Revista Científica
versão impressa ISSN 0798-2259
Rev. Cient. (Maracaibo) v.17 n.3 Maracaibo maio 2007
Levels of Immunoglobulin-A in Trachea,
Gut and Bile Samples of Chickens Vaccinated
Against Newcastle Disease.
Francisco Perozo 1, Giovanny Finol 1 y Yaneth Mavárez 2
1 Facultad de Ciencias Veterinarias, Universidad del Zulia. Apartado 15252. Maracaibo 4005-A Estado Zulia, Venezuela.
2 Veterinary Pathology Department, University of Georgia. USA. E-mail: frankperozo1@latinmail.com
ABSTRACT
Immunoglobulin-A (IgA) response to Newcastle diseases virus (NDV) vaccination was assessed using enzyme-linked immunosorbent assay. Total and NDV specific IgA levels were determined in tracheal washings, intestinal washings and bile. Chickens were primed with an in ovo recombinant avian adeno-associated vaccine coding for the NDV hemagglutinin-neuraminidase gene (rAAAV) and revaccinated with B1 or LaSota strains of the virus. The most suitable samples for IgA determinations were tracheal washings and bile. No NDV specific IgA response was elicited by the rAAAV alone. After revaccination with live NDV virus the IgA levels in the biological samples were significantly higher (P<0.005) than the levels in the unvaccinated controls. No strain dependant differences were observed in the NDV specific response after revaccination. No differences in total (unspecific) IgA levels between control and vaccinated birds were observed. In view of the importance of mucosal immune response to NDV infection, the assessment of local antibody levels can aid in the study of the host antiviral response.
Key words: Newcastle disease virus, immunoglobulin A, avian adeno-associated virus.
Niveles de inmunoglobulina A en muestras de tráquea, intestino y bilis provenientes
de aves vacunadas contra la enfermedad de Newcastle.
RESUMEN
Mediante la prueba de inmunoensayo ligado a enzimas se evaluó la respuesta de inmunoglobulina A (IgA) contra el virus de la enfermedad de Newcastle (VEN) en pollos. Se determinaron los niveles IgA total, así como los niveles de IgA específica contra el VEN en lavados traqueales, lavados intestinales y muestras de bilis. Las aves fueron inmunizadas in ovo con un virus adeno-asociado aviar recombinante expresando el gen de la hemaglutinina-neuraminidasa del VEN y fueron revacunadas con las cepas B1 o LaSota del virus. Los lavados traqueales y la bilis fueron las muestras más apropiadas para la determinación de los niveles de IgA específica para el VEN. El virus recombinante no estimuló la producción de IgA especifica contra el VEN. Los niveles de IgA en los fluidos analizados de las aves revacunadas con virus vivo fueron significativamente (P<0,05) más altos que los correspondientes a las aves control no vacunadas. No se observaron diferencias en los niveles de IgA especifica para el VEN, independientemente de la cepa utilizada para la revacunación. No se observaron diferencias en los niveles de IgA total (inespecíficos) entre los grupos vacunados y el control. Ante lo relevante de la respuesta inmune local contra la infección con el VEN, la determinación de los niveles de anticuerpos locales puede ser de ayuda para el estudio de la respuesta antiviral del hospedero.
Palabras clave: Virus de la enfermedad de Newcastle, IgA, Lasota, B1B1.
Recibido: 16 / 05 / 2006. Aceptado: 18 / 01 / 2007.
INTRODUCCIÓN
Newcastle Disease Virus (NDV) is a major concern to the poultry industry due to the costs of vaccination programs and the consequences of sub-clinical and clinical forms of the disease [1, 16, 17, 18, 24]. The classical approach to prevention of viral infectious diseases in poultry medicine has relied, almost exclusively, in the use of inactivated or live vaccines to elicit protective immune responses against pathogens [1, 7, 22, 24]. Recent advances in the understanding of the molecular basis of pathogenesis and in the mechanisms involved in the generation of protective immune responses have opened new avenues for prevention of infectious diseases [1, 10].
In ovo vaccination is popular due to increased speed, reduced labor costs, and uniform vaccination [13, 15]. However, since current NDV vaccines cause embryonic mortality, the alternative of using recombinant viruses to deliver the NDV immunogenic epítopes to the embryo has shown to be feasible [1].
The resistance to challenge with NDV virulent strains exhibited by chickens that display low levels of systemic antibodies is a frequent observation, meaning that systemic immunity is not enough and that local immune response (both cell mediated and humoral) is required for full protection [12, 20]. Local immunity acts as a barrier at surfaces where primary viral infections occur, thereby interfering with further spread of the virus [12, 21]. Local humoral immunity depends on locally produced antibodies, which are actively transported through the epithelial cells and released across the epithelium onto the mucosal surfaces [9, 19]. The IgA class predominates in most secretions and has been reported to be detectable in chicken tears, saliva, tracheal and intestinal washes and bile [4, 6, 9, 12, 21].
Monitoring systemic antibody responses can be performed using the hemagglutination inhibition test or the virus neutralization test. Lately the enzyme immunolinked assays (ELISA) have become the test of choice for antibody measurement, due to its sensitivity and reproducibility [2, 3, 4].
A recombinant avian adeno-associated virus (rAAAV) developed in the Poultry Diagnostic and Research Center of the University of Georgia, (Athens, Gerogia, USA), has been proven to be infective for a wide variety of tissues both in vivo and in vitro and can be used as a gene delivery system [8]. The type and extent of the immune response elicited by such recombinant product need to be further characterized. This research aims to compare the levels of total (unspecific) and NDV specific IgA in tracheal washes, intestinal washes and bile of chickens primed in ovo with the rAAAV coding for the hemmmaglutinin neuraminidase (HN) gene of NDV and revaccinated with B1 or LaSota strains to assess the chicken response to these vaccination protocols.
MATERIALS AND METHODS
Viruses
A rAAAV coding for the HN protein of the LaSota strain of NDV, constructed by simultaneous transfection of 293 human embryo kidney cells with a plasmid containing the HN gene, one the rep and cap genes of the avian adeno-associated virus and a pHelper plasmid was constructed and used for initial in ovo vaccination [8]. For the revaccination, commercial live vaccines containing LaSota and B1 strains of NDV (Merial Select, Inc. Gainesville, GA. USA.) were used following manufactures dose and recommendations.
Experimental design
A total of 96 specific-pathogen-free 18-day-old chicken embryos (Sunrise Farms, Catskill, NY. USA) were used. Three groups of 24 embryos were inoculated in ovo with 0.1 ml of the rAAAV (106 transfection units/ml). A fourth group (unvaccinated control) was inoculated using 0.1 ml of phosphate buffered saline. After hatch, the birds were placed in isolated units where appropriated husbandry was provided. At 28 and 38 days of age two of the groups (rAAAV + LaSota and rAAAV + B1) were revaccinated by ocular-oral route using LaSota or B1 live NDV vaccines, respectively. The third group (rAAAV) did not receive a secondary vaccination and was maintained to measure the effect of the recombinant virus alone. The fourth group remained as an unvaccinated control.
Bird Sampling
Three birds from each group were retrieved at 14, 21 28, 31, 35, 38, 42 and 45 days of age and bile, tracheal lavage samples and intestinal washes were obtained. Briefly, in order to avoid blood contamination of the trachea, chickens were euthanized using a CO2 chamber, the trachea was exposed aseptically at the pharyngeal region and clamped before the syrinx using an artery forceps, tracheal washes were obtained using a 16 gauge animal feeding needle (Popper & sons, inc., New York. USA) and 1 ml of PBS was flushed in and out the tracheal lumen 10 times with a syringe. Similar procedure was applied to an 8 centimeter portion of the duodenum to obtain the intestinal washings. Bile was collected from the gall bladder by direct puncture using a 22-gauge needle. All samples were placed in sterile containers and processed fresh.
Mucosal Immune Response
An indirect ELISA test using plates coated with NDV strain LaSota and a commercial goat anti-chicken IgA conjugate (Bethyl Laboratories, Inc. Montgomery, Texas. USA.) was used to determine the NDV specific IgA levels in the biological samples. The ELISA procedure was performed on bile, tracheal washings, and intestinal washes. Briefly, 100 µl of undiluted bile, tracheal and intestinal washes were added in duplicate (undiluted) to the NDV coated wells, NDV negative chicken serums were used as controls. After 45 minutes of incubation at room temperature (25°C) each well was washed five times using Tris buffer saline (TBS) with 0.05% of Tween 20. Chicken IgA binding to the coating antigen was detected with the anti chicken IgA conjugate. Finally, the plates were washed (five times) and bound conjugate was detected by staining for 20 minutes with Tetramethylbenzidine (TMB) substrate. The optical density at 650 (OD650) was measured using a precision microplate reader (Molecular Devices. Inc. New York, USA). Corrected optical density values were calculated by deducting the optical density values of NDV negative control samples from those of the test wells.
To estimate the total (unspecific) IgA production in the respiratory and intestinal epithelia, a commercial antigen capture ELISA chicken IgA quantitation kit (Bethyl Laboratories, Inc. Montgomery, Texas. USA.) was used following the manufactures recommendations. Checkerboard titrations established an optimal conjugate dilution of 1/10000.
Statistical analysis
The mean antibody titers were compared using Students T test. Statistical significance was established at the 0.05 level.
RESULTS AND DISCUSSION
No major effect in hatchability was observed in the in ovo vaccinated groups when compared with the controls, no embryo-toxicity can be attributed to the recombinant product. The safety of the in ovo vaccination procedure has been described previously [13, 15].
The importance of local immunity in the protection against infectious diseases has been properly established for mammals and chickens [3, 4, 5, 6, 9, 11, 12, 19, 21, 23, 25]. In response to NDV infection the IgA antibodies induce virus neutralization through its Fab fragment [3, 4, 9, 12]. IgA containing NDV immune complexes triggers the Fc receptors of several types of leukocytes and induces phagocytosis, antibody-dependant cell-mediated cytotoxicity and production of reactive oxygen intermediates, cytokines and other mediators of the inflammatory response [19, 22]. These pathways are evidence of the importance of quantifying the mucosal immune response to assess protection capabilities of NDV vaccines.
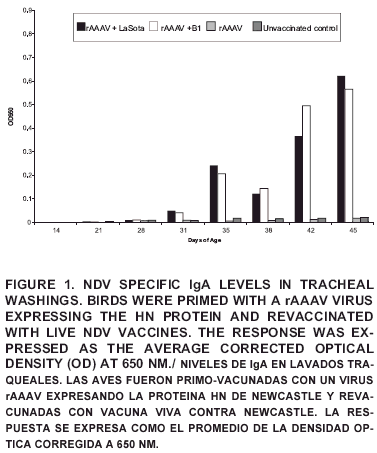
The IgA levels in trachea after revaccination with either LaSota or B1 strains of NDV (groups rAAAV + LaSota and rAAAV + B1) differed (P<0.05) from the unvaccinated controls after 35 days of age (FIG 1). These results are in agreement with previous researchers that have reported an increase in local IgA production to NDV infection or vaccination [4, 9, 12, 21].
When the effect of the strain used for revaccination was compared, no differences were observed in the IgA production regardless of the strain used, meaning no difference in the induction of IgA between LaSota and B1 strains. These results were unexpected, IgA production correlates positively with antigenic stimulation, and LaSota strain has been proven to be more immunogenic with a more aggressive epithelial tropism than B1. Strong post-vaccine reactions are considered a drawback for LaSota strain when applied to young birds with low levels of maternal antibodies [2, 9, 14, 16, 18].
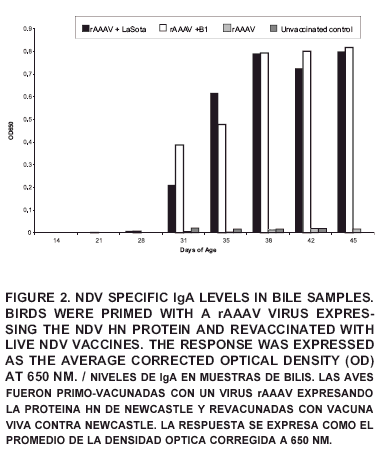
The mucosal response measured as IgA levels in bile was consistent with those of the tracheal washing results and can be observed in FIG 2. Nevertheless, the overall corrected optical density values (COD) in bile were higher than in other biological samples despite of the treatment, probably due to the physiological high concentration of all metabolites observed in the bile. Literature reports have established the link between bile and local protection against NDV showing the neutralizing capacity of bile obtained from immunized animals on NDV preparations in vitro, probably due to the presence of NDV specific neutralizing antibodies [14]. In mammals, the antibodies present in the bile are produced locally in the intestinal tract and transported via the portal vein to the liver, where IgA is actively transported through the epithelium [3].
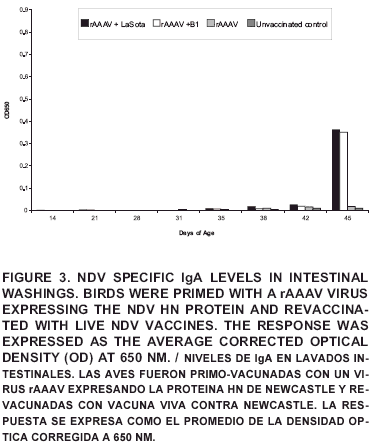
Levels of IgA in the intestinal washings were only significantly different from the control at day 45 and overall were very low (FIG 3). The diminished detection levels when compared with the bile and the tracheal washings of homologous birds may be explained by the dilution of local antibodies associated with the method of collection and due to the poor intestinal tropism of the strains used [12, 16].
No NDV specific IgA response was observed in any of the tested samples on days 14, 21 or 28 in birds primed in ovo with the rAAAV vaccine; only after revaccination with live viruses a measurable IgA response was observed. This means that the recombinant virus did not stimulate a measurable mucosal immune response by itself, probably due to the nature of the antigenic stimulation induced by the rAAAV which is a replication defective virus that relays in the host cell machinery to express the HN antigen, and due to the previously reported requirement of local viral replication in order to induce an IgA response [8, 9]. The rAAAV expressing the HN protein of NDV is a novel product and its complete mechanism of action for immunity is to be determined. Further research is being conducted to determine tissue localization after in vivo inoculation and mechanisms of immune response. Viral transduction studies using a murine model and adeno-associated virus (another member of the Parvoviridae family) reported genomic integration in 100% of the hepatocytes and striated muscle system after a single intravenous administration [11].
No significant differences (P<0.05) were observed in the levels of total IgA between the control and any of the vaccinated groups in none of the biological samples tested, suggesting that there is no effect of NDV vaccination in the overall IgA load of the mucosal tissue in chickens. This observation could be explained as the consequence of commensal and/or pathogenic colonization of the respiratory and intestinal epithelial surfaces which represent the putative site of initial antigen encounter [3, 6, 12, 19]. Furthermore, epithelial cells have been proved to provide co-stimulatory signals promoting terminal differentiation of B-cells oriented towards IgA production, generating relatively high and constant levels of the immunoglobulin [4, 5, 21, 22].
Birds have a well-developed mucosal immune system; its characteristics include local production and secretion of IgA antibodies and trafficking of IgA producing plasma cells, based on this the evaluation of IgA levels in the mucosal immune response is important and can aid in the NDV control strategies.
CONCLUSIONS
The most suitable samples for IgA determinations were tracheal washings and bile. No measurable NDV specific mucosal IgA levels response was elicited by the recombinant adeno-associated virus coding for the HN protein of NDV.
Mucosal IgA levels from revaccinated birds were significantly higher (P<0.005) than the levels in the control groups despite of the strain used for revaccination.
The evaluation of IgA levels in the mucosal immune response is important and can aid in the NDV control strategies.
BIBLIOGRAPHIC REFERENCES
1. ALEXANDER, D. Gordon Memorial Lecture. Newcastle disease. Br. Poult. Sci. 42(1): 5-22. 2001. [ Links ]
2. ALEXANDER, D. Newcastle Disease Virus and other Avian Paramyxoviruses. In: D. E. Swayne, (Ed.). A laboratory Manual for Isolation and Identification of Avian Pathogens, 4th. Ed. (235-240 pp). Kennett Square, PA: American. Association of Avian Pathologists. 220-238 pp. 1998. [ Links ]
3. AL-GARIB, S.; GIELKENS, A.; GRUYS, D.; KOCH, G. Review of Newcastle disease virus with particular references to immunity and vaccination. World. Poult Sci. J. 59(2): 185-200. 2003. [ Links ]
4. AL-GARIB, S.; GIELKENS, A.; GRUYS, D.; HARTOG, L.; KOCH, G. Immunoglobulin class distribution of systemic and mucosal antibody responses to Newcastle disease in chickens. Avian Dis. 47(2): 32-40. 2003. [ Links ]
5. BRANDTZAEG, P. Role of secretory antibodies in the defense against infections. Int. J. Med. Microbiol. 293(1): 3-15. 2003. [ Links ]
6. DHINAKAR, R.; JONES, R. Local antibody production in the oviduct and gut of hens infected with a variant strain of infectious bronchitis virus. Vet. Immunol and Immunopathol. 53(1): 147-161. 1996. [ Links ]
7. ERF, G. Cell mediated immunity in poultry. Poult. Sci. 83(2): 580-590. 2004. [ Links ]
8. ESTEVEZ, C.; VILLEGAS, P. Sequence analysis, viral rescue from infectious clones and generation of recombinant virion of the avian adeno-associated virus. Vir. Res. 105(1): 195-208. 2004. [ Links ]
9. EWERT, D.; BARGER, B.; EIDSON, C. Local antibody response in chickens: Analysis of the antibody synthesis to Newcastle disease virus by solid-phase radioimmunoassay and immunoflourescence with class specific antibody for chicken immunoglobulins. Infec. Immun. 24(1): 269-275. 1979. [ Links ]
10. JANENWAY, C. Humoral immune response. In Health and Diseases in Immunobiology. By Elsevior Science Ltd/ Cralang Publishing. 6th Ed. 363-376. pp. 2005. [ Links ]
11. GONCALVEZ, M. Adeno-associated virus: from defective virus to effective vector. Virol. J. 43(2):1186-1201. 2005. [ Links ]
12. JAYAWARDANE, G.; SPRADBROW, P. Mucosal immunity in chickens vaccinated with the V4 strain of Newcastle disease virus. Vet. Microbiol. 46(3): 69-77. 1995. [ Links ]
13. JOCHEMSEN, P.; JUEURISSEN, S. The localization and uptake of in ovo injected soluble and particle substances in the chicken. Poult. Sci. 81(1):1811-1817. 2004. [ Links ]
14. LEE, J.; HANSON, R. Effects of bile and gastrointestinal secretions on the infectivity of Newcastle disease virus. Infec. Immuni. 11(4): 692-697. 1975. [ Links ]
15. NEGASH, T.; AL-GARIB, S.; GRUYS, E. Comparison of in vivo and post-hatch vaccination with particular reference to infectious bursal disease. Vet. Quart. 26(2): 76-87. 2004. [ Links ]
16. NUNES, J.; VASCONCELOS, A.; JORGE, M. Comparative morphometric analysis of vaccine virulence of some lentogenic strains of Newcastle disease virus in tracheas of SPF chickens Arq. Bra. Med. Vet. Zootec. 54: 335-339. 2002. [ Links ]
17. OFFICE INTERNATIONAL DES EPIZOOTIES (OIE). Newcastle Disease. OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Paris 161-169. pp. 2004. [ Links ]
18. PEROZO, F.; NAVA, J.; RIVERA, S. Evaluation of two vaccination programs against Newcastle disease in Ross line broiler chickens reared under field conditions in Zulia State, Venezuela. 1. Productive parameters and posvaccine reaction. Rev. Científ. FCV-LUZ. XIV (2): 331-337. 2004. [ Links ]
19. PILETTE, Y.; QUADRHIRI, V.; GODDING, J.; SIBILLE, Y. Lung mucosal immunity: immunoglobulin-A revisited. Eur. Respir. J. 18(1): 571-588. 2001. [ Links ]
20. REYNOLDS, D.; MARAQA, A. Protective immunity against Newcastle disease: the role of cell-mediated immunity. Avian Dis. 44(2): 145-154. 2000. [ Links ]
21. RUSSELL, P. Newcastle disease virus: virus replication in the Harderian gland stimulates lachrymal IgA; the yolk sac provides early lachrymal IgG. Vet. Immunol. immunopathol. 37(3): 151-163. 1993. [ Links ]
22. SCOTT, T. Our current understanding of humoral immunity in poultry. Poult. Sci. 83920: 574-579. 2004. [ Links ]
23. SEAL, B.S.; KING, D.J.;. SELLERS, H. The avian response to Newcastle disease virus. Devel. Compar. Immunol. 24(2): 257-268. 1995. [ Links ]
24. VILLEGAS, P. Viral diseases of the respiratory system. Poult Sci 77(8): 1143-1145. 1998. [ Links ]
25. ZIGTERMAN, G.; VAN DE VEN, W.; VAN GEFFEN, C.; LOEFFEN, A.; PANHUIJZEN, J.; RIJKE, E.; VERMEULEN, A. Detection of mucosal immune responses in chickens after immunization or infection. Vet. Immunol. Immunopath. 36: 281-91. 1993. [ Links ]












 uBio
uBio