Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos Venezolanos de Farmacología y Terapéutica
versión impresa ISSN 0798-0264
AVFT vol.35 no.2 Caracas jun. 2016
A tale about perfect partners: New horizons in glimepiride and metformin Mechanisms of action
Una historia sobre socios perfectos: Nuevos horizontes en el mecanismo de acción de la Metformina y la Glimepirida
Joselyn Rojas, MD, MSc1,2, Roberto Añez, MD1, María Sofía Martínez, MD1, Maricarmen Chacín, MD1, Juan Salazar, MD1, María José Calvo, BSc1, Edward Rojas, MD, MgSc1,4, Sandra Wilches-Duran, MgSc5, Marco Cerda, MgSc5, Carlos Garicano, MD1, Modesto Graterol-Rivas, MgSc, PhD4, Julio Contreras-Velasquez, MgSc5, Juan Hernández-Lalinde, MgSc5, Valmore Bermúdez, MD, MSc, MPH, PhD1
1 Endocrine and Metabolic Diseases Research Center. University of Zulia, Maracaibo, Venezuela, 4004.
2 Brigham and Women's Hospital. The Lung Center. Harvard Institute of Medicine. Boston, USA
3 Rutgers, The State University of New Jersey. New Jersey Medical School. Newark, NJ, USA.
4 Economic Sciences School. University of Zulia. Maracaibo, Venezuela.
5 Altos Estudios de Frontera (ALEF) Research Group, Universidad Simón Bolívar, Cúcuta, Colombia
*Corresponding Author: Valmore J. Bermúdez, MD, MSc, MPH, PhD. Address for correspondence: The University of Zulia, Endocrine and Metabolic Diseases Research Center, 20th Avenue, Maracaibo 4004, Venezuela. Telephone/Fax Number: 58-261-7597279. E-mail: valmore@gmail.com
Abstract
The sustainability of the effects, the improvement of directly damaged and associated organ dysfunction, acceptable oral tolerability and lower side effects are probably the desired outcomes of any pharmacological therapy, especially for one that is used for long periods of time, such as T2DM pharmacotherapy. Biguanides and Sulfonylureas have a common development history, both associated with the economic difficulties associated with both World Wars and the impact caused by the discovery and application of Insulin for diabetes management. Glimepiride, the third generation sulfonylurea, is a KTP channel modulator, which also happens to influence plasma membrane dynamics, pro-inflammatory cytokine secretion and PPAR-π activation. The biological effects of metformin are widening every day, and they are not only related with the activation of AMP-dependent Kinase, but also with mitochondrial bioenergetics, glycogen and monocarbon metabolisms, epigenetic silencing and cell death pathways. The individual effects of glimepiride added to metformin´s could very much improve the patient´s metabolic and cardiovascular profiles, especially when such benefits are obtained with lower dosages than the standard. Such properties not only could guarantee adhesion to the oral medication but could enhance the prognosis of T2DM patients. The purpose of the following review is to describe the effects of glimepiride and metformin from a biological standpoint, including their recently pleiotropic effects.
Key words: metformin, glimepiride, lipid rafts, AMP-dependent Kinase, KATP channel, mitochondrial bioenergetics, glucose transporters.
Resumen
La sostenibilidad de los efectos, la mejora de la disfunción de orgánica, una tolerabilidad oral aceptable y pocos efectos secundarios son probablemente los resultados deseados de cualquier terapia farmacológica, especialmente, para una que se utiliza durante largos períodos de tiempo como los es la farmacoterapia con DM2. Las biguanidas y las sulfonilureas tienen una historia de desarrollo común, ambas asociadas con las dificultades económicas asociadas con ambas guerras mundiales y el impacto causado por el descubrimiento y la aplicación de insulina para el manejo de la diabetes. La glimepirida, la una sulfonilurea de tercera generación, es un bloqueador del canal KTP que también influye en la dinámica de las membranas plasmáticas, la secreción de citocinas pro-inflamatorias y la activación de receptores PPAR-gamma. Los efectos biológicos de la metformina se están ampliando cada día, y no sólo están relacionados con la activación de la quinasa dependiente de AMP, sino también con bioenergética mitocondrial, el metabolismo de glucógeno, silenciamiento epigenético y vías de muerte celular. Los efectos individuales de la glimepirida añadida a la metformina podrían mejorar mucho el perfil metabólico y cardiovascular del paciente, especialmente cuando estos beneficios se obtienen con dosis menores que las estándar. Tales propiedades no sólo podrían garantizar la adhesión al tratamiento, sino que podrían mejorar el pronóstico de los pacientes con DM2. El propósito de la siguiente revisión es describir los efectos de la glimepirida y la metformina desde un punto de vista biológico, incluyendo sus efectos pleiotrópicos recientemente descubiertos.
Palabras clave: metformina, glimepirida, balsas lipídicas, quinasa dependiente de AMP, canal KATP, bioenergética mitocondrial, transportadores de glucosa.
Mechanism of action of sulfonylurea ~ not as simple as it seems
The primary effect of sulfonylureas is to stimulate pancreatic insulin secretion1 and this property varies according to the generation of this drug class. The first developed sulphonylureas – called First Generation – were tolbutamide, acetohexamide, chlorpromide and tolazamide2. Second Generation class were gliclazide, glibenclamide (gliburide), glipizide, and Third Generation is currently glimepiride3. The drugs from the second and third generation are 20-50 more potent in their effects, with a prolonged half-life time when compared with chlorpromide4. These new sulfonylureas are associated with less side effects, included hyponatremia and disulfiram effect due to inhibition of hepatic alcohol dehydrogenase5.
As previously mentioned, insulin stimulation effect varies according to sulfonylurea, and its due to structural changes which would modify binding affinity to its receptor localized in the plasma membrane. The Sulfonylurea Receptor (SUR) belongs to the ATP-Binding Cassette family, described in Aguilar-Bryan et al.6 as a 140-170 kDa membrane protein. The SUR is structurally related to another protein called Inwardly Rectifying Potassium Channel and together they constitute the KATP channel, one of the major metabolic sensors in pancreatic beta cells7. This ion channel is an octamer of 4 SUR subunits and 4 Kir6.2 subunits, where the latter units are the porous aspect of the channel and the former are the regulatory elements8. The basic layout for sulfonylurea-mediated insulin secretion is briefly as follows (Figure 1). The SUR subunit acts via switch mechanism, so when a sulfonylurea is bound to SUR the ion channel closes, and when the sulfonylurea dislodges from the receptor, the channel opens again. The net result is increased concentrations of intracellular potassium, which progressively depolarizes the cell and induces insulin release9,10. Depolarization induces Voltagedependent calcium channels, increasing calcium influx towards the beta cells, activating cytoskeletal changes that end in the exocytosis of insulin vesicles10.
Sulfonylureas depend on genetically-determined receptors, where binding time and intensity of the effect varies with each drug generation. The SUR receptor family can be divided in 3 types11: a) SUR1, located mainly in the beta cell plasma membrane; b) UR2A and SUR2B derived from alternative splicing, are expressed in cardiac and skeletal muscle. SUR1-related polymorphisms have been associated with hyperinsulinemic hypoglycemia12 and neonatal diabetes mellitus13. In regards to receptor binding affinity, each drug class has peculiar properties. glibenclamide has 2.5-3 times more binding affinity than glimepiride14, which results in higher insulin secretion in humans and in dogs15. This higher affinity enhances the risk of oxidative stress, endoplasmic reticulum stress, and beta cell apoptosis, proven in dogs and humans16-18. Prolonged use of sulfonylureas (especially those with higher affinity to SUR) has been associated with less beta cell survival rate, suggesting that insulin replacement therapy is actually a better choice regarding preservation of pancreatic islet19.
Nevertheless, evidence has suggested that glimepiride may participate in the preservation of insulin secretion capacity during hyperglycemic states20. In fact, when glimepiride is combined with sitagliptine, islet diameter and proliferation markers seem to improve when compared with monotherapy21. Moreover, a clinical study conducted by our laboratory concluded that low dosages of glimepiride (0.5 mg/day) combined with metformin enhanced beta cell function, without an enhanced insulin secretion or downregulation of insulin receptors22. These findings show that glimepiride at low doses could exert non-pancreatic effects which influence beta cell function and improved 100% its performance. Therefore, we proposed that the extrapancreatic effects of sulfonylureas like glimepiride deserve further investigation, especially when combined with a pleiotropic drug such as metformin.
Non-Pancreatic effects of Glimepiride
The hypoglycemic effect of sulfonylureas has been mainly attributed to acute insulin secretion10, albeit, some extra-pancreatic effects have surfaced over the years, especially with glimepiride, which suggest novel pathways in the control of hyperglycemia in diabetic patients. First off, in vitro studies have confirmed that glimepiride intervenes in glycogen metabolism. Experiments in Hep-G2 cells have confirmed that the drug enhances glycogen production by 30-40% when used with insulin, apparently induced by increased insulin receptor recycling and Protein Kinase-C (PKC) pathway activation23. Moreover, a similar effect has been observed in myotubes mediated by Phosphatidylinositol-3-Kinase (PI3K), an effect that is not shared with glibenclamide24. Evidence has suggested that PKC might be related to this phenomenon23, especially with PKCε25 which is associated with insulin resistance in liver. In fact, PCKε is considered the culprit of lipid-induced insulin resistance via downregulation of insulin receptor expression26.
Other related miscellaneous effects have been described over cytokine production, such as the one reported by Mori et al.27, where glimepiride improved glucose tolerance and blunted expression of TNF-α in retroperitoneal adipose tissue, suggesting that glimepiride participates in the control of low grade inflammation and adiposopathy. Müller et al.28 published that glimepiride induced the expression of mRNA for Peroxisome Proliferator-Activated Receptor-gamma implying that this sulfonylurea is truly capable of modifying the expression on insulin-sensitizing factors such as adiponectin, subsequently enhancing insulin signaling in its dependent tissues28; these aspect will be further discussed in the next section. These two cytokines are important in the adiposopathy- related microenvironment, since they actively partake in the shaping of such inflamed tissue. TNF-α is known to phosphorylate and inhibit IRS-1/PI3K/Akt pathways which is immediately activated after insulin binds its receptor29. Meanwhile, adiponectin exerts the opposite effect, by activation of AMP-dependent Kinase (AMPK) which phosphorylates serine and theonine residues in IRS-1, improving insulin´s intracellular signaling30.
Glimepiride´s Insulin-like activity
The insulin-like effects of glimepiride have been investigated for the past 2 decades in adipose, skeletal muscle and liver cells. This sulfonylurea stimulates glucose influx in rat adipocyte, being associated with enhanced translocation of GLUT1 and GLUT towards the plasma membrane16. Interestingly, this effect is also observed in insulin resistant adipocytes and cardiomyocites31, partially explaining its benefits in T2DM patients31 and in ischemic cardiopathy32. Moreover, Müller et al.33 published that glimepiride also modulates the activity of several enzymes associated with glucose metabolism like cAMPspecific phosphodiesterase 3B and Protein Kinase A. Likewise, the drug is known to modify lipid metabolism by inhibiting lipolysis, as shown by the in vitro studies28. Finally, Takada34 reported that glimepiride induces PI3K and Akt activity, and these results were higher when compared with glibenclamide and tolbutamide. Enhancement of insulin downstream pathway seems vital in the pleiotropic effects of this sulfonylurea.
Glimepiride and plasma membrane dynamics
During the search for glimepiride´s long lost receptor, several in vitro studies were conducted using [3H]Glimepiride33,35, and during the analysis of several possible targets, plasma membrane proteins were marked with the tracer. Glycosylphosphatidylinositol 1/2 (GPI-1/2) were found to be covalently bound to glimepiride and preferably located in lipid raft domains36. These lipid raft domains contain GPI-anchored proteins in the outer side of the membrane, as well as Src kinases and prenilated small G Proteins36; Figure 2. GPI-anchored proteins are main components of the lipid rafts, and such structure can be disassembled via GPI-specific Phospholypase C (GPI-PLC)37. Interestingly, GPI-PLC is activated by glimepiride and to a lesser extent by insulin, interrupting such signaling cascades38-40 and enhancing glucose transporter translocation86. In fact, these effects are also observed during exercise, resulting in lower insulin secretion and decreased levels of C peptide41.
The exact mechanisms for lipid rafting membrane control are still not completely understood, especially the regulation of outer membrane GPI-proteins and dually acylated signaling proteins (also known as NRTK) in the inner membrane locations28. It seems that it all depends on the electrochemical interactions between the fatty acid long chains of GPI-proteins and the dually acylated proteins which involves caveolin domains as scaffolds42,43. The caveolin scaffolding domain (CSD) conveys the presence of caveolin-1 fragments associated with cholesterol rich membranes, which are essential in receptor platforms where homo-oligomerization is key44.
If this theory is correct, then insulin/insulin receptor signaling must has a complex lipid raft like this, which would aid in the homo-phosphorylation of more insulin receptors (amplification) and coordinates the hetero-phosphorylation of IRS-1/2 as second messengers. Müller et al.45 published that insulin signaling required a dynamic lipid raft composed of NRTKs, Lyn and Fak, in raft microdominions called hydrophobic detergent-insoluble Glycolipid-enriched raft microdomain (DIG)46. It seems that when insulin binds its receptor, caveolin is phosphorylated while Lyn gets dissociated from the raft, and this phenomenon is also imitated by glimepiride. The NRTK are Non-receptor Tyrosine Kinases that act as switch mechanisms controls for several signaling platforms, commonly called cytoplasmatic enzymes47; the family comprises 32 NRTK in human genome, including Lyn and Fak. The former, is part of the Src family of cytoplasmatic enzymes, intimately associated with PI3K and Phospholypase Cγ2 48. The latter, Fak (Focal Adhesion-binding domain Kinase), is associated with plasma membrane shaping, focal adhesions and cytoskeleton remodeling49.
Further research has demonstrated that glimepiride and caveolin are also associated in insulin secretion stimulation, where depletion of caveolin-1 in beta cells blunts sulfonylurea- induced insulin secretion, suggesting that caveolin-rich microdomains might also be associated with SUR/Kir6.2 assembly50. Sun and Hu51 reported that the cardiac KATP channel required caveolin-3 scaffolding domains to properly assemble with SUR2A. Likewise, Davies et al.52 KATP Kir6.1/ SUR2B activity in vascular smooth muscle cells depends on caveolin-1 rich microdomains. This information suggests that KATP control is a two-way street, relying not only on the peptidic structure of its ensemble, but also on the lipid-based structure which is embedded in the plasma membrane, further enhancing the role of lipid raft in metabolic control53.
Novel anti-diabetic effects of glimepiride – beyond the plasma membrane and onwards to the nucleus
The PPAR´s belong to a subfamily of nuclear receptors, with 3 different isoforms coded by 3 separate genes: PPARα (PPARA), PPARβ/δ (PPARD) and PPARγ (PPARG)54,55. PPAR receptors control gene expression for several gene clusters involved in processes such as adipogenesis56,57, lipid metabolism58, inflammation58,59 and bone turnover60. These receptors are activated by lipophylic ligands like fatty acids and derived metabolites, heaving as an intracellular lipid content sensor, and ergo capable of redirecting intermediary metabolism61.
Receptor activation is similar for all 3 PPAR proteins. After the ligand has bound to the receptor it heterodimerizes with another nuclear receptor, the X-Retinoid receptor, forming the PPAR-RXR complex. This dimeric complex colocalizes to the promoter site of target genes, activating assembly and progression by recruitment of several transcription coactivators62,63; nevertheless, all PPAR isoforms have specific tissue distribution and different roles in energy metabolism. For example, PPARα are expressed in skeletal muscle, liver, heart muscle and kidneys, whose principal function is lipid and lipoprotein metabolism control64. Likewise, the PPARβ/δ are expressed ubiquitously albeit with lower levels in liver, and has been associated with energy balance in adipose tissue and skeletal muscle65. Finally, the PPARγ has two alternate splicing products, PPARγ1 and PPARγ2. The former is expressed in adipose tissue, small intestine and hematopoietic cells, while the latter is expressed in white and brown adipose tissue66-70. Endogen ligands for PPARs include fatty acids and prostanoids such as 15-deoxy-12,14-prostaglandin-J2, 9- and 13-cis-hydroxy-octadecaenoic acids, and lysophosphatidic acid, acting as weak agonists when compared with thiazolidinediones (TZD), certain NSAIDs and the Angiotensin II receptor blocker Telmisartan71-78. A possibility is open concerning the existence of an endogenous ligand with higher affinity than those previously mentioned, and perhaps this type of ligand performs in a promiscuous manner in order to detect small changes in intracellular lipid concentrations79,80.
In humans, these receptors are incredibly important in order to regulate glucose and lipid homeostasis in liver, muscle and adipose tissue, the differentiation process of adipocytes, especially from pre-adipocytes to mature adipocytes, and achieving adipocyte functional differentiation maturity by controlling the expression of major cytokines such as ADPN, leptin, resistin, TNF-α, and insulin signaling platform-related proteins such as GLUT4 and CAP81-89. Interestingly, PPARγ participates in the immune system, particularly in antigen presenting cells such as macrophages and dendritic cells, controlling lipid partition, inflammation and cell proliferation. In fact, such anti-atherosclerotic properties have also been observed in animal models using TZDs, demonstrating expression pattern changes in TNF-a, IL-1b and IL-6, suppression of inducible Nutric Oxide Synthase (iNOS) and reduction of free radical production90-92.
As mentioned in previous sections, several sulfonylureas have been proven to generate extra-pancreatic effects, especially with glimepiride93. Most cited articles show sensitizing effects with increased glucose uptake in skeletal muscle and adipose tissue via higher density of GLUT4 in plasma membrane94,95. Even though these studies proved the association, they did not show how these effects came to be until the XXI Century, when reports of glimepiride, glibenclamide and telmisartan agonist activity of PPARγ surfaced. Fukuen et al.96 published a classic manuscript describing how glimepiride was capable of inducing PPAγ activity (25% potency of Pioglitazone) in HEK293 cells, with parallel increase in DRIP205 co-activator participation and dissociation of co-repressors NCoR/SMRT. Likewise, this study also revealed that glimepiride assembles complexes with these receptors, competing with Rosiglitazone for its binding site. Moreover, this glimepiride-PPARγ complex modified mRNA levels of PPARγ-gene targets in 3T3-L1 adipocytes, including ADPN96. Such findings were confirmed by Tsunekawa et al.97 which reported that ADPN expression is enhanced in T2DM patients when treated with glimepiride.
An important aspect concerning sulfonylureas, its the proapoptotic effects reported with glibenclamide, glimepiride and repaglinide treatment in in vitro studies with pancreatic beta cells98-100. In fact, the UKPDS (United Kingdom Prevalence Diabetes Study) published that glibenclamide and chlorpropamide were associated with better glycemic control and lower incidence of microangiopathic complications. However, even though improved HOMA-βcell indexes are observed, further down the line there is a progressive decline of beta cell function with literal fatigue of this cell, observed in almost all sulfonylureas10. In vitro and animal studies have suggested that beta cell decline is associated with activation of Akt, NADPH oxidase and increased oxidative stress, and these outcomes seem to de dose-dependent102,103. Del Guerra et al.104 analyzed that glucose stimulated insulin secretion, insulin content, islet apoptosis and GLUT1 expression in human Langerhans islets exposed to glimepiride (10 μM), glibenclamide (10 μM) and chlorpropamide (600 μM). Insulin content diminished after the exposure with the three drugs, albeit glucose stimulated insulin secretion was steady on the cells exposed to glimepiride, not with the other 2 sulfonylureas104.
In fact, Remedi and Nichols105 evaluated prolonged pancreatic hyperexcitability with implants that were able to measure depolarization impulses in beta cells when exposed to glibenclamide. They reported a progressive decline in production of and insulin secretion, effects that were reverse after glibenclamide treatment was removed. Moreover, immunostaining of pancreatic islet cells demonstrated normal-sized α and β cells when further evaluating them using TUNEL technique (Terminal deoxynucleotidyl transferase dUTP Nick End Labeling)106. These findings are similar to those found on our laboratory using low doses of glimepiride in combination with metformin22,106. Taking all this information into account, its conceivable that glimepiride´s biological profile is more favorable than other sulfonylureas, especially due to its insulin-mimnetic effects, anti-inflammation properties and recently published anti-apoptotic effects via Bcl/BclXL and protein 14-3-3ε 103,107-109.
Metformin – the oldest new kid on this block
As previously mentioned, metformin´s IUPAC name is N,NDimethylimidodicarbonimidic diamide (Figure 1), is positively charged at physiological pH, with a pKa of 2.8 and 11.51 110. Its molecule has five nitrogen groups, making it electronically possible to form square planar complexes with transition metals like copper and nickel by acting as a bidentate ligand coordinator in a 1:2 ratio manner111. Such structural conformations require the two imino groups, serving as primary, secondary and tertiary amino groups as electron donors during the conformation of the π bond111. Since copper bears functional importance due to mitochondrial bioenergetics112, coordinated complexes with copper have extensively analyzed. Zhu et al.113 published that deprotonated metformin forms a stable complex with deprotonated copper, described as [Cu(C4H10N5)2]8H2O, with a resulting copper atoms between a twofold rotation axis held together by van der Wall forces114.
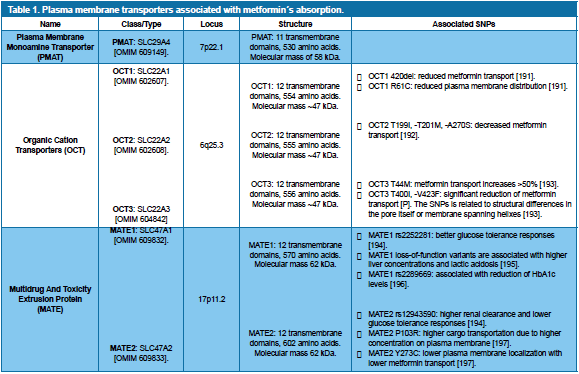
Absorption and distribution and metformin is quite complex and requires the intervention of small intestine, the liver and kidneys. The drug has an oral bioavailability of 40-60% and is completely absorbed during the first 6 hours after ingestion115, with plasma levels between 54 – 4133 ng/mL116. The varying plasma levels are associated with a variety of plasma membrane transporters belonging to 3 transporter families, whose affinity guarantee metformin´s level of absorption (Table 1). Once inside the target cell, metformin will influence several aspects of its functionality, including mitochondrial energetics and DNA metabolism. Sun et al.117 published a signaling pathway network analyzing which genes/proteins were relevant in metformin´s network. They found that the drug targets 65 upstream genes and some 355 downstream genes, and includes 7 fundamental genetic targets that are partially responsible for its antidiabetic and anticancer effects: CDKN1A, ESR1, MAX, MYC, PPARGC1A, SP1 and STK11117. The signaling cascades that are needed to activate these target proteins can be academically divided in two major groups: AMP-Dependent Kinase (AMPK)-dependent and AMPK-independent; which will be explained shortly.
AMP-Dependent Kinase (AMPK)-dependent mechanism of action of Metformin
The Adenosin Mono-Phosphate-dependent Kinase (AMPK) is perhaps the most important piece in metformin´s intracellular signaling pathways. This αβγ heterotrimeric complex is constituted by 3 subunits: alpha-subunit which is the catalytic fraction beta-subunit118 which functions as a scaffold protein119; and the gamma-subunit which is the AMP/ATP sensor120. When metformin enters the target cell, it activates AMPK and its subsequent downstream signaling which includes the following net effects: lower Acetyl-CoA Carboxylase (ACC) activity, lower expression of lipogenic enzymes and induction of fatty acid oxidation121. The activation of AMPK relies basically in two pathways: Mitochondrial-dependent and -independent mechanisms. Even though the mechanisms will be explain separately, they are indeed intertwined and will be discussed further.
The Mitochondrial dependent pathway requires the uncoupling of respiratory chain from oxidative phosphorylation, modifying overall cellular energetics122. Metformin inhibits Complex I due to interaction with deprotonated cupper using molecule chelating mechanisms113,114, and such property has been proposed as a viable anticancer therapy due to cancer cell´s upregulation of OCT1 and weaker intracellular redox status122,123. Moreover, inhibition of this Complex was associated with enhanced superoxide production by 260%, increasing oxidative cellular damage and apoptosis induction, key processes in cancer control124. Uncoupling between the respiratory chain and Complex V shifts cellular bioenergetics, increasing glycolysis and lactate production122, with a resulting overall decrease of ATP levels and progressive elevation of intracellular AMP. Once the ATP/AMP ratio shifts, the gamma-subunit from AMPK changes the cargo in its adenosine- binding sites, switching from ATP to AMP (considered a primed inactive state)122, which loses the beta-subunit myristoylated tail from the alpha-subunit, making it susceptible to phosphorylation from upstream AMPK kinases like LKB1 and CaMKK (activated state)122,125,126; Figure 3. In accordance to this line of thought, AMPK non-catalytic subunits are more important than previously considered, suggesting that actually beta-subunit is the gatekeeper during metabolic stress sensing and AMPK activation127.
Among the Mitochondrial-independent proposed mechanisms for AMPK activation, Ouyang et al.128 published that metfotmin inhibited the enzyme ADP Deaminase, responsible for the conversion of AMP to inosine monophosphate (IMP), increasing the availability of AMP. These findings were corroborated by Vytla and Ochs129 that this biguanide increases free AMP and ADP availability, enhanced fatty acid oxidation and gluconeogenesis inhibition, placing the AMPD1 enzyme in a novel role as insulin sensitizer and probable pharmacological target130. In fact, modulation of the one-carbon metabolism and interference with folate availability, suggests that metformin actually mimics antifolate drugs by accumulating 5-formimino-THF131,132. The sudden increase in AMP levels has been also associated with inhibition of the Adenilate cyclase (AC) family of enzymes (EC 4.6.1.1). Miller et al.133 published that the inhibition of AC by AMP134 results in a blunted Protein Kinase A signal and therefore abrogates glucagon hyperglycemic signals and favors metabolic homeostasis in insulin resistant and diabetic patients134, especially those with hyperglucagonemia135.
AMPK downstream signaling
Once this secondary messenger has been activated, the end results can be classified into 2 groups: a) nuclear effects, which include the modulation of genetic expression of specific set of genes, induction of sirtuins and autophagia, and final modification of metabolic memory programming, and b) cytosolic effects, encompassing glucose transporter-4 (GLUT4) mobilization towards the plasma membrane; Figure 3.
Overall Nuclear Effects
The Cyclic-AMP (cAMP) responsive element (CRE)-binding protein depends on the phosphorylation of Ser133 (active state) in the KID domain of the protein via PKA136. The CRTCs (cAMP-regulated transitional Co-activators) are cytosolic proteins which mobilize towards the nucleus after cAMP induces dephosphorylation via inhibition of SIK137. These co-activators have multiple phosphorylation sites, including one for AMPK, the Ser171 which serves as a negative modulator136. The CREB and its DNA binding site CRE are responsible for the expression control of gluconeogenic genes such as Pyruvate Carboxylase, Phosphoenolpyruvate Carboxykinase 1 and Glucose- 6-phosphatase136-140. AMPK is a major genetic modulator, having major participation in several protein expression machineries, including transporting families, cytoskeleton-related proteins, enzymes, and housekeeping genes.
Indeed, several have made the assumption that AMPK and suirtuins may work hand in hand in managing metabolic stress, starvation and proper response141. In fact, AMPK and Sirtuin-1 share some common functions, such as inducing GLUT4 translocation142 and fatty acid oxidation induction143, both scenarios being associated with the activation of Peroxisome Proliferator-Activated Receptor-gamma Coactivator 1-alpha. It´s been demonstrated that metformin itself induces SIRT1 activity by incrementing intracellular NAD+ levels, resulting in increased SIRT1 activity, including three especial targets: PGC1α, Forkhead Box O1 and Forkhead Box O3 144. The biguanide´s actions rely on AMPK´s ability to increase the expression of Nicotinamide Phosphoribosyltransferase, the key enzyme during NAD+ salvage pathways145, favoring sirtuin activity. Finally, a novel pathway between AMPK and SIRT1 has been described, and it relates to the decreased availability of p53 via metformin-mediated inhibition of MDM2 (murin double minute 2), the ubiquitin-ligase responsible for p53 ubiquitinization and destruction146. Metformin induces AMPK activity in high and low glucose concentrations, yet SIRT1 is only induced by the drug during high-glucose conditions146. Such effect on p53 could impact in the activation of senescence, DNA repair and aging mechanisms associated with hyperglycemic states. As a side note, another sirtuins seems to participate.
In regards to glucose uptake via insulin-dependent tissues, the Facilitated Glucose Transporter member 4, otherwise known as GLUT4, is perhaps the most important glucose transporter during the postprandial phase, since it correlates with insulin sensitivity and glucose deposition147. Grisouard et al.148 have proven that metformin increases the expression of GLUT4 mRNA but not GLUT1 mRNA via AMPK, favoring glucose uptake in insulin-dependent tissues such as adipose and skeletal muscle tissues. Almost 20 years ago, Lenzen et al.149 demonstrated that metformin could modify the expression of glucose transporters in the small intestine, with increased expression of GLUT5 and SGLT1. By 2005 Walker et al.150 reported that AMPK induced GLUT2 translocation towards the brush-border membrane (BBM), suggesting that metformin might be involved in other glucose transporter systems. Five years later, Sakar et al.151 confirmed that metformin via-AMPK does redistribute glucose transporters GLUT2 towards the BBM, whereas reducing SGLT1´s concentration in this cellular region.
Overall Cytosolic Effects
The translocation of GLUT4 to the plasma membrane has been reviewed elsewhere152, but certain aspects will be detailed in order to describe the effects of metformin in this process. Insulin downstream pathways depict the phosphorylation and activation of Phosphoinositide-3-kinase, key enzyme in insulin metabolic effects. Once PosphoInositol-3,4,5 is generated, this serves as an anchor and allosteric activator for the serine/threonine kinase 3-Phosphoimositide-dependent Protein Kinase and PDK2. Both PDK enzymes have Akt/Protein Kinase B as target, but they phosphorylate different targets153,154: PDK2 associates with mTOR/Rictor in order to phosphorylate Ser473, and afterwards, PDK1 phosphorylates Thr308. Once Akt is activated, phosphorylates AS160, a GTPase protein which is known to block VAMP2-vesicles towards the plasma membrane by favoring the generation of Rab-GDP155. Lee et al.156 reported that metformin increases phosphorylation of AS160 and enhances the activity of Rab4-GTPase activating protein and even increases phosphorylation of Protein Kinase C-zeta (PKCζ), modulating insulin-dependent GLUT mobilization towards the plasma membrane and overall improvement of glucose uptake, notions that were previously reported by Thong et al. in 2007 157. The PKCζ is known to be involved in the dynamics of vesicle traffic via actin remodeling158, suggesting that metformin also influences cytoskeleton networking. Moreover, Lee et al.159 also published that metformin stimulates an alternate vesicle-traffic inducing pathways, the AMPK/Src/Cbl axis. The Cbl-CAP-CrkII-C3G-TC10 pathway is an alternate pathway for GLUT4-vesicle mobilization towards the plasma membrane160.
As a final note, there are other miscellaneous effects attributed to metformin, including modulation of mitochondrial shuttles, like the Glycerol phosphate shuttle. This mechanism of reducing equivalent mobilization is important during keep the respiratory chain active using Complex II as the catapult161. Madiraju et al.162 confirmed that metformin inhibits the Glycerophosphate Dehydrogenase, which modifies hepatic redox states, decreasing the rate of gluconeogenesis: albeit increasing the chance for lactoacidosis since the conversion of lactate to glucose is seriously blunted in a dose-dependent manner. Similarly, this shuttle seems to be prone to electron leak and could be considered an important site for ROS production using intermediaries such as flavin or semiquinone163. Moreover, in 2003 Otto et al.164 reported that hepatocytes incubated with metformin had impaired gluconeogenesis and interrupted glycogen synthesis as well. AMPK´s activity seems to be modulated by carbohydrate- binding via its beta-subunit, and this chemical property seems to be important in glycogen metabolism. During metabolic stress, acute exposure to AMPK is bound to block glycogen synthesis in favor of glucose oxidation and ATP production by phosphorylating Glycogen Synthase at Ser8 165. However, chronic activation of AMPK has been known to increase glycogen synthesis due to increased availability of glucose-6-phosphate166. In fact, AMPK is also known to esoterically associate with Glycogen-phosphorylase164 and Glycogen debranching enzyme167 and such interactions seem to depend on the beta-subunit and autophosphorylation of βThr148 residue168.
Together is better
The use of glimepiride and metformin in this day and age of new anti-diabetic drugs such as SGLT2 inhibitors169, can still be justified by the numerous synergistic effects of these old school drugs, especially considering their respective pleiotropic effects, albeit considerable lesser side effect. Using an animal model of streptozotocin + high fat diet to induce diabetes in rats, Saad et al.170 reported that metformin increased ADPN levels, while glimepiride was more powerful when concerning lowering nonesterified fatty acids, suggesting that both drugs exerted cardiovascular protection functions. In fact, metformin/glimepiride combination showed lower crude incidence rated of cardiovascular mortality (20.7 [19.7-21.7]) and lower rates of cardiovascular death (9.6 [8.9-10.3]) when compared with metformin/glibenclamide and metformin/glipizide171. When comparing effect over HbA1c between metformin/ glimepiride fixed doses vs. metformin tittering doses, the combination was significantly superior in lowering basal levels172, even when metformin was up to 2550 mg/day173. In fact, there´s a plateau effect of metformin at 1500 mg, after which no additional response has been observed174,175.
The combination of low dose of glimepiride and up to 1500mg of metformin has been proven to be effective in managing not only insulin resistance in T2DM, but to properly wield pleiotropic effects that enhance glycemic control. Our laboratory published in 2007 22 that 0.5 mg/day of glimepiride with 1500 mg/day of metformin daily is associated with powerful reduction of fasting, postpandrial levels, associated with improved HOMA-IR and HOMA-βcell indexes. These findings have been observed in further clinical studies such as the one from Keiko et al.176, reporting significant and sustained glycemic control when using these drug quantities, even when compared with triple-medication therapy (sitagliptine, metformin and sulfonylurea). Likewise, González-Ortiz et al.177 reported that 1 mg of glimepiride in combination with metformin (500 mg/day) was more efficacious than glibenclamide (5 mg/day) with metformin in achieving glycemic control and sustained management of uncontrolled T2DM.
Concluding remarks
The sustainability of the effects, the improvement of directly damaged and associated organ dysfunction, acceptable oral tolerability and lower side effects are probably the desired outcomes of any pharmacological therapy, especially for one that is used for long periods of time, such as T2DM pharmacotherapy. Several anti-diabetic drugs have been approved178, new ones are recently169 and certainly more will be devised. The pharmacological goal is to standardize which is a better monotherapy and how to choose add-on medication onwards, reflected in the latest management guide sponsored by the American and European diabetes agencies179. The individual effects of glimepiride over metformin could very much improve the patient´s metabolic and cardiovascular profiles, especially when such benefits are obtained with lower dosages than the standard170-177. Such properties not only could guarantee adhesion to the oral medication but could enhance the prognosis of T2DM patients, especially in the younger patients.
Acknowledgments
This work was supported by Research Grant no.
Disclosure
There are no financial or other contractual agreements that might cause conflict of interests.
References
1. Loubatieres A. The hypoglycemic sulfonamides: history and development of the problem from 1942 to 1955. Ann N Y Acad Sci. 1957;71:4-11. [ Links ]
2. Groop LC. Sulfonylureas in NIDDM. Diabetes Care. 1992;15:737-54. [ Links ]
3. Melander A, Bitzen PO, Faber O, Groop L. Sulphonylurea antidiabetic drugs, an update of their clinical pharmacology and rational therapeutic use. Drugs. 1989;37:58-72. [ Links ]
4. Ylitalo P, Oksala H, Pitkajarvi T. Comparison of acute and prolonged effects of glibenclamide and chlorpropamide in patients with non-insulin-dependent diabetes. Arzneimittelforcomposed schung. 1985;35:1596-9. [ Links ]
5. Matz R. Hyponatremia and sulfonylureas. Diabetes Care. 1984;7:201-2. [ Links ]
6. Aguilar-Bryan L., Nichols CG, Wechsler SW, Clement JP IV, Boyd AE III, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423-6. [ Links ]
7. Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470-6. [ Links ]
8. Shi NQ, Ye B, Makielski JC. Function and distribution of the SUR isoforms and splice variants. J Mol Cell Cardiol. 2005;39:51-60. [ Links ]
9. Proks P, Reimann F, Green N, Gribble F, Ashcroft F. Sulfonylurea stimulation of insulin secretion. Diabetes. 2002;51(suppl 3):S368– S376. [ Links ]
10. Bryan J, Aguilar Bryan L. Sulfonylurea receptors: ABC trans- porters that regulate ATP-sensitive K+ channels. Biochim Biophys Acta. 1999;1461:285-303. [ Links ]
11. Loffler-Walz C, Hambrock A, Quast U. Interaction of K(ATP) Control channel modulators with sulfonylurea receptor SUR2B: implication for tetramer formation and allosteric coupling of subunits. Mol Pharmacol. 2002;61:407-14. [ Links ]
12. Thomas PM, Cote GJ, Wohllk N, Haddad B, Mathew PM, Rabl W, Aguilar-Bryan L, Gagel RF, Bryan J. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;268:426-9. [ Links ]
13. Tarasov AI, Nicolson TJ, Riveline JP, Taneja TK, Baldwin SA, Baldwin JM, Charpentier G, Gautier JF, Froguel P, Vaxillaire M, Rutter GA. A rare mutation in ABCC8/SUR1 leading to altered ATP-sensitive K+ channel activity and beta-cell glucose sensing is associated with type 2 diabetes in adults. Diabetes. 2008;57:1595-604. [ Links ]
14. Geisen K. Special pharmacology of the new sulfonylurea glimepiride. Arzneimittelforschung. 1988; 38:1120-30. [ Links ]
15. Nguyen C, Pan J, Charles MA. Preclinical studies of glimepiride. Drugs Today (Barc). 1998; 34(5):391-400. [ Links ]
16. Efanova IB, Zaitsev SV, Zhivotovsky B, Kohler M, Efendic S, Orrenius S, Berggren PO. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells: a process dependent on intracellular Ca2+ concentration. J Biol Chem. 1998; 273: 33501-7. [ Links ]
17. Iwakura T, Fujimoto S, Kagimoto S, Inada A, Kubota A, Someya Y, Ihara Y, Yamada Y, Seino Y. Sustained enhancement of Ca(2+) influx by glibenclamide induces apoptosis in RINm5F cells. Biochem Biophys Res Commun. 2000;271:422-8. [ Links ]
18. Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY. Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab. 2005;90:501-6. [ Links ]
19. Del Guerra S, Marselli L, Lupi R, Boggi U, Mosca F, Benzi L, Del Prato S, Marchetti P. Effects of prolonged in vitro exposure to sulphonylureas on the function and survival of human islets. J Diabetes Complications. 2005;19:60-4. [ Links ]
20. Lupi R, Del Prato S. β-cell apoptosis in type 2 diabetes: quantitative and functional consequences. Diabetes Metab. 2008;34:S56-64. [ Links ]
21. Mohamed NA, Zaitone SA, Moustafa YM. Effect of sitagliptin in combination with glimepiride on glycemic control and islet cell diameter/ proliferation in a model of type 2 diabetic rats. IOSR Journal Of Pharmacy 2013;3:72-80. [ Links ]
22. Bermúdez-Pirela VJ1, Cano C, Medina MT, Souki A, Lemus MA, Leal EM, Seyfi HA, Cano R, Ciscek A, Bermúdez-Arias F, Contreras F, Israili ZH, Hernández-Hernández R, Valasco M. Metformin plus low-dose glimeperide significantly improves Homeostasis Model Assessment for insulin resistance (HOMA(IR)) and beta-cell function (HOMA(beta-cell)) without hyperinsulinemia in patients with type 2 diabetes mellitus. Am J Ther 2007;14:194-202. [ Links ]
23. Hribal ML, DAlfonso R, Giovannone B, Lauro D, Liu YY, Borboni P, Federici M, Lauro R, Sesti G. The sulfonylurea glimepiride regulates intracellular routing of the insulin-receptor complexes through their interaction with specific protein kinase C isoforms. Mol Pharmacol. 2001;59:322-30. [ Links ]
24. Haupt A, Kausch C, Dahl D, Bachmann O, Stumvoll M, Häring HU, Matthaei S. Effect of glimepiride on insulin-stimulated glycogen synthesis in cultured human skeletal muscle cells: a comparison to glibenclamide. Diabetes Care. 2002;25:2129-32. [ Links ]
25. Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739-45. [ Links ]
26. Dasgupta S, Bhattacharya S, Maitra S, Pal D, Majumdar SS, Datta A, Bhattacharya S. Mechanism of lipid induced insulin resistance: activated PKCε is a key regulator. Biochim Biophys Acta. 2011;1812:495-506. [ Links ]
27. Mori Y, Komiya H, Kurokawa N, Tajima N. Comparison of the effects of glimepiride and glibenclamide on adipose tissue tumour necrosis factor-alpha mRNA expression and cellularity. Diabetes Obes Metabol. 2004;6:28. [ Links ]
28. Müller G. The mode of action of the antidiabetic drug glimepiride beyond insulin secretion. Curr Med Chem Immun Endoc Metab Agents. 2005;5:499-518. [ Links ]
29. Hotamisligil GS. Molecular mechanisms of insulin resistance and the role of the adipocyte. Int J Obes Relat Metab Disord. 2000;24:S23. [ Links ]
30. Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52:1355. [ Links ]
31. Bähr A, von Holtey M, Müller G, Eckel J. Direct stimulation of myocardial glucose transport and glucose transporter-1 (GLUT1) and GLUT4 protein expression by the sulfonylurea glimepiride. Endocrinol. 1995;136:2547. [ Links ]
32. Hausenloy DJ, Wynne AM, Mocanu MM, Yellon DM. Glimepiride treatment facilitates ischemic preconditioning in the diabetic heart. J Cardiovasc Pharmacol Ther. 2013;18:263-9. [ Links ]
33. Müller G., Geisen K. Characterization of the molecular mode of action of the sulfonylurea, glimepiride, at adipocytes. Horm Metab Res. 1996;28:469. [ Links ]
34. Takada Y, Takata Y, Iwanishi M, Imamura T, Sawa T, Morioka H, Ishihara H, Ishiki M, Usui I, Temaru R, Urakaze M, Satoh Y, Inami T, Tsuda S, Kobayashi M. Effect of glimepiride (HOE 490) on insulin receptors of skeletal muscles from genetically diabetic KK-Ay mouse. Eur J Pharmacol. 1996;308:205. [ Links ]
35. Kramer W, Müller G, Girbig F, Gutjahr U, Kowalewski S, Hartz D, Summ HD. Differential interaction of glimepiride and glibenclamide with the β-cell sulfonylurea receptor II. Photoaffinity labeling of a 65 kDa protein by [3H]glimepiride. Biochim Biophys Acta. 1994;1191:278-90. [ Links ]
36. Anderson RGW, Jacobsen K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 2002;296:1821. [ Links ]
37. Müller G, Dearey EA, Pünter J. The sulphonylurea drug, glimepiride, stimulates release of glycosylphosphatidylinositol-anchored plasma-membrane proteins from 3T3 adipocytes. J Biochem J. 1993;289:509-21. [ Links ]
38. Movahedi S, Hooper N. Insulin stimulates the release of the glycosyl phosphatidylinositol-anchored membrane dipeptidase from 3T3- L1 adipocytes through the action of a phospholipase C. Biochem J. 1997;326:531. [ Links ]
39. Müller G, Dearey EA, Korndörfer A, Bandlow W. Stimulation of a glycosyl-phosphatidylinositol-specific phospholipase by insulin and the sulfonylurea, glimepiride, in rat adipocytes depends on increased glucose transport. J Cell Biol 1994;126:1267. [ Links ]
40. Müller G, Welte S. Concepts and options for current insulin research and future anti-diabetic therapy. Recent Res Develop Endocrinol. 2002;3:401. [ Links ]
41. Massi-Benedetti M, Herz M, Pfeiffer C. The effects of acute exercise on metabolic control in type II diabetic patients treated with glimepiride or glibenclamide. Horm Metab Res. 1996;28:451. [ Links ]
42. Gabrielsson BG, Karlsson AC, Lönn M, Olofsson LE, Johansson JM, Torgerson JS, Sjostrom L, Carlsson B, Eden S, Carlsson LM. Molecular characterization of a local sulfonylurea system in human adipose tissue. Mol Cell Biochem. 2004;258:65. [ Links ]
43. Müller G, Hanekop N, Kramer W, Bandlow W, Frick W. Interaction of phosphoinositolglycan(-peptides) with plasma membrane lipid rafts of rat adipocytes. Arch Biochem Biophys 2002;408:17. [ Links ]
44. Hoop CL, Sivanandam VN, Kodali R, Srnec MN, van der Wel PC. Structural characterization of the caveolin scaffolding domain in association with cholesterol-rich membranes. Biochemistry. 2012;51:90-9. [ Links ]
45. Müller G, Jung C, Wied S, Welte S, Frick W. Insulin-mimetic signaling by the sulfonylurea glimepiride and phosphoinositolglycans involves distinct mechanisms for redistribution of lipid raft components. Biochemistry. 2001;40:14603-20. [ Links ]
46. Harder T1, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929-42. [ Links ]
47. The ENZYME entry: EC 2.7.10.2. Available at http://enzyme.expasy.org/EC/2.7.10.2. Last accessed june 2015. [ Links ]
48. Rickles RJ, Botfield MC, Weng Z, Taylor JA, Green OM, Brugge JS, Zoller MJ. Identification of Src, Fyn, Lyn, PI3K and Abl SH3 domain ligands using phage display libraries. EMBO J. 1994;13:5598-604. [ Links ]
49. Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56-68. [ Links ]
50. Puddu A, Salani B, Cordera R, Viviani GL, Maggi D. Caveolin-1 is essential for glimepiride-induced insulin secretion in the pancreatic betaTC-6 cell line. Biochem Biophys Res Commun. 2008;375:235-7. [ Links ]
51. Sun W, Hu K. Role for SUR2A in coupling cardiac K(ATP) channels to caveolin-3. Cell Physiol Biochem. 2010;25:409-18. [ Links ]
52. Davies LM, Purves GI, Barrett-Jolley R, Dart C. Interaction with caveolin- 1 modulates vascular ATP-sensitive potassium (KATP) channel activity. J Physiol. 2010;588: 3255-66. [ Links ]
53. Dart C. Indirect Channel Regulation by Cholesterol: The Role of Caveolae and Caveolins in Regulating KATP Channel Function. In Cholesterol Regulation of Ion Channels and Receptors (eds I. Levitan and F. J. Barrantes), John Wiley & Sons, Inc., Hoboken, NJ, USA, 2012. [ Links ]
54. Laudet V, Hänni C, Coll J, Catzeflis F, Stéhelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992;11:1003–13. [ Links ]
55. Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645-50. [ Links ]
56. Medina-Gomez G, Gray SL, Yetukuri L, Shimomura K, Virtue S, Campbell M, Curtis RK, Jimenez-Linan M, Blount M, Yeo GS, Lopez M, Seppänen-Laakso T, Ashcroft FM, Oresic M, Vidal-Puig A. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet 2007;3:e64. [ Links ]
57. Gervois P, Torra IP, Fruchart JC, Staels B. Regulation of lipid and lipoprotein metabolism by PPAR activators. Clin Chem Lab Med. 2000;38:3-11. [ Links ]
58. Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab. 2012;23:351-63. [ Links ]
59. Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta 2011;1812:1007-22. [ Links ]
60. Lecka-Czernik B. PPARγ, an essential regulator of bone mass: Metabolic and molecular cues. IBMS BoneKEy. 2010;7:171-81. [ Links ]
61. Lee Chih-Hao, Olson Peter and Evans Ronald M. Minireview: Lipid Metabolism, Metabolic Diseases, and Peroxisome Proliferator-Activated Receptors. Endocrinology. 2003;144:2201-7. [ Links ]
62. Gearing KL, Gottlicher M, Teboul M, Widmark E, Gustafsson JA. Interaction of the peroxisome-proliferator-activated receptor and retinoid X receptor. Proc Natl Acad Sci USA 1993; 90:1440-4. [ Links ]
63. Yu S, Reddy JK. Transcription coactivators for peroxisome proliferator- activated receptors. Biochim Biophys Acta. 2007;1771:936-51. [ Links ]
64. Vidal-Puig AJ, Considine RV, Jimenez-Liñan M, Werman A, Pories WJ, Caro JF, Flier JS. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J Clin Invest. 1997;99:2416-22. [ Links ]
65. Fredenrich A, Grimaldi PA. PPAR delta: an uncompletely known nuclear receptor. Diabetes Metab. 2005;31:23-7. [ Links ]
66. Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354-66. [ Links ]
67. Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman B. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224-34. [ Links ]
68. Vidal-Puig A, Jimenez-Liñan M, Lowell BB, et al. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest. 1996;97:2553-61. [ Links ]
69. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147-56. [ Links ]
70. Sears IB, MacGinnitie MA, Kovacs LG, Graves RA. Differentiationdependent expression of the brown adipocyte uncoupling protein gene: regulation by peroxisome proliferator-activated receptor gamma. Mol Cell Biol. 1996;16:3410-19. [ Links ]
71. Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813-9. [ Links ]
72. Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803-12. [ Links ]
73. Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator- activated receptors alpha and delta. Proc Natl Acad Sci USA. 1997;94:4312-17. [ Links ]
74. Dussault I, Forman BM. Prostaglandins and fatty acids regulate transcriptional signaling via the peroxisome proliferator activated receptor nuclear receptors. Prostaglandins Other Lipid Mediat. 2000;62:1– 13. [ Links ]
75. McIntyre TM, Pontsler AV, Silva AR, St Hilaire A, Xu Y, Hinshaw JC, Zimmerman GA, Hama K, Aoki J, Arai H, Prestwich GD. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc. Natl. Acad. Sci. U. S. A. 2003;100:131-6. [ Links ]
76. Rocchi S, Picard F, Vamecq J, Gelman L, Potier N, Zeyer D, Dubuquoy L, Bac P, Champy MF, Plunket KD, Leesnitzer LM, Blanchard SG, Desreumaux P, Moras D, Renaud JP, Auwerx J. A unique PPARgamma ligand with potent insulin-sensitizing yet weak adipogenic activity. Mol Cell. 2001;8:737-47. [ Links ]
77. Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997;272:3406-10. [ Links ]
78. Goyal SN, Bharti S, Bhatia J, Nag TC, Ray R, Arya DS. Telmisartan, a dual ARB/partial PPAR-γ agonist, protects myocardium from ischaemic reperfusion injury in experimental diabetes. Diabetes Obes Metab. 2011;13:533-4. [ Links ]
79. Wang L, Waltenberger B, Pferschy-Wenzig EM, Blunder M, Liu X, Malainer C, Blazevic T, Schwaiger S, Rollinger JM, Heiss EH, Schuster D, Kopp B, Bauer R3, Stuppner H, Dirsch VM, Atanasov AG. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review. Biochem Pharmacol. 2014;92:73–89. [ Links ]
80. Kliewer SA, Xu HE, Lambert MH, Willson TM. Peroxisome proliferator- activated receptors: from genes to physiology. Recent Prog Horm Res. 2001;56:239-63. [ Links ]
81. Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229-40. [ Links ]
82. Lemberger T, Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: a nuclear receptor signaling pathway in lipid physiology. Ann Rev Cell Dev Biol. 1996;12:335-63. [ Links ]
83. Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649-88. [ Links ]
84. Rangwala SM, Lazar MA. Peroxisome proliferator-activated receptor γ in diabetes and metabolism. Trends Pharmacol Sci 2004;25:331-6. [ Links ]
85. Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879-87. [ Links ]
86. Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355-61. [ Links ]
87. Feige JN, Auwerx J. Transcriptional coregulators in the control of energy homeostasis. Trends Cell Biol. 2007;17:292-301. [ Links ]
88. Fruchart JC. Peroxisome proliferator-activated receptor-alpha (PPARalpha): at the crossroads of obesity, diabetes and cardiovascular disease. Atherosclerosis. 2009;205:1-8. [ Links ]
89. Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA. 1997;94:4318-23. [ Links ]
90. Cuaranta-Monroy I, Kiss M, Simandi Z, Nagy L. Genome-wide effects of PPAR gamma in macrophages and dendritic cells – revealing complexity through systems biology. Eur J Clin Invest. 2015 DOI: 10.1111/eci.12491. [ Links ]
91. Schneider C, Nobs SP, Kurrer M, Rehrauer H, Thiele C, Kopf M. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat Immunol. 2014;15:1026-37. [ Links ]
92. Chawla A. Control of Macrophage Activation and Function by PPARs. Circulation Res. 2010;106:1559-69. [ Links ]
93. Müller G, Satoh Y, Geisen K. Extrapancreatic effects of sulfonylureas a comparison between glimepiride and conventional sulfonylureas. Diabetes Res Clin Pract. 1995;28:S115–S137. [ Links ]
94. Müller G, Wied S. The sulfonylurea drug, glimepiride, stimulates glucose transport, glucose transporter translocation, and dephosphorylation in insulin-resistant rat adipocytes in vitro. Diabetes. 1993;42:12:1852-67. [ Links ]
95. Overkamp D, Volk A, Maerker E, Heide PE, Wahl HG, Rett K, Häring HU. Acute effect of glimepiride on insulin-stimulated glucose metabolism in glucose-tolerant insulin-resistant offspring of patients with type 2 diabetes. Diabetes Care. 2002;25:2065-73. [ Links ]
96. Fukuen S, Iwaki M, Yasui A, Makishima M, Matsuda M, Shimomura I. Sulfonylurea agents exhibit peroxisome proliferator-activated receptor γ agonistic activity. J Biol Chem. 2005;280:23653-9. [ Links ]
97. Tsunekawa T, Hayashi T, Suzuki Y, Matsui-Hirai H, Kano H, Fukatsu A, Nomura N, Miyazaki A, Iguchi A. Plasma adiponectin plays an important role in improving insulin resistance with glimepiride in elderly type 2 diabetic subjects. Diabetes Care. 2003;26:285-9. [ Links ]
98. Takahashi A, Nagashima K, Hamasaki A, Kuwamura N, Kawasaki Y, Ikeda H, Yamada Y, Inagaki N, Seino Y. Sulfonylurea and glinide reduce insulin content, functional expression of K(ATP) channels, and accelerate apoptotic beta-cell death in the chronic phase. Diabetes Res Clin Pract. 2007;77:343-50. [ Links ]
99. Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY. Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab. 2005;90:501-6. [ Links ]
100. Efanova IB, Zaitsev SV, Zhivotovsky B, Köhler M, Efendić S, Orrenius S, Berggren PO. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells. A process dependent on intracellular Ca2+ concentration. J Biol Chem. 1998;273:33501-7. [ Links ]
101. UK Prospective Diabetes Study (UKPDS) Group. Intensive bloodglucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-53. [ Links ]
102. Sawada F, Inoguchi T, Tsubouchi H, Sasaki S, Fujii M, Maeda Y, Morinaga H, Nomura M, Kobayashi K, Takayanagi R. Differential effect of sulfonylureas on production of reactive oxygen species and apoptosis in cultured pancreatic β-cell line, MIN6. Metabolism. 2008;57:1038-45. [ Links ]
103. Ueba H, Kuroki M, Hashimoto S, Umemoto T, Yasu T, Ishikawa SE, Saito M, Kawakami M. Glimepiride induces nitric oxide production in human coronary artery endothelial cells via a PI3-kinase-Akt dependent pathway. Atherosclerosis. 2005;183:35-9. [ Links ]
104. Del Guerra S, Marselli L, Lupi R, Boggi U, Mosca F, Benzi L, Del Prato S, Marchetti P. Effects of prolonged in vitro exposure to sulphonylureas on the function and survival of human islets. J Diabetes Complications 2005;19:60-4. [ Links ]
105. Remedi MS, Nichols CG. Chronic antidiabetic sulfonylureas in vivo: reversible effects on mouse pancreatic β-cells. PLoS Med. 2008;5:e206. [ Links ]
106. Bermúdez V, Cano R, Cano C, Bermúdez F, Leal E, Acosta K, Mengual E, Arraiz N, Briceño C, Gómez J, Bustamante M, Aparicio D, Cabrera M, Valdelamar L, Rodriguez M, Manuel V, Hernández R. Homeostasis model assessment (HOMA) as surrogate insulinization criteria in patients with type 2 diabetes. Am J Ther. 2008;15:409-16. [ Links ]
107. Chen K. Athero-protective actions of two oral antidiabetic drugs: Suppression of inflammation and oxidative stress. J Cardiovasc Dis Res. 2012;3:3-4. [ Links ]
108. Wu JS, Lin TN, Wu KK. Rosiglitazone and PPAR-gamma overexpression protect mitochondrial membrane potential and prevent apoptosis by upregulating anti-apoptotic Bcl-2 family proteins. J Cell Physiol. 2009;220:58-71. [ Links ]
109. Fong WH, Tsai HD, Chen YC, Wu JS, Lin TN. Anti-apoptotic actions of PPAR-gamma against ischemic stroke. Mol Neurobiol. 2010;41:180-6. [ Links ]
110. Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996; 30: 359-371. [ Links ]
111. Rao NK, Annapurna MM. Copper and nickel complexes of metformin: synthesis, characterization and pharmacodynamic evaluation. JASA 2007;3:43-6. [ Links ]
112. Horn D, Barrientos A. Mitochondrial copper metabolism and delivery to cytochrome c oxidase. IUBMB Life. 2008;60:421-9. [ Links ]
113. Zhu M, Lu L, Yang P, Jin X. Bis(1,1-di¬methyl¬biguanido)copper(II) octahydrate. Acta Cryst. 2002;E58:m217-m219. [ Links ]
114. Rozas I, Sánchez-Sanz G, Alkorta I, Elguero J. Solvent effects on guanidinium-anion interactions and the problem of guanidinium Yaromaticity. J Physical Organic Chem. 2013;26:378-85. [ Links ]
115. Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996;30:359-71. [ Links ]
116. Christensen MM, Brasch-Andersen C, Green H, Nielsen F, Damkier P, Beck-Nielsen H, Brosen K. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet Genomics. 2011;21:837-50. [ Links ]
117. Sun J, Zhao M, Jia P, Wang L, Wu Y, Iverson C, Zhou Y, Bowton E, Roden DM, Denny JC, Aldrich MC, Xu H, Zhao Z. Deciphering Signaling Pathway Networks to Understand the Molecular Mechanisms of Metformin Action. PLoS Comput Biol. 2015;11:e1004202. [ Links ]
118. Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273:35347-54. [ Links ]
119. Iseli TJ, Walter M, van Denderen BJ, Katsis F, Witters LA, Kemp BE, Michell BJ, Stapleton D. AMP-activated protein kinase beta subunit tethers alpha and gamma subunits via its C-terminal sequence (186- 270). J Biol Chem. 2005;280:13395-400. [ Links ]
120. Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274-84. [ Links ]
121. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167-74. [ Links ]
122. Andrzejewski S, Gravel SP, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014;2:12. [ Links ]
123. Luengo A, Sullivan LB, Heiden MG. Understanding the complex-Ity of metformin action: limiting mitochondrial respiration to improve cancer therapy. BMC Biol. 2014;12:82. [ Links ]
124. Matsuzaki S, Humphries KM. Selective inhibition of deactivated mitochondrial complex I by biguanides. Biochemistry. 2015;54:2011-21. [ Links ]
125. Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420-5. [ Links ]
126. Meng S, Cao J, He Q, Xiong L, Chang E, Radovick S, Wondisford FE, He L. Metformin activates AMP-activated protein kinase by promoting formation of the αβγ heterotrimeric complex. J Biol Chem. 2015;290:3793-802. [ Links ]
127. Oakhill JS, Chen ZP, Scott JW, Steel R, Castelli LA, Ling N, Macaulay SL, Kemp BE. β-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK). Proc Natl Acad Sci U S A. 2010;107:19237-41. [ Links ]
128. Ouyang J, Parakhia RA, Ochs RS. Metformin activates AMP kinase through inhibition of AMP deaminase. J Biol Chem. 2011;286:1-11. [ Links ]
129. Vytla VS, Ochs RS. Metformin increases mitochondrial energy formation in L6 muscle cell cultures. J Biol Chem. 2013;288:20369-77. [ Links ]
130. Cheng J, Morisaki H, Toyama K, Sugimoto N, Shintani T, Tandelilin A, Hirase T, Holmes EW, Morisaki T. AMPD1: a novel therapeutic target for reversing insulin resistance. BMC Endocr Disord. 2014;14:96. [ Links ]
131. Corominas-Faja B, Quirantes-Piné R, Oliveras-Ferraros C, Vazquez-Martin A, Cufí S, Martin-Castillo B, Micol V, Joven J, Segura-Carretero A, Menendez JA. Metabolomic fingerprint reveals that metformin impairs one-carbon metabolism in a manner similar to the antifolate class of chemotherapy drugs. Aging (Albany NY). 2012;4:480-98. [ Links ]
132. Menendez JA, Joven J. One-carbon metabolism: an aging-cancer crossroad for the gerosuppressant metformin. Aging (Albany NY). 2012;4:894-8. [ Links ]
133. Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494:256-60. [ Links ]
134. Johnson RA. Adenyl Cyclases. In Encyclopedia of Molecular Pharmacology, Editors Dr Stefan Offermanns and Dr. Walter Rosenthal. 2008, pp 28-37. DOI 10.1007/978-3-540-38918-7_3. [ Links ]
135. Cao J, Meng S, Chang E, Beckwith-Fickas K, Xiong L, Cole RN, Radovick S, Wondisford FE, He L. Low concentrations of metformin suppress glucose production in hepatocytes through AMP-activated protein kinase (AMPK). J Biol Chem. 2014;289:20435-46. [ Links ]
136. Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12:141-51. [ Links ]
137. Bittinger MA, McWhinnie E, Meltzer J, Iourgenko V, Latario B, Liu X, Chen CH, Song C, Garza D, Labow M. Activation of cAMP response element-mediated gene expression by regulated nuclear transport of TORC proteins. Curr Biol. 2004;14:2156-61. [ Links ]
138. Leahy P, Crawford DR, Grossman G, Gronostajski RM, Hanson RW. CREB binding protein coordinates the function of multiple transcription factors including nuclear factor I to regulate phosphoenolpyruvate carboxykinase (GTP) gene transcription. J Biol Chem. 1999;274:8813-22. [ Links ]
139. Thonpho A, Sereeruk C, Rojvirat P, Jitrapakdee S. Identification of the cyclic AMP responsive element (CRE) that mediates transcriptional regulation of the pyruvate carboxylase gene in HepG2 cells. Biochem Biophys Res Commun. 2010;393:714-9. [ Links ]
140. Kodama S, Moore R, Yamamoto Y, Negishi M. Human nuclear pregnane X receptor cross-talk with CREB to repress cAMP activation of the glucose-6-phosphatase gene. Biochem J. 2007;407:373-81. [ Links ]
141. Fulco M, Sartorelli V. Comparing and Contrasting the Roles of AMPK and SIRT1 in Metabolic Tissues. Cell Cycle. 2008;7:3669-79. [ Links ]
142. Breen DM, Sanli T, Giacca A, Tsiani E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem Biophys Res Commun. 2008;374:117-22. [ Links ]
143. Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC- 1alpha. EMBO J. 2007;26:1913-23. [ Links ]
144. Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056-60. [ Links ]
145. Caton PW, Nayuni NK, Kieswich J, Khan NQ, Yaqoob MM, Corder R. Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J Endocrinol. 2010;205:97-106. [ Links ]
146. Nelson LE, Valentine RJ, Cacicedo JM, Gauthier MS, Ido Y, Ruderman NB. A novel inverse relationship between metformin-triggered AMPK-SIRT1 signaling and p53 protein abundance in high glucoseexposed HepG2 cells. Am J Physiol Cell Physiol. 2012;303:C4-C13. [ Links ]
147. Gibbs EM, Stock JL, McCoid SC, Stukenbrok HA, Pessin JE, Stevenson RW, Milici AJ, McNeish JD. Glycemic improvement in diabetic db/db mice by overexpression of the human insulin-regulatable glucose transporter (GLUT4). J Clin Invest. 1995;95:1512-8. [ Links ]
148. Grisouard J, Timper K, Radimerski TM, Frey DM, Peterli R, Kola B, Korbonits M, Herrmann P, Krähenbühl S, Zulewski H, Keller U, Müller B, Christ-Crain M. Mechanisms of metformin action on glucose transport and metabolism in human adipocytes. Biochem Pharmacol. 2010;80:1736-45. [ Links ]
149. Lenzen S, Lortz S, Tiedge M. Effect of metformin on SGLT1, GLUT2, and GLUT5 hexose transporter gene expression in small intestine from rats. Biochem Pharmacol. 1996;51:893-6. [ Links ]
150. Walker J, Jijon HB, Diaz H, Salehi P, Churchill T, Madsen KL. 5-aminoimidazole- 4-carboxamide riboside (AICAR) enhances GLUT2- dependent jejunal glucose transport: a possible role for AMPK. Biochem J. 2005;385:485-91. [ Links ]
151. Sakar Y, Meddah B, Faouzi MA, Cherrah Y, Bado A, Ducroc R. Metformin- induced regulation of the intestinal D-glucose transporters. J Physiol Pharmacol. 2010;61:301-7. [ Links ]
152. Klip A, Sun Y, Chiu TT, Foley KP. Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation. Am J Physiol Cell Physiol. 2014;306:C879-86. [ Links ]
153. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098-101. [ Links ]
154. Tsuchiya A, Kanno T, Nishizaki T. PI3 kinase directly phosphorylates Akt1/2 at Ser473/474 in the insulin signal transduction pathway. J Endocrinol. 2013;220:49-59. [ Links ]
155. Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab. 2008;295:E29-37. [ Links ]
156. Lee JO, Lee SK, Jung JH, Kim JH, You GY, Kim SJ, Park SH, Uhm KO, Kim HS. Metformin induces Rab4 through AMPK and modulates GLUT4 translocation in skeletal muscle cells. J Cell Physiol. 2011;226:974-81. [ Links ]
157. Thong FS, Bilan PJ, Klip A. The Rab GTPase-activating protein AS160 integrates Akt, protein kinase C, and AMP-activated protein kinase signals regulating GLUT4 traffic. Diabetes. 2007;56:414-23. [ Links ]
158. Luiken JJ, Ouwens DM, Habets DD, van der Zon GC, Coumans WA, Schwenk RW, Bonen A, Glatz JF. Permissive action of protein kinase C-zeta in insulin-induced CD36- and GLUT4 translocation in cardiac myocytes. J Endocrinol. 2009;201:199-209. [ Links ]
159. Lee JO, Lee SK, Kim JH, Kim N, You GY, Moon JW, Kim SJ, Park SH, Kim HS. Metformin regulates glucose transporter 4 (GLUT4) translocation through AMP-activated protein kinase (AMPK)-mediated Cbl/CAP signaling in 3T3-L1 preadipocyte cells. J Biol Chem. 2012;287:44121-9. [ Links ]
160. Liu J, DeYoung SM, Hwang JB, OLeary EE, Saltiel AR. The roles of Cbl-b and c-Cbl in insulin-stimulated glucose transport. J Biol Chem. 2003;278:36754-62. [ Links ]
161. Brisson D, Vohl MC, St-Pierre J, Hudson TJ, Gaudet D. Glycerol: a neglected variable in metabolic processes? Bioessays. 2001;23:534-42. [ Links ]
162. Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, Camporez JP, Lee HY, Cline GW, Samuel VT, Kibbey RG, Shulman GI. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542-6. [ Links ]
163. Mráček T, Drahota Z, Houtěk J. The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim Biophys Acta. 2013;1827:401-10. [ Links ]
164. Otto M, Breinholt J, Westergaard N. Metformin inhibits glycogen synthesis and gluconeogenesis in cultured rat hepatocytes. Diabetes Obes Metab. 2003;5:189-94. [ Links ]
165. Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta. 1989;1012:81-6. [ Links ]
166. Hunter RW, Treebak JT, Wojtaszewski JF, Sakamoto K. Molecular mechanism by which AMP-activated protein kinase activation promotes glycogen accumulation in muscle. Diabetes. 2011;60:766-74. [ Links ]
167. Sakoda H, Fujishiro M, Fujio J, Shojima N, Ogihara T, Kushiyama A, Fukushima Y, Anai M, Ono H, Kikuchi M, Horike N, Viana AY, Uchijima Y, Kurihara H, Asano T. Glycogen debranching enzyme association with beta-subunit regulates AMP-activated protein kinase activity. Am J Physiol Endocrinol Metab. 2005;289:E474-81. [ Links ]
168. Oligschlaeger Y, Miglianico M, Chanda D, Scholz R, Thali RF, Tuerk R, Stapleton DI, Gooley PR, Neumann D. The recruitment of AMPactivated protein kinase to glycogen is regulated by autophosphorylation. J Biol Chem. 2015;290:11715-28. [ Links ]
169. Haas B, Eckstein N, Pfeifer V, Mayer P, Hass MD. Efficacy, safety and regulatory status of SGLT2 inhibitors: focus on canagliflozin. Nutr Diabetes. 2014;4:e143. [ Links ]
170. Saad MI, Kamel MA, Hanafi MY. Modulation of Adipocytokines Production and Serum NEFA Level by Metformin, Glimepiride, and Sitagliptin in HFD/STZ Diabetic Rats. Biochem Res Int. 2015;2015:138134. [ Links ]
171. Mogensen UM, Andersson C, Fosbøl EL, Schramm TK, Vaag A, Scheller NM, Torp-Pedersen C6, Gislason G7, Køber L. Metformin in combination with various insulin secretagogues in type 2 diabetes and associated risk of cardiovascular morbidity and mortality--a retrospective nationwide study. Diabetes Res Clin Pract. 2015;107:104- 12. [ Links ]
172. Kim HS, Kim DM, Cha BS, Park TS, Kim KA, Kim DL, Chung CH, Park JH, Jang HC, Choi DS. Efficacy of glimepiride/metformin fixeddose combination vs metformin uptitration in type 2 diabetic patients inadequately controlled on low-dose metformin monotherapy: A randomized, open label, parallel group, multicenter study in Korea. J Diabetes Investig. 2014;5:701-8. [ Links ]
173. Charpentier G, Fleury F, Kabir M, Vaur L, Halimi S. Improved glycaemic control by addition of glimepiride to metformin monotherapy in type 2 diabetic patients. Diabet Med. 2001;18:828-34. [ Links ]
174. Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: results of a double-blind, placebocontrolled, dose-response trial. Am J Med. 1997;103:491-7. [ Links ]
175. Fujioka K, Brazg RL, Raz I, Bruce S, Joyal S, Swanink R, Pans M. Efficacy, dose-response relationship and safety of once-daily extended- release metformin (Glucophage XR) in type 2 diabetic patients with inadequate glycaemic control despite prior treatment with diet and exercise: results from two double-blind, placebo-controlled studies. Diabetes Obes Metab. 2005;7:28-39. [ Links ]
176. Arai K, Maeda H, Shirabe S, Yamamoto R, Kumakura A, Yamauchi M, Hirao T, Hirao S, Hirao K. Both glimepiride and high-dose metformin are important for sustained glucose lowering in Japanese type 2 diabetes patients on glimepiride-sitagliptin-metformin therapy: subanalysis of a single-center, open-label, randomized study. Diabetes Technol Ther. 2014;16:442-6. [ Links ]
177. González-Ortiz M, Guerrero-Romero JF, Violante-Ortiz R, Wacher-Rodarte N, Martínez-Abundis E, Aguilar-Salinas C, Islas-Andrade S, Arechavaleta-Granell R, González-Canudas J, Rodríguez-Morán M, Zavala-Suárez E, Ramos-Zavala MG, Metha R, Revilla-Monsalve C, Beltrán-Jaramillo TJ. Efficacy of glimepiride/metformin combination versus glibenclamide/metformin in patients with uncontrolled type 2 diabetes mellitus. J Diabetes Complications. 2009;23:376-9. [ Links ]