Revista Científica
versão impressa ISSN 0798-2259
Rev. Cient. (Maracaibo) v.19 n.2 Maracaibo mar. 2009
BURSAL RESTORATION AFTER INTERMEDIATE AND INTERMEDIATE PLUS INFECTIOUS BURSAL DISEASE VIRUS VACCINATION.
María Castro1, Elsy Saume1, Carmen Díaz2, Judith García3 y Francisco Perozo4
1Instituto Nacional de Investigaciones Agrícolas. Maracay, Venezuela. mcastro@inia.gob.ve ; esaume@inia.gob.ve
2Facultad de Ciencias Veterinarias. carmenteresad@yahoo.com
3Facultad de Ciencias Agronómicas. garciaj@agr.ucv.ve , Universidad Central de Venezuela.
4Facultad de Ciencias Veterinarias. Universidad del Zulia. Apartado 15252.Maracaibo 4005-A, Venezuela fperozo@uga.edu .
ABSTRACT
Infectious bursal disease (IBD) is an acute, contagious, viral disease of young chickens characterized by diarrhea, vent picking, trembling, incoordination, inflammation followed by atrophy of the bursa of Fabricius and by variable degrees of immunosuppression. The diseases is caused by the infectious bursal disease virus (IBDV) which upon its antigenic characteristics and pathogenicity has been classified as classic (mild, intermediate and intermediate plus) strains, very virulent IBDV (vvIBDV) and variant strains. With the widespread presence of vvIBDV, the poultry industry has resorted to the use of less attenuated vaccines raising the concern about bursal integrity after vaccination. IBD vaccination using intermediate plus vaccine strains can temporarily deplete the bursal follicles and interrupt the normal B-cell development; if the damage is reversible this process can be followed by B-cell repopulation and histological regeneration. In order to assess this bursal restoration process, specific pathogen free birds were vaccinated with intermediate and intermediate plus IBDV vaccine and bursas were evaluated by histopathology and immunohistochemistry. Both B and T cells were detected in the recovering bursas. At the end of the trial, signs of bursal regeneration and B cell repopulation were observed in the intermediate IBDV vaccinated birds. The bursal restoration process was impaired or delayed in the intermediate plus vaccine group. Relevance of B and T cell repopulation is discussed.
Key words: Infectious bursal disease virus, vaccination, restoration.
Restauración de la bolsa posterior a la vacunación con cepas intermedias e intermedias plus del virus de la enfermedad infecciosa de la bolsa.
RESUMEN
La enfermedad infecciosa de la bolsa (por sus siglas en Inglés IBD) es una enfermedad viral aguda y contagiosa que afecta a los pollos jóvenes, caracterizada por diarrea, picado de la cloaca, temblores, incoordinación, inflamación seguida de atrofia de la bolsa de Fabricious y por grados variables de inmunosupresión. La enfermedad es causada por el virus de la enfermedad infecciosa de la bolsa (por sus siglas en Inglés IBDV) que basado en sus características antigénicas y de patogenicidad ha sido clasificado en cepas clásicas (virus suaves, intermedios e intermedios plus), IBDV muy virulento y cepas variantes. Debido a la amplia presencia de IBDV muy virulento, la industria avícola ha implementado la utilización de vacunas menos atenuadas, lo que genera preocupación por la integridad de la bolsa posterior a la vacunación. La vacunación contra IBD utilizando vacunas intermedias plus puede despoblar los folículos de la bolsa e interrumpir el desarrollo normal de las células B, si el daño es reversible este proceso puede ser seguido de la repoblación de la bolsa con células B y de regeneración histológica. Con la finalidad de evaluar este proceso de restauración, se vacunaron aves libres de patógenos específicos con vacunas intermedia e intermedia plus contra IBDV y se evaluaron las bolsas mediante histopatología e inmunohistoquímica. En las bolsas en recuperación se detectaron tanto células B como células T. Al final del experimento, en las aves vacunadas con la cepa intermedia se observaron signos de regeneración de la bolsa y repoblación de células B. El proceso de restauración de la bolsa se vio comprometido o retrasado en el grupo vacunado con la cepa intermedia plus. Se discute la relevancia de la repoblación de la bolsa con células T y B.
Palabras clave: Virus de la enfermedad infecciosa de la bolsa, vacunación, restauración.
Recibido: 27 / 11 / 2007. Aceptado: 29 / 02 / 2008.
INTRODUCTION
Infectious bursal disease (IBD) is a highly infectious viral disease of chickens that induces bursal atrophy and consequently immunosuppression. This infection has a worldwide distribution and the virus can be detected in most commercial farms [12]. The infectious bursal disease virus (IBDV) is a member of the Birnaviridae family, and is classified into the genus Avibirnavirus [10]. The genome of IBDV consists of two segments of double-stranded RNA. The viral particles are single shelled, non-enveloped with icosahedral symmetry [15, 16]. The bursa of Fabricius is the main target organ for IBDV replication [7]. After the virus enters the host, IBDV causes acute lytic infections and high titers of anti-IBDV antibodies [5, 14]. IBDV replicates in actively dividing IgM+ B cells in the bursa of Fabricius. Moreover, IBDV is able to replicate in non-bursal locations, such as spleen, thymus and liver [9]. Infection results in lymphoid depletion and severe atrophy of the bursa as the predominant feature of the pathogenesis of this disease [13].
In addition to mortality, the birds infected with IBDV may develop immunosuppression; this condition may enhance the susceptibility to other infections such as inclusion body hepatitis [3], infectious bronchitis [17], infectious laryngotracheitis [21], salmonellosis and colibacilosis [23]. Furthermore, the birds previously infected with IBDV may elicit a suboptimal immune response to live attenuated vaccines as it has been reported with the vaccinations against infectious bronchitis and Newcastle disease [9, 12, 13].
The IBDV infection elicits an elevated anti-IBDV antibody response. This antibody response is believed to play a major role in the defense against the disease. Therefore, attenuated live and inactivated IBDV vaccines are selected on their ability to induce antibodies and protection [13]. The immunization of breeder flocks is especially important to transmit immunity from the dam to the progeny via the yolk of the egg; this passive immunity can protect chickens against clinical signs at early age [11]. To induce high titers of maternally derived antibodies that persist over the whole laying period, breeders are vaccinated with inactivated oil-emulsified vaccines. However, vaccination of the broilers is also necessary to protect them against clinical disease after hatching due to the high infectivity of IBDV [9, 12]. There are different types of live vaccines available; the differences are based on the virulence and antigenic characteristics of the vaccine strains. The factors that may have an impact on the efficacy of the vaccination on the farm include the appropriate vaccination time, the level of maternal antibodies, virulence of the vaccine virus and the route of administration [14, 20].
The primary role of the avian bursa of Fabricius is to provide an essential microenvironment for B-lymphocytes to diversify their immunoglobulin genes by gene hyperconversion [22]. After vaccination, the severity of the bursal lesions may be varied from transitional to irreversible depending on the pathogenicity of the virus strains. In cases, when the damage is reversible, the histological regeneration of the bursa of Fabricius has been reported [6]. The aim of this work was to assess the bursal tissue damage induced by IBDV vaccines and the extent of bursal recovery and repopulation during commercial rearing time.
MATERIALS AND METHODS
Viruses
IBDV-Lukert intermediate and intermediate plus commercial vaccines were used following manufactures recommendations. The following live commercial vaccines were given by intra-conjunctiva instillation at 6 and 14 days of age respectively: intermediate strain Lukert and intermediate plus strain Lukert 2 (Bursine Fort Dodge Animal Health, Inc., Fort Dodge, IA. USA).
Experimental design
A total of 90 specific pathogen free (SPF) birds were used to assess the effects of two IBDV vaccines of different pathogenicity applied at the dose and time recommended by the manufacturer. Birds were divided in three groups of 10 each with three replicates and raised in isolation up to 42 days of age. The treatments applied were: T1 = IBDV intermediate at day 6; T2 = IBDV intermediate plus at day 14; T3 = non-vaccinated. Ten days post vaccination and at the end of the experiment, 15 birds in each group were randomly selected and euthanized by cervical dislocation for sampling.
Histopathology
Bursas were collected and fixed by immersion in 10% neutral buffered formalin for 24 hours. Tissues were processed and embedded in paraffin using routine histological techniques. The paraffin-embedded tissues were sectioned, mounted, stained with hematoxylin and eosin (H&E) and examined by light microscopy to grade bursal lesions as previously reported [4]. Briefly, grade 0: no changes; grade 1: mild multifocal lymphoid necrosis; grade 2: moderate to severe lymphoid necrosis up to 20% of the follicles, presence of cists in the parenchyma; grade 3: up to 50% of the follicles are affected and there are abundant cist formations; grade 4: between 60 and 80% of the follicles are affected by lymphoid depletion and cysts.
Immunohistochemistry (IHC) identification of bursal lymphocyte subpopulation
In order to evaluate bursal restoration and to differentiate cell subtypes during repopulation, bursal tissue sections were cut (5 ìm) from paraffin-embedded samples and mounted on charged glass slides. Immunohistochemical staining was carried out using the avidin-biotin immunoperoxidase technique with the polyvalent immunoperoxidase kit (Omnitag, Lipshaw, MI, USA with DAB Chromogen) according to the manufacturers recommendations. For B cell identification a CD20 (IgG2a clone L26, Termo Shadon, USA) mouse monoclonal antiserum was used as primary antibody. For T cell identification a primary antibody was a CD45RO (IgG2a clone UCHL1, Termo Shadon, USA) mouse monoclonal antibody. The secondary antibody was a biotin labeled polymer conjugated to anti-mouse immunoglobulins. DAB was used as substrate for reaction development. The proportion of B and T cells was calculated by counting 100 cells in each one of five randomly chosen fields using light microscope.
RESULTS AND DISCUSSION
The main target cell for IBDV is the actively dividing B-lymphocyte, replicating virus depletes the bursal follicles, inhibiting the expansion of the immune repertoire. Ivan et al. [6] suggested a model for the immunosuppression and restoration of the immune response following IBDV vaccination that was tested in this trial.
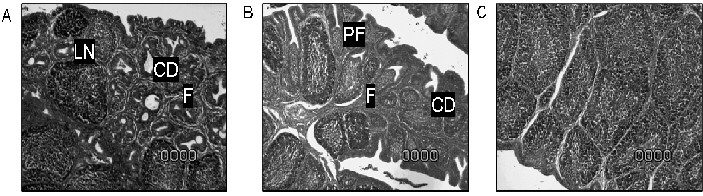
In this experiment, the intermediate vaccine group presented moderate to severe damage to the bursal tissue (grade 3) ten days post vaccination. In the intermediate plus vaccinated group severe lymphoid depletion of the follicles, increased amount of stoma between follicles and severe follicular atrophy lesions were observed (grade 4). The extent of the lesions is compared with the unvaccinated control group in FIG. 1. These results are in agreement with previous reports on the damage induced by less attenuated IBDV strains [13, 18].
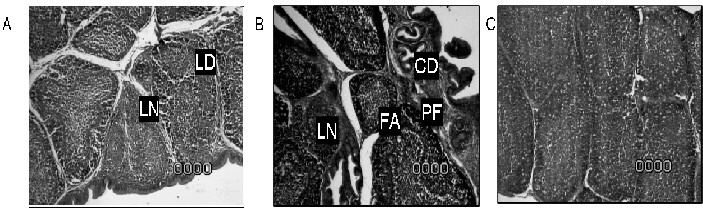
At the end of the experiment (42 days of age), signs of bursal restoration represented by repopulation of the follicles and a more normal appearance of the bursa were observed in the intermediate vaccine group, a grade 1 lesion score was assigned. Severe damage to the bursa remained present in the intermediate plus vaccine group to which a grade 4 lesion score was assigned; the lesions are observed in FIG. 2.
IBDV is a lymphotropic virus that can cause humoral and to a lower degree, cell-mediated immunosuppression in chickens infected before 3 weeks of age [9]. There are reports that the duration of immunosuppression and restoration of the humoral immune response seems to be correlated with the histological regeneration of the bursa of Fabricius. Edwards et al. [2], investigated the relationship between the bursal damage and the depression of humoral immune response to Brucella abortus in SPF chickens caused by IBDV and suggested that chickens are unlikely to be fully immunocompetent until approximately 50% of the bursa is fully repopulated. Intermediate and intermediate plus IBDV vaccination has been reported to induce histopathological lesions in primary lymphoid organs, compromising in some extent the humoral immune response [18].
Cell subtypes in the bursal repopulation process were assessed by immunohistochemistry; the proportion of B and T cell lymphocytes is of functional relevance. The proportion of B and T cells are shown in TABLE 1, in this trial, proportion of B and T cells in the non-vaccinated controls was of 74 and 2%, respectively, this results are in agreement with Kim et al. [8], whom reported that between 63 and 84% the cells in the bursa of naïve chickens were B lymphocytes and less than 4% were T cells, seven days post vaccination with an intermediate IBDV strain the proportions inverted showing near 80% T lymphocytes.
The results from the present experiment demonstrated at ten days post-vaccination (PV) 12% of B cells and 58% of T cells for the intermediate vaccinated group. At the end of the experiment (42 days of age) the proportion was 21 and 46% for B and T cells, respectively, indicating B cell repopulation. For the intermediate plus vaccinated group, the proportion ten days PV was 12 and 75% for B and T cells, respectively. At 42 days of age, B lymphocytes remained low at 8% and T cells represented 75% of the cells counted indicating less B cell repopulation. In similar studies Rautenschlein et al. [19], reported that after IBDV infection the number of IgM+ cells drop precipitously as the virus replicated within bursal follicles. However, the appearance of viral antigen in the bursa is accompanied by a dramatic infiltration of T cells in and around the site of virus replication the infiltrating T cells can be detected as early as day one PV and persisted until at least 12 weeks, although the viral antigen had disappeared by three weeks PV.
FIGURE 1. EFFECT OF INTERMEDIATE AND INTERMEDIATE PLUS IBDV VACCINATION ON BURSAL TISSUE 10 DAYS POST VACCINATION. (A) = INTERMEDIATE IBDV VACCINATION INDUCED FOLLICULAR ATROPHY (F), LYMPHOID NECROSIS (LN) AND CYSTIC DEGENERATION (CD) WERE OBSERVED (SCORE 3). (B) = INTERMEDIATE PLUS VACCINATED CHICKENS, SAME TYPE OF LESIONS IN LARGER SURFACE OF THE BURSAS (SCORE 4) WERE OBSERVED. (C) =NONVACCINATED CONTROLS NO LESIONS WERE OBSERVED (SCORE 0) / EFECTO DE LA VACUNACIÓNINTERMEDIA E INTERMEDIA PLUS CONTRA IBDV EN EL TEJIDO DE LA BOLSA 10 DÍAS POST-VACUNACIÓN. (A) = LA VACUNACIÓN CON LA CEPA INTERMEDIA DE IBDV INDUJO ATROFIA FOLICULAR, NECROSIS LINFOIDE Y DEGENERACIÓN QUÍSTICA (NIVEL 3). (B) = LAS AVES VACUNADAS CON LA CEPA INTERMEDIA PLUS MOSTRARON EL MISMO TIPO DE LESIONES SÓLO QUE MÁS EXTENDIDAS EN LA BOLSA (NIVEL 4). (C) = CONTROLES NO VACUNADOS, NO SE OBSERVARON LESIONES.
B-cell lysis caused by the replicating virus is probably a random process; consequently, those lymphocytes which respond to the IBDV antigens might also be affected to a certain level. However, surviving B-cells produce sufficient IBDV-specific antibody to provide an effective immune response [1], the later has been proved not to be true for the immune response against several other common poultry pathogens after IBDV infection [3, 17, 21, 23]. After restriction of virus replication, histological regeneration allows the bursa to resume to its original function and serve as an efficient primary lymphoid organ. The spleen and the peripheral lymphoid organs are then recolonized, which may contribute to the restitution of normal humoral responses [9].
Pathogenesis studies have looked into the role of T cells after infection and the mechanism of recovery from acute infection, the dramatic influx of T cells at the site of viral replication, suggest that T cells may be involved in limiting viral spread and thus initiating the recovery process [22]. Nevertheless, it is possible that IBDV-induced T cells may enhance viral lesions. For example, cytotoxic T cells may exasperate virus-induced cellular destruction by lysing cells expressing viral antigens. T cells may also promote the production of inflammatory factors that may accentuate tissue destruction. Nitric oxide (NO) produced by macrophages activated by T cell cytokines (e.g. IFN-ã) may promote cellular destruction [19]. Together with an increased virus replication due to higher virulence the T cell effects may explain the diminished recovery of the intermediate plus vaccinated bursas in this trial.
FIGURE 2. EFFECT OF INTERMEDIATE AND INTERMEDIATE PLUS IBDV VACCINATION ON BURSAL TISSUE AT 42 DAYS OF AGE. BIRDS IN THE INTERMEDIATE VACCINE GROUP (A) = RECOVERED TO A SCORE 1, SOME LYMPHOID NECROSIS (LN) AND DEPLETION (LD) WERE OBSERVED. INTERMEDIATE PLUS VACCINATED CHICKENS (B) REMAINED AS A SCORE 4 WITH FOLLICULAR ATROPHY (FA), LYMPHOID NECROSIS (LN) AND CYSTIC DEGENERATION (CD) AND PERIFOLLICULAR FIBROSIS. (C) = NON-VACCINATED CONTROLS, NO LESIONS WERE OBSERVED (SCORE 0) / EFECTO DE LA VACUNACIÓN INTERMEDIA E INTERMEDIA PLUS CONTRA IBDV EN EL TEJIDO DE LA BOLSA A LOS 42 DÍAS DE EDAD. LAS AVES EN EL GRUPO DE LA VACUNA INTERMEDIA (A) = SE RECUPERARON A NIVEL 1, AUNQUE SE OBSERVÓ ALGÚN GRADO DE NECROSIS LINFOIDE Y DEPLECIÓN LINFOIDE. LAS AVES VACUNADAS CON LA CEPA INTERMEDIA PLUS PERMANECIERON EN EL NIVEL 4 CON ATROFIA FOLICULAR, NECROSIS LINFOIDE DEGENERACIÓN QUÍSTICA Y FIBROSIS PERIFOLICULAR. (C) = CONTROLES NO VACUNADOS, NO SE OBSERVARON LESIONES.
TABLE I
IMMUNOHISTOCHEMICAL DETECTION OF BURSAL LYMPHOCYTE SUBPOPULATION / DETECCIÓN POR INMUNOHISTOQUÍMICA DE LA POBLACIÓN DE LINFOCITOS PRESENTES EN LA BOLSA DE FABRICIOUS
| Antibodies used for B and T cell identification | % Positively stained cells | ||||||
| 10 days post-vaccination | 42 days of age | ||||||
| Non-vaccinated | IBDV | IBDV intermediate plus | Non-vaccinated | IBDV | IBDV intermediate plus | ||
| CD20 | 74 | 12 | 12 | 73 | 21 | 8 | |
| CD45RO | 2 | 58 | 75 | 2 | 46 | 75 | |
CONCLUSIONS
Increased histopathological bursal damage and delayed recovery was observed in the intermediate plus vaccinated group when compared with the intermediate strain. Signs of bursal restoration were observed at 42 days of age, mainly in the intermediate vaccinated group.
BIBLIOGRAPHIC REFERENCES
1.BURKHARDT, E.; MÜLLER, H. Susceptibility of chicken blood lymphoblasts and monocytes to infectious bursal disease virus (IBDV). Arch. Virol. 94: 97-303. 1987. [ Links ]
2.EDWARDS, K.; MUSKETT, J.; THORNTON, D. Duration of immunosuppression caused by a vaccine strain of infectious bursal disease. Res. Vet. Sci. 32:79-83. 1982. [ Links ]
3.FADLY, A.; WINTERFIELD, R.; OLANDER, H. Role of the bursa of Fabricius in the pathogenicity of inclusion body hepatitis and infectious bursal disease virus. Avian Dis. 20:467-477. 1976. [ Links ]
4.HENRY, C.; BREWER, R.; EDGAR, S.; GRAY, B. Studies on Infectious Bursal Disease in Chickens. 2. Scoring Microscopic Lesions in the Bursa of Fabricius, Thymus, Spleen, and Kidney in Gnotobiotic and Battery Reared White Leghorns Experimentally Infected with Infectious Bursal Disease Virus. Poult. Sci. 59:1006-1007. 1980. [ Links ]
5.HITCHNER, S.. Persistence of parental infectious bursal disease antibody and its effects on susceptibility of young chickens. Avian Dis. 15:894-900. 1971 [ Links ]
6.IVAN, J.; NAGY, N.; MAGYAR, A.; KACSKOVICS, I.; MESZAROS, J.. Functional restoration of the bursa of fabricius following in ovo infectious bursal disease vaccination. Vet. Immunol. immunopath. 79:235-248. 2001. [ Links ]
7.KAUFER, I.; WEISS, E. Significance of bursa of Fabricius as target organ in infectious bursal disease of chickens. Infect. Immun. 27:364-367. 1980. [ Links ]
8.KIM, I.; YOU, S.; KIM, H.; YEH, H.; SHARMA, J. Characteristics of bursal T lymphocytes induced by infectious bursal disease virus. J. Virol. 74:8884-8892. 2000. [ Links ]
9.LASHER, H; SHANE, S. Infectious bursal disease. Worlds Poult. Sci. J. 50:133-166. 1994. [ Links ]
10.LEONG, J. C; BROWN, D; DOBOS, P; KIBENGE, F, S; LUDERT, J. E; MÜLLER, H;MUNDT, E; NICHOLSON, B. Family Birnaviridae. In: Virus Taxonomy. Seventh Report of the International Committee on the taxonomy of Viruses. San Diego. Academic Press. 481-490 pp. 2000. [ Links ]
11.LUCIO, B; HITCHNER, S. Infectious bursal disease emulsified vaccine: effect upon neutralizing antibody levels in the dam. Avian Dis. 23:466-478. 1979. [ Links ]
12.LUKERT, P. D; SAIF, Y. M. Infectious Bursal Disease. In: SAIF, Y. M; BARNES, H. J; GLISSON, J. R; FADLY, A. M; MCDOUGALD, L. R; SWAYNE, D. E. (Eds), Diseases of Poultry, 11th Ed. Blackwell Publishing Co. 161-179 pp. 2003. [ Links ]
13.MÜLLER, H; ISLAM, M.; RAUE, R. Research on infectious bursal disease the past, the present and the future. Vet. Microbiol. 97:153-165. 2003. [ Links ]
14.NAQI, S.; MARQUEZ, B.; SAHIN, N. Maternal antibody and its effect on infectious bursal disease immunization. Avian Dis. 27:623-631. 1981. [ Links ]
15.NICK, H.;CURSIEFEN, D.; BECHT, H. Structural and growth characteristics of infectious bursal disease virus. J. Virol. 18:227-234. 1976. [ Links ]
16.OZEL, M.; GELDERBLOM, H. Capsid symmetry of viruses of the proposed birnavirus group. Arch. Virol. 84:149-161. 1985. [ Links ]
17.PEJKOVSKI, C.; DAVELAR, F.; KOUWENHOVEN, B. Immunosuppressive effect of infectious bursal disease virus on vaccination against infectious bronchitis. Avian Pathol. 8:95-106. 1979. [ Links ]
18.RAUTENSCHLEIN, S.; YEH, Y.; SHARMA, J. Comparative immunopathogenesis of mild, intermediate, and virulent strains of classic infectious bursal disease virus. Avian Dis. 47:66-78. 2003. [ Links ]
19.RAUTENSCHLEIN, S.; YEH, Y.; SHARMA, J. The role of T cells in protection by an inactivated infectious bursal disease virus vaccine. Vet Immunol Immunopathol. 89:159-167. 2002. [ Links ]
20.ROSALES, A.; VILLEGAS, P.; LUKERT, P.; FLETCHER, O.; MOHAMED, M.; BROWN, J. Pathogenicity of recent isolates of infectious bursal disease virus in specific-pathogen-free chickens. Avian Dis. 33:729-734. 1989. [ Links ]
21.ROSENBERGER, J.; GELB, J. Response to several avian respiratory viruses as affected by infectious bursal disease virus. Avian Dis. 22:95-105. 1978. [ Links ]
22.SHARMA, J.; KIM, J.; RAUTENSCHLEIN, S.; YEH, Y. Infectious Bursal disease virus of chickens: pathogenesis and immunosuppression. Devel. & Comp. Immunol. 24: 223-235. 2000. [ Links ]
23.WYETH, P. J. Effect of infectious bursal disease on the response of chickens to S. typhymurium and E. coli infection. Vet. Rec. 96:238-243. 1975. [ Links ]












 uBio
uBio