subtype i/o; m2AChR, muscarinic receptor type 2; m3AchR, muscarinic receptor type 3; NP, Natriuretic Peptides; NPR-GC, Natriuretic Peptides Receptor Guanylyl Cyclase; NPR-A, Natriuretic Peptides Receptor Guanylyl Cyclase type A ; NPR-B, Natriuretic Peptides Receptor Guanylyl Cyclase type B; PTX, Pertussis Toxin; sGC, soluble Guanylyl Cyclase; SNP, sodium nitroprusside; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; PDE, cyclic nucleotide phosphosdi-esterase; TCA, tricloroacetic acid and BTSM, Bovine tracheal smooth muscle.
Key words: Tracheal smooth muscle; carbachol; mastoparan; soluble guanylyl cyclase; Natriuretic Peptide Receptor-guanylyl cyclase.
Resumen
La activación muscarínica del músculo liso de las vías aéreas relacionada a la contracción de dicho músculo liso esta asociada a la generación de dos señales de GMPc (20 y 60 s), siendo la señal de los 20s relacionado a la activación de la guanililciclasa soluble mientras que el pico de los 60s a la guanililciclasa unida membranas y sensible a péptidos natriuréticos (NPR-GC). En este trabajo, nosotros mostramos que la pre-incubación de fragmentos del músculo liso traqueal de bovino (BTSM) con mastoparan y su análogo superactivo (mastoparan 7), en una forma dosis dependiente, son capaces de disminuir de manera significativa la actividad contráctil dependiente de agentes muscarinicos. Adicionalmente, mastoparan (50 nM) inhibió completamente la respuesta contráctil muscarinica del BTSM y afectó dramáticamente los picos de GMPc asociados a la activación muscarinica siendo la primera señal inhibida en un 63 ± 5%, mientras que la segunda señal desapareció completamente. Esta inhibición del mastoparan de la activación muscarínica es especifica ya que otros espamogenos como la serotonina y la histamina fueron capaces de inducir respuestas máximas en presencia del mastoparan y su análogos. Este efecto del mastoparan sobre los niveles del GMPc fue evaluado en presencia de otros agentes generadores de este segundo mensajero como son el nitroprusiato de sodio (SNP) que activa la guanililciclasa soluble sensible a NO y los péptidos natriureticos como el CNP-53 (CNP) activador de la NPR-GC asociada a membranas plasmáticas. Tanto, el SNP como el CNP aumentaron en mas de 50 veces los niveles de GMPc a los 30-40 s en forma bifasica, siendo estos incrementos inhibidos de manera significativa por el mastoparan. Ademas, se sugiere la participación de proteínas Gi/o en los efectos inhibitorios del mastoparan, porque la Toxina pertussis revertió los efectos inhibitorios. Estos resultados indican que la cascada de activación muscarinica que conduce a la contracción del músculo liso de las vías aéreas involucra a 2 diferentes guanililciclasas y ambas son reguladas por proteínas G sensibles al mastoparan.
Palabras claves: Músculo liso traqueal, carbamilcolina, mastoparan, guanililciclasa soluble, guanililciclasa sensible a péptidos natriúreticos.
Recibido: 20/09/2011 Aceptado: 21/01/2012
Introduction
Muscarinic activation is physiologically responsible for the contraction of the airways smooth muscle (ASM)1. Thus, the interaction between ACh with its muscarinic receptors (mAChRs), causes the contraction of the ASM, the mucous secretion stimulation and increase in the ion transport through epithelia of the airways, which can be associated to bronchial hyper-reactivity presents in bronchial asthma2.
The muscarinic-dependent contraction of the ASM is initiated by mAChRs activation at the smooth muscle sarcolemma leading to the generation of the second messengers such as cGMP3, being this cyclic nucleotide involves, in both, the contraction3,4 or relaxation5 of the ASM. Airway smooth muscle cells have two sources of cGMP, one is associated with the activation of an hemoprotein, NO-sensitive guanylyl cyclase (sGC) mainly located at the cytoplasm and the second ones is related to Natriuretic Peptide Receptor guanylyl cyclases (NPR-GC) at the ASM sarcolemma6.
Two mAChRs (m2 and m3) subtypes are present at tracheal smooth muscle with m2/m3 (4/1) ratio in most of smooth muscles studied7,8. These two receptors can interact, in opposite way, with the NPR-GC located at smooth muscle sarcolemma6,9. Thus, m2AChRs and m3AChRs are coupled to Gq16 and Gi/o proteins respectively, which mediate the NPR-GC stimulation, via Gq16 10 and via Gi/o proteins, the inhibition of this NPR-GC6. These two G proteins are sensitive to mastoparan, a cationic tetradecapeptide isolated from the wasp venom Vespula lewisii11. Mastoparan activates directly G proteins increasing the GTP/GDP exchange activity, which mimics the GDP/GTP Exchange Factor (GEF) activity associated with some G proteins coupled receptors (GPCR)12. Therefore, muscarinic activation of bovine tracheal smooth muscle (BTSM) is mediated by G-proteins and mastoparan can influence this activation.
Thus, in the present work, we studied the effects of mastoparan on the muscarinic activation of BTSM, specifically on some biological activities induced by muscarinic agonist (carbachol): 1.- The ASM contractile responses and 2.- the cGMP signals associated with such activation. Furthermore, the mastoparan effects were evaluated in the presence of others tracheal smooth muscle cGMP elevating substances such as Sodium Nitroprusside (SNP), which is a NO donor and a classic activator of the sGC13 and the Natriuretic Peptides (CNP-53) to stimulate the plasma membrane bound NPR-GC-B14 being both enzymes locate in this smooth muscle type.
Materials and Methods
Materials
Atropine, carbachol, EDTA, glucose, histamine, serotonin and Pertussis toxin were purchased from Sigma Chemical Company. CNP, mastoparan and mastoparan 7 were obtained from American Peptide Co. Chemical reagents and salts were obtained from Merck and Fisher Co. Cyclic [8,5-3H]GMP (25-50 Ci/mmol) and Liquifluor were purchased from New England Nuclear. Diethylether from BDH-Chemicals GPR.
Biotra Assay System TRK 500 for determination of cGMP from Amersham-GE.
Preparation of Bovine tracheal smooth muscle
Tracheal smooth muscle was dissected from fresh bovine tracheas obtained from the local slaughterhouse and transferred to the laboratory in solution Krebs Ringer- Bicarbonate (KRB), the composition of this KRB in mM is : NaCl 118.5; KCl 4.47; MgS04 1.18; KH2P04 1.18; CaCl2 2.54; NaHC03 24.9; Glucose 10; pH: 7.4. Once dissected the smooth muscle was placed in the buffer KRB bubbled with a mixture of 95% O2/5% CO2 at room temperature. Fresh KRB was replaced each 30 min. BTSM strips were employed within 3 hours, after dissection.
Incubation of Smooth Muscle Fragments
The evaluation of smooth muscle contraction and nucleotide concentration was performed using two procedures as previously described4.
Procedure 1: Briefly, smooth muscle fragments were placed into an organ bath (20 mL) and equilibrated for 1 hr in KRB with 95% O2 and 5% CO2 (pH 7.4) at 37°, with medium replacement every 30 min. Strips were loaded with 1 g of tension, and the contraction was expressed as an increase in tension of these preparations, measured isometrically by using a force displacement transducer (Grass model FT03) attached to a polygraph (Grass model 7-B). After 1 hr of incubation, the different pharmacological agents (less than 20 μL) were added. Later, the bath was drained rapidly, and the strip was frozen in liquid nitrogen. The latter step took around 5 s.
Procedure 2: Smooth muscle strips were placed into a specially designed multi-organ chamber with a volume of 400 mL. This chamber has a system of aeration with 95% O2 and 5% CO2, and it is able to hold simultaneously some 16 strips at 37°, at 1g of tension. After addition of drugs, individual fragments were removed from the chamber, every 10 s and placed into liquid nitrogen (within less than 1s). Samples were kept in liquid nitrogen until nucleotide extraction was performed.
Measurement of Cyclic GMP
Briefly, frozen samples were thawed and homogenized in 6% TCA as previously described4. TCA extractions were performed twice, and the insoluble material was removed by centrifugation at 1500x g for 10 min at 4°. The insoluble material was processed for protein determination as described later. The acid supernatants were combined, extracted twice with water-saturated diethylether to remove TCA, frozen in liquid nitrogen, and lyophilized. The acid-soluble lyophilized material was dissolved in a small volume of 50 mM Tris, 4 mM EDTA, pH 7.4, that was named the acid-soluble nucleotide extract, which was kept frozen at -80°. In each experiment, some untreated frozen strips were used to evaluate the cyclic nucleotide recovery following the procedure described latter. For this assay, 0.4 pmol of [3H]cGMP was added to some samples, and the recovery was between 95 and 98% for this labeled nucleotide. This recovery rate was assumed to be the same for all samples. cGMP was determined using a radioimmunoassay as previously described4 with a commercial kit (TRK 500) from Amersham. TCA-insoluble material was dissolved in 2 mL of 1 N NaOH and incubated at room temperature during overnight and later was diluted five times to determine total tissue protein content by using a procedure described elsewhere15.
Cyclic nucleotide values are presented as pmoles cGMP /mg of total tissue protein.
There were no differences in the cyclic nucleotide responses to the agents tested under the two experimental procedures as above described.
Results
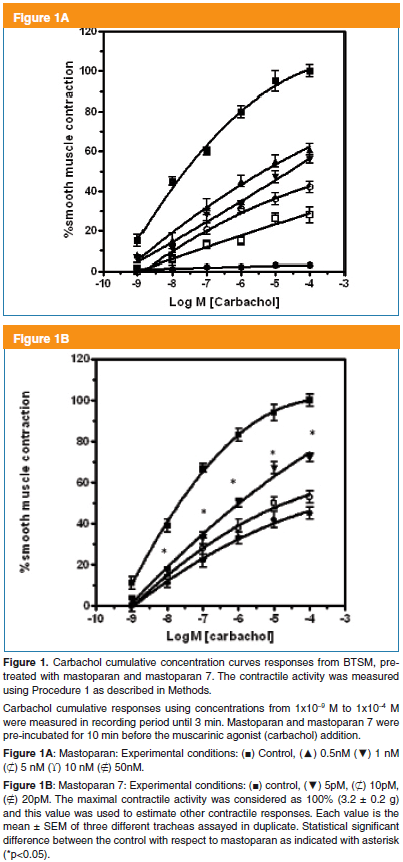
BTSM strips, after 1 hr of stabilization using Procedure 1, were pre-incubated for 15 min under three different experimental conditions. Thus, the first condition was in the presence of mastoparan (MP), a second with super-active mastoparan analog as mastoparan 7 (MP-7) and a third, without mastoparans (Control condition). After, this pre-treatment, carbachol (CC) cumulative concentrations curves of the smooth muscle contractile activities were measured during 3 min, after each agonist addition. In Control condition, at CC amounts higher than 1 x 10-5 M, the smooth muscle contraction reached a plateau being maximal at 1 x 10-4 M CC. These carbachol concentration dependent activation curves as Control condition are shown in Figure 1A, B. In addition, the carbachol-dependent smooth muscle contractile responses were significantly affected by MP (Fig 1A) and MP-7 (Fig 1B). Moreover, both tetradecapeptides decreased, in a dose dependent manner, the BTSM maximal contractile activities. However, we estimated EC50 values for CC in all curves with values around EC50 = 1.0 ± 0.3 x 10-7 M. In respect to MP ability to alter BTSM contractile activities, it was found that MP-7 acting in the pM range was more powerful than MP, in the nM range. In this sense, MP (50nM) inhibited completely the BTSM contraction induced by carbachol (1x10-4M). These maximal concentrations of mastoparan and carbachol were used through this study.
These results suggest that mastoparans behave as non-competitive inhibitors of smooth muscle contraction induced by muscarinic agonists.
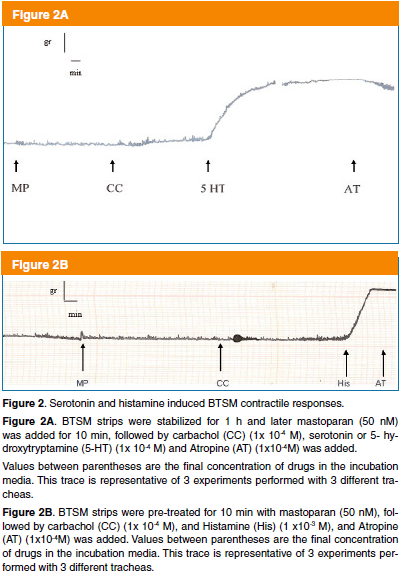
The powerful inhibition exerts by mastoparans on BTSM muscarinic activation suggests that mastoparans may be affecting the BTSM contractile machinery. To test that proposition using Procedure 1, a set of experiments performed with other classic spasmogens of TSM as 5 HT or histamine, which were assayed with the same BTSM strip, in which mastoparan blocked the muscarinic agonist activation. In this sense, the serotoninergic and histaminergic contractile responses were evaluated. A BTSM powerful contraction induced by 5-hydroxytryptamine (5-HT) or serotonin (1 x 10-4 M) is shown in Figure 2A. Similar set of experiments were performed with histamine (1x 10-3 M) and a mastoparan-insensitive potent histamine contractile response was observed, which is shown in Figure 2B. Both the serotoninergic and histaminergic contractile responses of BTSM were not affected by muscarinic antagonist atropine (1 x 10–4 M). However, selective 5 HT or histamine antagonists were not assayed to block these serotoninergic and histaminergic contractile responses because our main interest was to show that BTSM still physiologically active. These results suggest that mastoparan seems to be a specific inhibitor of this muscarinic activation system without affecting the smooth muscle sarcolemma integrity and the contractile machinery functionality.
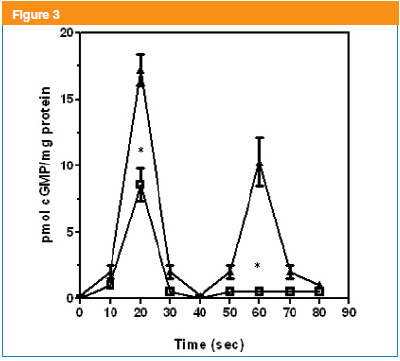
Muscarinic activation of BTSM induced two cGMP signal peaks being the first peak at 20s and the second at 60 s as previously described4. In order to evaluate, these cGMP signals linked to muscarinic activation, several kinetic studies (0-70s) were undertaken. In this sense, mastoparan (50 nM) significantly altered the first signal (20s), that was partially inhibited 63 ± 2 % and the second signal (60s) disappeared (Figure 3). These results suggest that mastoparan is altering, both cGMP signals exerting a more profound effect on the 60 s peak, which is associated with the NPR-GC. Interestingly, the first signal (20s) was significantly inhibited by this tetradecapeptide. Taking into consideration, that mastoparan affected the two cGMP signals; it was decided to explore the origin of these two signals, which are related to specific guanylyl cyclases as above mentioned.
The dramatic disappearance of the second peak (60s) was initially investigated. This second signal of cGMP is product of a cascade coupling mAChRs to NPR-GC9,10.
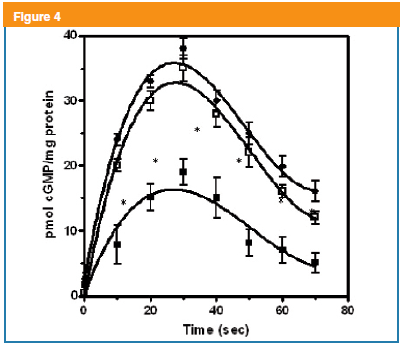
CNP-53 is the specific ligand activator for the NPR-GC-B in BTSM14,16. From previous experiments, it was found that CNP-53 (1x10-7 M) induced the maximal cGMP levels in the intact BTSM. Interestingly, in the basal condition, a cGMP binary pattern emerged under CNP (1x10-7 M) action, with a fast rise at 30 s in cGMP levels reaching maximal values (37 ± 2 pmol/mg total tissue protein), followed by a slow decline remaining higher at 70s (Figure 4). However, in the presence of mastoparan, this distinct behavior remained but significant lower values were observed (Figure 4). It has been reported that NPR-GC-B is coupled to PTX and mastoparan-sensitive heterotrimeric Gi/o proteins9,10,16. Interestingly, PTX pre-treatment reversed the mastoparan inhibitory effect on the production of cGMP induced by CNP-53 at intact BTSM strips (Figure 4).
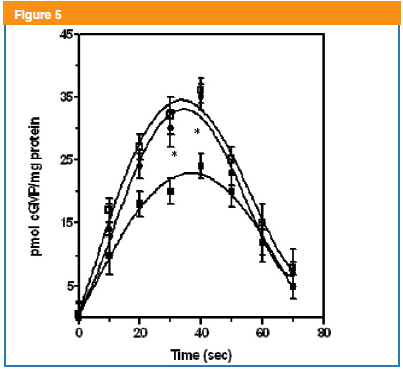
On the other hand, the first cGMP signal (20s) is linked to mAChR activation at the plasma membrane triggering a signal transducing cascade that leads to the stimulation of an ODQ-sensitive sGC as previously reported4. This NO-sensitive sGC was evaluated using a NO donor such as Sodium Nitroprusside (SNP)13. Thus, SNP (50 µM) in BTSM strips produced a cGMP parabolic-like binary pattern reaching maximal values (35 ± 3 pmol/mg total tissue protein) around 40 s, followed by cGMP decrement to basal levels at 70s (Figure 5). Mastoparan (50nM) pre-incubation diminished in a significant way the levels of cGMP induced by SNP (Figure 5). Trying to unravel, these mastoparan effects on this cGMP pool linked to a NO sensitive GC, that a new approach was undertaken. At this step, we have to postulate the existence of a novel mastoparan-sensitive mAChR signaling transducing cascade at plasma membrane, which can induce the inhibition of a NO-sensitive sGC. It is well known that mAChR are GPCR systems coupled to PTX-sensitive G proteins17. In this sense, PTX pre-incubation reversed the mastoparan inhibition of the SNP-induced increments in isolated BTSM fragments, which is shown in Figure 5. These data suggest that an ODQ-sensitive NO-stimulated GC coupled to PTX and mastoparan-sensitive Gi/o proteins are present in BTSM cells.
Discussion
It is important to point out, that this work was performed in the absence PDE inhibitors.
Here, we evaluated the effect of mastoparan11,12 in nM range and super-active analog mastoparan 718, in pM range, on the BTSM contraction induced by muscarinic agonist as carbachol (CC). Our original findings indicated that Mastoparans decreased the contractile maximal responses induced by CC without changing its EC50. One explanation for the decrement on the contractile maximal responses by mastoparans may be related to the ability of these tetradecapeptides to disturb the function and the contractile machinery of the BTSM type through cytotoxic mechanisms, which have been described in other biological models, specifically in the μM concentration19,20. This assumption is not supported by our results on the effects of classic TSM spasmogens as 5-HT and histamine21, which produced potent contractions even in the presence of mastoparan (nM). These findings can be explained since these bioactive amines have been claimed to exert their physiological effects on TSM through specific GPCRs. These receptors are the 5-HT2A for 5-HT and the H1HR for histamine. It is well known that 5-HT induced activation of 5-HT2A receptors mediates the functional effects of serotonin through activation of the Gq/11 protein and its downstream effector phospholipase C (PLC) leading to intracellular phosphatidyl- inositol turnover and Ca2+mobilization22. Likewise, the H1 histamine receptor (H1HR) mediates the functional effects of histamine through activation of the Gq/11-PLC pathway results in the synthesis of inositol 1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol, which in turn stimulate an increase in intracellular Ca2+ and the activation of protein kinase C (PKC) respectively21,23. These Gq/11 proteins are mastoparan-insensitive ones. The last facts can explain the mastoparan-insensitivity of the serotoninergic and histaminergic transducing cascades at BTSM. Furthermore, our results demonstrated that mastoparan inhibits selectively the muscarinic activation without altering other spasmogens transducing cascades at BTSM.
Mastoparan may affect specifically the signal cascades associated with the muscarinic activation at BTSM sarcolemma, which are initiated with a mAChRs coupled to heterotrimeric G-proteins17. These two molecular entities were evaluated. It found that mastoparan in nM range and mastoparan 7 in pM range did not change the muscarinic receptor activity expressed as [3H]QNB binding in plasma membranes fractions isolated from this same BTSM (data not shown). These results indicate that the most like candidates implicated in mastoparan effects are the heterotrimeric G-proteins coupled to these mAChRs.
The G protein involvement on the mastoparan inhibition on BTSM muscarinic activation is supported by the ability of this G-protein activator to alter the generation of the two GMPc signals at 20s and 60s as previously described4. Mastoparan (50 nM) induced a potent inhibition of these cGMP signals, that is correlated with a significant reduction on the contractile maximal responses as here reported. After mastoparan preincubation, the kinetics of cGMP intracellular levels was evaluated following the muscarinic agonist exposure. Interestingly, the first cGMP signal (20s) decreased in more than 60% and the second signal peak (60s) vanished.
The dramatic disappearance of the second peak of cGMP (60s) was under intense scrutiny. This cGMP peak is product of two opposite mAChRs-G-protein linked signaling cascades acting on a NPR-GC9,10. In this sense, m3AChR coupled to Gq16 protein activates24 whereas, m2AChR coupled to Gi/o proteins inhibits this NPR-GC9,10. Recently, we further recognized that muscarinic agonist and mastoparan activations of NPR-GC in isolated BTSM plasma membranes fraction involve this Gq16 protein9.
This NPR-GC was evaluated in intact BSTM strips by a direct stimulation using CNP-53, selective ligand activator for this transmembrane GC enzyme as previously described16,14,24. On basal conditions, CNP increased in more than 18 fold the cGMP production reaching maximal values at 30 s and these cGMP levels decreased slowly and remained high at 60 s. These results are similar to ones reported in guinea pig TSM, where CNP caused a dose dependent rise in the tissue cGMP level, with a peak at 1 min, which is correlated to its smooth muscle relaxant effects25.
The participation of Gi/o-proteins coupled to NPR-GC in mastoparan action was further supported using mastoparan pre-incubated BTSM strips and later exposed to CNP.
Under these experimental conditions, there was a significant reduction, more than 50%, of cGMP-dependent-CNP increments. However, PTX exposure, priori to, mastoparan and later CNP-53 addition, reverses mastoparan inhibition of CNP-dependent cGMP formation. These mastoparan-PTX sensitive changes in cGMP levels linked to NPR-GC may be explained since more than 50% of the NPR-GC activity presents in BTSM sarcolema seems to be coupled to Gi/o proteins as showed in previous work16.
The Gq16 and Gi/o proteins are activated by mastoparan, being the former G-protein, an activator and the latter, an inhibitor of NPR-GC as previously reported10,24. It is intriguing that the Gi/o inhibitory effects on NPR-GC remained after 15 min, after mastoparan addition. It is possibly that the higher abundance of m2AChR/m3AChR ratio (4:1)6 coupled to Gi/o vs Gq16 may explain the persistence of Gi/o inhibition in intact BTSM. Moreover, it can be speculate that Gi/o subunits have more affinity than Gq16 subunits for the putative GPRM on NPR-GC16. However, more research has to be done to clarify these exciting results. All these evidences indicated that mastoparan inhibition of the second cGMP signal linked to NPR-GC is mediated by PTX-sensitive Gi/o proteins as previously mentioned.
Muscarinic activation of BTSM displays a first peak of cGMP (20s), which is a product of an ODQ-sensitive-GC, stimulated by a novel transducing cascade that does not involved the generation of NO, as previously reported4. This novel cascade involves a mAChR coupled to a G-protein, which targets a specific effector possibly an ODQ-sensitive-GC, anchored to the internal face of the BTSM sarcolemma. Experimental evidences from our group indicate that an ODQ-sensitive NO-stimulated GC activity is translocated to the plasma membrane during muscarinic activation of BTSM26. These evidences seem to be similar to ones reported in other biological systems, where NO-sensitive GCs activities have been described at plasma membrane from cardiomyocytes27 and neurons28. Recently, it has been suggested that the α2β2 isoform of GC at plasma membrane site, may provide a localized pool of cGMP29, which seems to be in a similar trend suggested by our work. To assure that the mastoparan inhibition of the first peak is related to a NO sensitive GC, some further experiments were performed. On basal conditions, a NO donor such as SNP increased cGMP in BTSM strips, as expected. Hence, SNP induced a binary response, wherein, cGMP intracellular levels increased around 18-fold at 40 s followed by a reduction of the intracellular levels to basal levels around 70s being the maximal values (35 ± 2 pmoles cGMP/mg total tissue protein). Thus, in the absence of PDE inhibitors, comparable experimental results using SNP and other NO donors have been reported in canine TSM. These authors reported a concentration-related increase in cGMP content in about 18-fold above basal levels within 2 min, that was accompanied by a concentration-dependent relaxation of canine TSM30.
Interestingly, these SNP-dependent cGMP responses were affected by mastoparan, but this peptide did not affect the binary pattern but significantly decreased the maximal responses in about 45 ± 3 %. It is possible to postulate that this decrement on cGMP levels may be related to the membrane-bound NO-sensitive GC isoform in this TSM.
These original findings on mastoparan affecting a NO-sensitive GC system and the fact that mastoparan can affect PTX-sensitive Gi/o-proteins31, induced us to perform further experiments with PTX. Under the last experimental conditions, PTX reversed the mastoparan inhibition on the cGMP augmentation-induced by SNP. These results suggest that a PTX-sensitive Gi/o protein may be responsible for this mastoparan inhibition of the SNP actions. The identification of mAChR subtype, the PTX-sensitive Gi/o-protein coupled to NO-sensitive sGC isoform, that are responsible of the first cGMP signal peak is under research in our group.
The BTSM muscarinic activation is blocked by mastoparan as here described. This inhibition may be explained since an early exposure of intact TSM strips to mastoparans, previous to muscarinic agonist addition, can disrupt the muscarinic signal cascade. It is well known that mastoparan directly activates G proteins stimulating the GTP/GDPexchange12,31, which mimics the GEF activity associated with some agonist activated receptors31, which seems to be our case. Thus, this G-protein activator can dissociate these two G-proteins (Gi/o and Gq16) into their respective GTP and subunits. This latter dissociation process may produce a transient desensitization of the muscarinic agonist signal transducing cascade machinery presents in BTSM. Under these last conditions, the activated mAChRs are not longer able to signal down inside the BTSM cells. Consequently, the BTSM became unresponsive to muscarinic agonist addition as described in this work. During the completion of this work, similar results by mastoparan disrupting another cyclic nucleotide (cAMP) system as β-adrenoceptor-G(s) signaling present in1321N1 human astrocytoma cells have been reported20. These authors suggest that mastoparan changes the localization of Gα(s) from plasma membranes (lipid rafts) into the cytoplasm, where it is not available to activate membrane-adenylyl cyclase. Whatever is the molecular mechanism of mastoparan action, it is well know its ability to dissociate this crucial heterotrimer GDPβinto their respective GTP and the dimmer32 leaving these subunits to interact freely with their intracellular specific targets.
Until now, the BTSM muscarinic activation is unique biological system that involves two cGMP signals as second messengers4,10. In addition, this activation is a highly regulated biological process, which starts with m2/m3AChRs coupled to two mastoparan-sensitive G proteins, leading to a fine time regulation and stimulation of two guanylyl cyclases that accomplish the generation of these two cGMP signal peaks.
Based in our results, it can be postulated that the first signal (20s) is a product of the activation of one mAChR subtype coupled to a novel mastoparan and PTX-sensitive-G protein that leads to the activation of the heterodimer NO stimulated-hemoprotein guanylyl cyclase possibly anchored to plasma membrane and the second signal peak is a product of a the activation of m3AChR coupled to the stimulation of mastoparan-sensitive PTX insensitive-Gq1624 to turn on a transmembrane-homodimer as NPRGC being inhibited by a mastoparan and PTX sensitive Gi/o proteins9,10.
In addition, specific cGMP phosphodiesterases33,34 may be involved in the production of these two sharp and short life cGMP signal peaks.
Nevertheless, in the BTSM system, when each guanylyl cyclase type is directly stimulated by its selective ligands such as CNP-53 for NPR-GC14,16 and SNP for the NO sensitive GC13, a binary pattern emerged. Both activators induced a fast increment in more than 50 fold of cGMP, reaching maximal values between 30-40s, decreasing to basal values faster in the case of SNP-linked cGMP elevations, possibly under the action of powerful cyclic nucleotide PDE-5 as reported in this BTSM33,34.
It is interesting that both SNP and CNP stimulations reach a maximal values around 35-37 pmoles cGMP/mg total tissue protein. It can be speculated that the latter cGMP intracellular concentration range seems to act as a threshold to trigger a fast cGMP hydrolyzing PDEs33-35, enzymes responsible for the cGMP levels declines to basal values as here described.
Finally, this work unravels some of complex molecular mechanisms associated with the muscarinic activation of ASM. This latter activation is the most physiologically and pharmacologically relevant because its a neurotransmitter-linked stimulation of ASM.
A disfunction of these mAChR signal transducing cascades has been implied in the pathophysiological mechanisms of bronchial asthma36 and Chronic Obstructive Pulmonary Disease (COPD)37. In that sense, this work opens new pharmacological and therapeutical approaches for the treatment of these chronic respiratory diseases, in which, the ASM is involved.
Acknowlegments:
This work was supported by Grants from Consejo de Desarrollo Científico Humanístico UCV, PI 09.00.6464.2006/2 and PG 09.7410.2008/1 to (RGA), 09.7726.2009 (ILB).
WalidHassan is Graduate Student at Curso de Postgrado en Ciencias Fisiológicas. Facultad de Medicina. Universidad Central de Venezuela and he holds a fellowship from Misión Ciencia from Ministerio del Poder Popular para la Ciencia, Tecnología e Industrias Intermedias (MPPCTII) at Venezuela.
References
1. Barnes PJ. Airway smooth muscle receptors. Recent Prog Med. 1990;81:184-192. [ Links ]
2. Jacoby DB, Fryer AD. Anticholinergic therapy for airway diseases. Life Sci. 2001; 68: 2565-2572. [ Links ]
3. Katsuki S, Murad F. Regulation of adenosine cyclic 3,5-monophosphate and guanosine cyclic 3,5-monophosphate levels and contractility in bovine tracheal smooth muscle. Mol Pharmacol. 1977;13: 330-341. [ Links ]
4. Guerra de Gonzalez L, Misle A, Pacheco G, Napoleon de Herrera V, Gonzalez de Alfonzo R, Lippo de Becemberg I, Alfonzo MJ. Effects of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and Nomega(6)-nitro-L-arginine methyl ester (NAME) on cyclic GMP levels during muscarinic activation of tracheal smooth muscle. Biochem Pharmacol 1999; 58:563-574. [ Links ]
5. Carvajal J, Germain A, Huidobro-Toro J, Weiner C. Molecular Mechanism of cGMP-mediated Smooth Relaxation. J of Cell Physiology 2000;184: 409-420. [ Links ]
6. Lucas K, Pitari C, Kazcrounian S, Ruiz-Stewart l, Park J, Schulz S, Chepenik K, Waldman SA. Guanylyl Cyclases and Signaling by Cyclic GMP. Pharmacol Rev 2000; 52: 375-413. [ Links ]
7. Roffel A, Meurs H, Elzinga C, Zagsma J. Characterization of the muscarinic receptor subtype involved in phosphoinositide metabolism in bovine tracheal smooth muscle. Br J Pharmacol 1990; 99:293-296. [ Links ]
8. Misle AJ, Lippo de Becemberg I, Gonzalez de Alfonzo R, Alfonzo MJ. Methoctramine binding sites sensitive to alkylation on muscarinic receptors from tracheal smooth muscle. Biochem Pharmacol. 1994; 48:191-195. [ Links ]
9. Alfonzo MJ, Lippo de Becemberg I, Sanchez de Villaroel S, Napoleon de Herrera V, Misle AJ, Gonzalez de Alfonzo R. Two opposite signal transducing mechanisms regulate a G-protein-coupled guanylyl cyclase. Arch Biochem Biophys. 1998; 350:19-25. [ Links ]
10. Alfonzo M, Guerra L, Sánchez S, Francis G, Misle A, Napoleón de Y, Gonzalez R, Lippo I. Signal transduction pathways through mammalian guanylyl cyclases. New Advances in Cardiovascular Physiology and Pharmacology. 1998; 147-175. [ Links ]
11. Hirai Y, Yasuhara T, Yoshida H, Nakajima T, Fujino M, Kitada C. A new mast cell degranulating peptide mastoparan in the venom of Vespula lewisii. Chem Pharm Bull (Tokyo). 1972; 27: 1942-1944. [ Links ]
12. Higashijima T, Uzu S, Nakajima T, Ross E. Mastoparan a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins). J Biol Chem 1988; 263: 6491-6494. [ Links ]
13. Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3:5-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977; 74: 3203-3207. [ Links ]
14. Borges A, Sánchez S, Winand N, Lippo I, Alfonzo MJ, González R . Molecular and Biochemical Characterization of a CNP-sensitive Guanylyl Cyclase in Bovine Tracheal Smooth Muscle. Am J Respir Cell Mol Biol 2001; 25: 98-103. [ Links ]
15. Bensadoun A, Weinstein D. Assay of proteins in the presence of interfering materials. Analytical Biochemistry 1976; 70: 241-250. [ Links ]
16. Alfonzo MJ, de Aguilar EP, de Murillo AG, de Villarroel SS, de Alfonzo RG, Borges A, de Becemberg IL. Characterization of a G protein-coupled guanylyl cyclase B receptor from bovine tracheal smooth muscle. J Recept Signal Transduct Res. 2006; 26:269-297. [ Links ]
17. Murthy KS, Makhlouf GM. Differential coupling of muscarinic m2 and m3 receptors to adenylyl cyclases V/VI in smooth muscle. Concurrent M2-mediated inhibition via Galphai3 and m3-mediated stimulation via Gbetagammaq. J Biol Chem. 1997; 272:21317-21324. [ Links ]
18. Bavec A. Novel features of amphiphilic peptide Mas 7 in signalling via heterotrimeric G-proteins. J Pept Sci. 2004; 10: 691-699. [ Links ]
19. Jones S, Howl J. Charge delocalization and the design of novel mastoparan analogues: enhanced cytotoxicity and secretory efficacy of [Lys5, Lys8, Aib10]MP. Regul Pept. 2004;15;121:121-128. [ Links ]
20. Sugama J, Yu JZ, Rasenick MM, Nakahata N. Mastoparan inhibits betaadrenoceptor-G(s) signaling by changing the localization of Galpha(s) in lipid rafts. Cell Signal 2007; 19: 2247-2254. [ Links ]
21. Pype JL, Mak JC, Dupont M, Verleden GM, Barnes PJ. Desensitization of the histamine H1-receptor and transcriptional down-regulation of histamine H1-receptor gene expression in bovine tracheal smooth muscle. Br J Pharmacol 1998;125:1477-84. [ Links ]
22. Shi J, Zemaitaitis B, Muma NA. Phosphorylation of Gq11 Protein Contributes to Agonist-Induced Desensitization of 5-HT2A Receptor Signaling. Mol Pharmacol. 2007; 71:303-313. [ Links ]
23. Leurs R, Smit MJ, Timmerman H. Molecular Pharmacological aspects of histamine receptors. Pharmacol Ther. 1995;66:413-463. [ Links ]
24. Bruges G, Borges A, Sanchez de Villarroel S, Lippo de Becemberg I, Francis de Toba G, Placeres F, Gonzalez de Alfonzo R, Alfonzo MJ. Coupling of M3 acetylcholine receptor to Gq16 activates a natriuretic peptide receptor guanylyl cyclase. J Recept Signal Transduct Res. 2007; 27:189-216. [ Links ]
25. Takagi K, Araki N, Suzuki K. Relaxant effect of C-type natriuretic peptide on guinea-pig tracheal smooth muscle. Arzneimittelforschung. 1992; 42:1329-1331. [ Links ]
26. Placeres Uray F., Gonzalez de Alfonzo R., Lippo de Becemberg I. and Alfonzo MJ. Muscarinic agonists acting through M2 acetylcholine receptors stimulate the migration of an NO-sensitive guanylyl cyclase to the plasma membrane of bovine tracheal smooth muscle. J Recept Signal Transduct Res. 2010; 30: 10-23. [ Links ]
27. Agulló L, Garcia-Dorado D, Escalona N, Ruiz-Meana M, Mirabet M, Inserte J, Soler-Soler J. Membrane association of nitric oxide-sensitive guanylyl cyclase in cardiomyocytes. Cardiov. Res 2005; 68:65-74. [ Links ]
28. Bidmon HJ, Mohlberg H, Habermann G, Buse E, Zilles K, Behrends S. Cerebellar localization of the NO-receptive soluble guanylyl cyclase subunits-alpha(2)/beta (1) in non-human primates. Cell Tissue Res. 2006; 326:707-714. [ Links ]
29. Belligham M, Evans TJ. The α2β2 isoform of guanylyl cyclase mediates plasma membrane localized nitric oxide signaling. Cellular Signalling, 2007, 19: 2183-2193. [ Links ]
30. Zhou HL, Torphy TJ. Relationship between cyclic guanosine monophosphate accumulation and relaxation of canine trachealis induced by nitrovasodilators. J Pharmacol Exp Ther. 1991;258:972-978. [ Links ]
31. Higashijima T, Burnier J, Ross EM. Regulation of Gi and Go by mastoparan, related amphiphilic peptides, and hydrophobic amines. Mechanism and structural determinants of activity. J Biol Chem. 1990; 265:14176-14186. [ Links ]
32. Oldham WM, Hamm HE. How do receptors activate G proteins? Adv Protein Chem. 2007;74: 67-93. [ Links ]
33. Shahid M, van Amsterdam RG, de Boer J, ten Berge RE, Nicholson CD, Zaagsma J. The presence of five cyclic nucleotide phosphodiesterase isoenzyme activities in bovine tracheal smooth muscle and the functional effects of selective inhibitors. Br J Pharmacol. 1991;104: 471-477. [ Links ]
34. Guerra de Gonzalez L, González de Alfonzo R, Lippo de Becemberg I, Alfonzo MJ. Cyclic nucleotide-dependent phosphodiesterases (PDEI) inhibition by muscarinic antagonists in bovine tracheal smooth muscle. Biochem Pharmacol. 2004; 68:651-658. [ Links ]
35. Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003; 93:280-291. [ Links ]
36. Coulson FR, Fryer AD. Muscarinic acetylcholine receptors and airway diseases. Pharmacol Ther. 2003; 98:59-69. Review. [ Links ]
37. Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res. 2006; 7:73-86. Review. [ Links ]