Revista Científica
versão impressa ISSN 0798-2259
Rev. cient. (Maracaibo) v.20 n.2 Maracaibo mar. 2010
ULTRASTRUCTURAL STUDY OF THE HYPOTHALAMUS IN MICE CHRONICALLY TREATED WITH MANGANESE
Virginia Villalobos 1, Juan Carlos Martínez 1, Alan Castellano 2, Juan Pablo Hernández-Fonseca 2,4,
Shirley Medina-Leendertz 3 y Ernesto Bonilla 3,4*
1 Facultad Experimental de Ciencias, Departamento de Biología. Universidad del Zulia (Venezuela). 2 Instituto de Investigaciones Biológicas. Facultad de Medicina. Universidad del Zulia (Venezuela). 3 Instituto Venezolano de Investigaciones Científicas (IVIC), (Venezuela). 4 Instituto de Investigaciones Clínicas Dr. Américo Negrette. Facultad de Medicina. Universidad del Zulia, Maracaibo. Apartado 23. Venezuela. * (0414-6144972). E-mail: embonilla2008@yahoo.com
ABSTRACT
Manganese (Mn) is an essential metal that is an integral part of some metalloproteins and acts as a cofactor of several enzymes. Mn is able to cross the blood-brain barrier and enter the nervous system. It has a low toxicity but exposure to high concentrations or for prolonged periods of time produce neurological disorders in humans that initially cause hallucinations and compulsive behaviour followed by stiffness, muscle weakness, ataxia, memory loss and a tremor resembling Parkinsons disease. This study assessed the ultrastructural alterations produced in the hypothalamus of male albino mice injected intraperitoneally with MnCl2 (5 mg Mn/Kg/day) and a control group injected with NaCl 0.9% (0.1 mL) daily for 9 weeks. The animals were sacrificed by cervical dislocation. The hypothalamus was extracted and subsequently processed to be observed on the conventional transmission electron microscope at 2, 4, 6 and 9 weeks of treatment. After 2 weeks it was observed a slight disruption of the Golgi apparatus and the myelin fibers. After 4 weeks the disorganization was accentuated and dilatation of the endoplasmic reticulum (ER) and alterations of mitochondria were observed. After 6 weeks the normal pattern of the myelin sheath was lost. After 9 weeks of treatment it was found swollen mitochondria with lost of crystae, a marked dilatation of rough and smooth endoplasmic reticulum and dendrites with a high degree of swelling. These results suggest that the neurotoxic effect of Mn increases as time of exposure passes and produces ultrastructural alterations of nerve cells in the hypothalamus.
Key words: Manganese toxicity, hypothalamus, ultrastructural alterations.
Estudio ultraestructural del hipotálamo de ratones tratados crónicamente con manganeso
RESUMEN
El manganeso (Mn) es un metal esencial que forma parte de algunas metaloproteínas y actúa como cofactor de varias enzimas. El Mn es capaz de atravesar la barrera hematoencefálica e ingresar al sistema nervioso. Presenta baja toxicidad, pero la exposición a altas concentraciones o por tiempos prolongados produce alteraciones neurológicas en humanos que inicialmente provocan alucinaciones y conducta compulsiva seguidas por rigidez, debilidad muscular, ataxia, pérdida de la memoria y temblor, síntomas similares a los de la enfermedad de Parkinson. En el presente estudio se evaluaron los efectos tóxicos del Mn sobre la ultraestructura del hipotálamo de ratones. Se inyectaron intraperitonealmente ratones albinos machos con MnCl2 (5 mg Mn/Kg/día durante 9 semanas). El grupo control recibió NaCl 0,9% (0,1 mL/dosis). Los animales se sacrificaron por dislocación cervical, extrayéndose y disecándose el hipotálamo, que posteriormente se procesó para realizar observaciones al microscopio electrónico de transmisión convencional a las 2; 4; 6 y 9 semanas de tratamiento. A las 2 semanas, se observó ligera desorganización en el aparato de Golgi y en las fibras mielínicas. A las 4 semanas, se acentuó la desorganización y se comenzó a observar dilatación del retículo endoplasmático liso y rugoso asi como mitocondrias alteradas. A las 6 semanas, se encontró pérdida del patrón normal de la cubierta mielínica. Finalizadas las 9 semanas de tratamiento, se observaron mitocondrias hinchadas con pérdida de las crestas, dilatación acentuada del retículo endoplasmático rugoso y liso y dendritas con cierto grado de edema. Estos resultados parecen indicar que el efecto neurotóxico del Mn aumenta a medida que transcurre el tiempo de exposición para producir alteraciones ultraestructurales de las células nerviosas del hipotálamo.
Palabras clave: Intoxicación por manganeso, hipotálamo, alteraciones ultraestructurales.
INTRODUCTION
Manganese (Mn) is an essential metal in several biological functions because it is an integral part of a series of metalloproteins such as hydrolase, kinases, decarboxylases and transferases; it also acts as a cofactor for some enzymes [8] such as glutamine synthetases (selectively expressed by astroglial cells) which use 80% of the available Mn in the central nervous system, phosphoenol piruvate carboxylase (oxidative chain) and mitochondrial super-oxide dismutase (anti-oxidant in neuronal mitochondria) [2, 31,41].
The main routes by which Mn enters the organism are inhalation and ingestion [8, 19], this is done by means of retrograde axonal transportation through the pre-synaptic terminals of the nerves located in the nasal cavity from where they pass directly to the neurons of the central nervous system. This is the main route of exposure to high concentrations of metal [36].
Mn is able to cross the blood-brain barrier and enter the nervous system; the cerebrospinal fluid transports it to other regions of the central nervous system through the axonal transport system [36]; and its elimination, after having entered the brain, is extremely slow (Average time = 56 – 260 days) [19].
Although Mn has low toxicity, it can produce intoxication when exposed to low concentrations of metal during prolonged time periods or high doses [3, 8]. The intoxication with Mn is known as manganism and is usually associated to prolonged occupational exposure in environments with high atmospheric concentrations [8].
Certain pesticides such as Maneb or Mancozeb form Mn organic compounds; another widely used derivate is methylcyclopentadienyl manganese tricarbonyl (MMT), an anti-detonate lead substitute in some ecological gasoline [1, 17]. Mn has also ample applications in the siderurgical industry (steel production), paint fabrication, ceramics, cell batteries, magnetic tapes, water purification, bactericides and fungicides, among others [8].
From an anatomy-pathological point of view, the most characteristic injury observed during Mn intoxication is neuronal destruction in the globus pallidus, caudate nucleus, putamen, cerebral cortex and the cerebellum of rats (Rattus norvegicus, cepa Sprague-Dawley) intoxicated with Mn; besides neuronal alterations of the hippocampus, neostriatum, midbrain and pons have been described [8,11,12].
Because psychiatric and neurological manifestations dominate the clinical chart of chronic manganism, most experimental studies have been concentrated in the alterations produced in the cerebral metabolism (catecholamines, serotonine, acetylcholine, GABA, among others) and at the level of receptors to the neurotransmitters [7-9, 15, 16, 30, 40]. In this regard, Mn has been found to produce an increase, a decrese or no alteration in the binding characteristics of ligands to dopaminergics [4, 45], gabaergics [15, 16, 32], muscarinic [4, 20, 28, 43] and adenosine receptors [44].
The hypothalamus is a tiny and complex neural structure, responsible for many behavioral aspects, such as the basic biological impulses, motivation and emotions. Nerve tracts connect it to all the regions of the brain, the erogenous zones, viscera and limbic system; therefore it integrates the emotional and physical signals that proceed from the whole body, adapting the responses [34].
The hypothalamus fulfills an import role in some physic and psychomotor functions, such as certain conditions of hyperexcitability or depression owed to functional derangement of the hypothalamic centers. Also, the symptoms of emotional states such as palpitations, excessive tears, salivation, vomiting, blushing, among others, are regulated by it [25, 34].
Neural degeneration has been described in the caudate nucleus, putamen, globus pallidus, olfactory bulb, substantia nigra, and the hypothalamus of experimental animals and humans, intoxicated with manganese [8, 11, 12, 22, 46]. Ultrastructural changes have also been described in sub-cellular organelles such as synaptic vesicles, swollen mitochondria, dilated endoplasmic reticulum, disorganized Golgi apparatus, lysosomes [39]. Some alterations of the plasma membrane have also been reported in neurons as well as in neuroglia which have been exposed to Mn [5, 10, 26, 42].
The ultrastructural changes of nervous cells of the hypothalamus of mice exposed to Mn, during different exposure periods are described in this study using a transmission electron microscope (Jeol, JEM-1010, Japan). In a previous study the effect of manganese toxicity on the ultrastructure of the olfactory bulb was evaluated [10]. After 4 weeks of treatment, degenerated neurons with dilated cysternae of rough endoplasmic reticulum and swollen mitochondria appeared. Some glial cells showed disorganization of the Golgi apparatus. On week 9, an increase in the number of astrocytes was found and neurons appeared degenerated with swollen mitochondria and a vacuolated cytoplasm [10].
MATERIALS AND METHODS
Animal treatment
Male albino mice (Mus musculus) (NMRI-IVIC) with an average weight between 25-30 grs were used, these animals were fed ad libitum with Ratarina® (Protinal) and distilled water, and were kept at a temperature of 25°C (Labcraft, Brand, United Kingdom) under a 12 hours light/darkness scheme. The animals were divided into two (2) groups: the first group was intraperitoneally injected with MnC12 (5 mg Mn/Kg weight/day), and the second group (control group) was injected with a saline solution (NaCl2 0.9%). Both groups received a daily dose of 0.1 mL of the corresponding solution during 9 weeks (5 days/week). The selected manganese (Mn) dose for these experiments was based on previous studies [9].
Sample selection
After 2; 4; 6 and 9 weeks of treatment, 5 animals per group were chosen, which were sacrificed by means of cervical dislocation. The brain was removed, and the hypothalamus was dissected according to the technique described by Glowinski and Iversen [21].
Tisular manganese determination
The hypothalamic manganese content was determined by Atomic Absorption spectrum-photometry with graphite furnace according to the procedure described by Bonilla [6].
Processing for transmission electron microscope
The extracted hypothalamus, of each one of the exposure periods, as well as the controls, were initially fixed in glutaraldehide at 3% in buffer cacodylate 0.1 M; pH 7.4 at 4°C during 3 hours; they were washed with the same solution during 10 minutes and were then post-fixed in osmium tetraoxide at 1% in buffer cacodylate during 1 hour. The samples were then washed in buffer cacodylate solution 0.1 M, pH 7.4 during 10 minutes. The tissues were dehydrated in increasing concentrations of ethanol (50; 70; 80; 90 and 100%) and in two successive passages of propylene oxide. Thereafter the samples were included in araldite 502 [13].
Sections were made with a thickness of 0.5 - 2 microns, which were dyed with toluidine blue and observed under a Olympus microscope (Model CX21-FS1, Japan) in order to delimitate the sample. Ultrathin 20-40 nm sections were obtained, using a Sorvall (Model MT-5000) ultra-microtome, and were dyed with uranyl acetate and lead citrate [13]. Afterwards they were observed and photographed in a transmission electron microscope (Jeol JEM-1010, Japan) at different magnifications.
RESULTS AND DISCUSSIONS
Control group
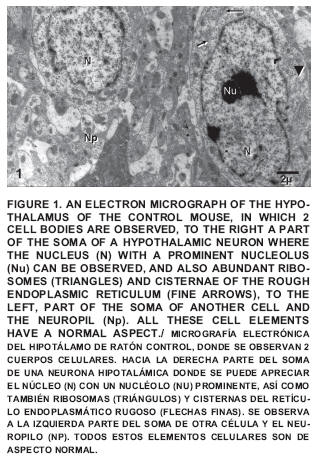
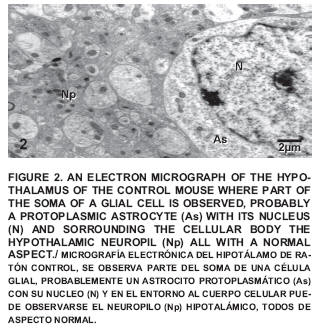
Within this group an important number of nerve cells are described, all having a normal aspect (FIGS. 1 and 2).
The presence of nucleus (N), nucleolus (Nu), ribosomes (R), cisterns of the rough endoplasmic reticulum (RER), mitochondria (Mt), among other normal cytoplasmic elements could be observed which are part of the hypothalamic nervous cells.
Treated group
The distribution of Mn in the rats brains according to atomic absorption spectrometry studies shows high concentrations in the hypothalamus and pineal gland. Following the intra-peritoneal injection of Mn the brain starts to show a gradual increase in the retention after 30 days of treatment [4]. It was found a concentration of 2.37 ± 0.08 µg/g dry weight of Mn in the hypothalamus of normal mice and 11.07 ± 1.14 µg/g in mice treated intraperitoneally with MnCl2 during 9 weeks. The difference was highly significant (P<0.01).
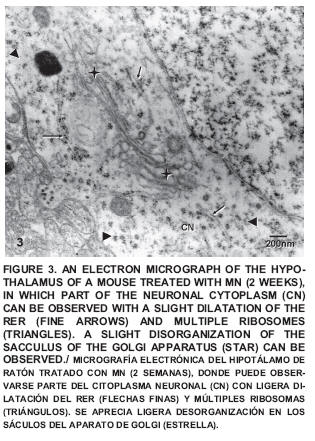
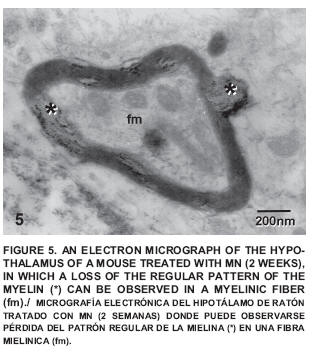
After two weeks of exposure some small alterations could be observed such as a certain degree of disorganization in the sacculus of the Golgi apparatus (FIG. 3) and a neuropile with little changes (FIG. 4), which exhibited certain alterations of the regular pattern of myelin (FIG. 5).
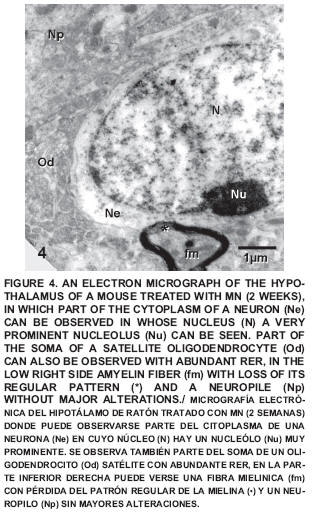
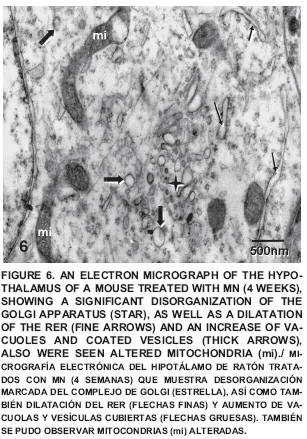
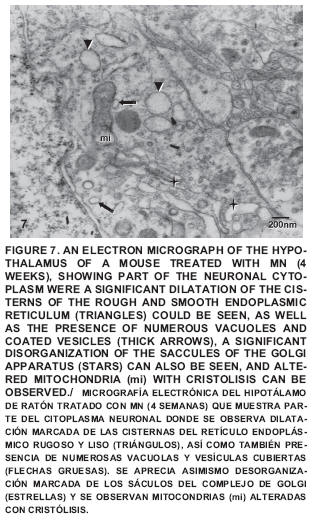
After four weeks of treatment a noticeable disorganization can be seen in the sacculus of the Golgi apparatus, as well as the presence of dilated cisternae of the RER and smooth endoplasmatic reticulum (SER), coated vesicles and numerous vacuoles; also seen were altered mitochondria with cristolisis (FIG. 6), Amyelinic nervous fibers in the neuropile were observed and some changes in the conformation of the myelin cover and altered mitochondria with loss of crests were also detected (FIG. 7).
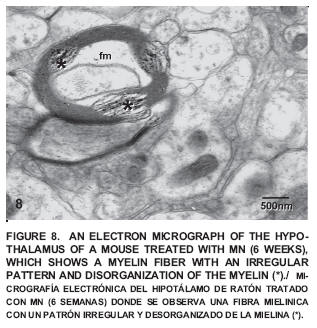
After six weeks of exposure to Mn a neuropile exhibiting myelinic fibers with a clear disorganization of the regular pattern of myelin was observed (FIG. 8).
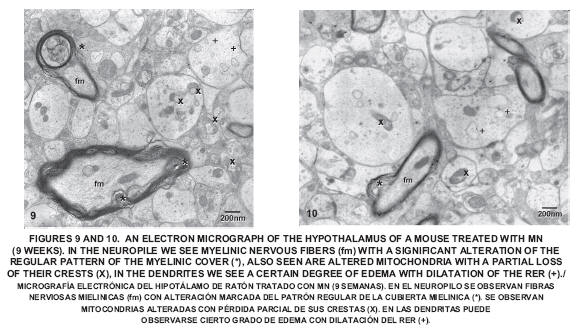
After nine weeks of exposure to Mn the disorganization of the myelin increased. Several altered mitochondria with loss of cristae could be observed, as well as dendrites with a certain degree of edema; also seen was the dilatation of the RER and the disorganization of the Golgi apparatus (FIGS. 9 and 10).
The hypothalamus is one of the most significant regions of the brain; it fulfills important homeostatic regulatory functions, and relates to the behavior and emotions. It is closely connected to the endocrine system, the autonomic nervous system and the limbic system. The consequences of an alteration in this region can vary depending on the affected zone [34].
High concentrations of Mn have been reported in the hypothalamus of rats treated with this metal [7]. In mice, after 30 days of the intra-peritoneal injection of Mn, the brain starts to show a gradual increase in the retention of this metal [26].
It has been demonstrated that the levels of Mn in the brain of rats treated with MnCL2 .4H20 via ingestion shows an increase in the concentration of the metal in different regions, especially in the striatum, the hypothalamus and the hippocampus [27]. There is also an increase of Mn in all the subcellular organelles; the ones showing the highest concentrations are the nucleus, the mitochondria and the synaptosomes [8].
In this study, after 2 weeks exposure to Mn, it was observed that the morphological changes of the cells were characterized by alterations in the normal pattern of the myelin. These changes are similar to those described by Bonilla et al [10] in the olfactory bulb of mice treated with manganese, and in agreement with the observations made by Towler et al [42] whom reported that Mn produced alterations in the microsomes of the Golgi apparatus by showing disorganization and fragmentation in mammalian cells.
After 4 weeks of treatment with Mn, the same previous changes in the myelin cover could be seen although there was evidence of a slight increase in the degree of severity. An increase in disorganization could be seen in the Golgi apparatus. The RER and SER showed dilatation in their cisternae; the mitochondria were shown swollen with alterations in their crests. Bonilla et al [10] found these alterations in the mitochondria of the olfactory bulb. Shukakidze et al [38] in studies made in the caudate nucleus, the frontoparietal cortex and substancia nigra of mice treated during 30 days with 20 mg of MnCL2 .4H20 showed a cytotoxic effect of the manganese ions on the astrocytes causing the destruction of the mitochondria, the dilatation of the canaliculus and cisterns of the endoplasmatic reticulum, an accumulation of glycogen granules and reduction of the oxidative processes in the nervous system.
The accentuated disorganization of the regular pattern of myelin in mice treated with Mn during 6 weeks has been described by Castejón et al. [14] in brain edema, in which the edema fluid disorganizes the periodicity of the myelin sheath.
There is a very pronounced disorganization of the myelin cover after 9 weeks of treatment with Mn; areas can be found were there is a significant alteration of the nervous fibers; therefore it could affect the propagation of the nervous impulses. These findings were also described in the olfactory bulb by Bonilla et al. [10] whom reported a significant alteration of the myelin cover, evidencing that the myelin sheath is one of the most affected areas by the neurotoxic effects of Mn.
Multiple sclerosis is a demyelinating illness the etiology of which is attributed to an auto-immune phenomenon, in which the outstanding histopathological characteristic is the presence of demyelinization and inflammatory perivascular infiltration of the central nervous system cells [18]. Studies made in biopsy tissues have revealed an anatomic-pathological heterogeneity in which four different demyelinization patterns are described; the first two, show a loss of myelin with perivascular infiltration, with preservation of oligodendrocytes and remyelinization. Patterns III and IV show the death of the oligodendrocytes with little evidence of remyelinization [18]. It should be interesting to establish if an experimental model based upon the neurotoxic effect of Mn on the myelin cover would provide relevant information regarding multiple sclerosis.
The disorganization of the Golgi apparatus, the dilatation of the RER and SER cisterns, the mitochondrial cristolisis, as well as the edematization of the dendrites have also been observed in the olfactory bulb of mice exposed to Mn with a higher degree of severity. These changes are accompanied by an increase in the number of astroglial cells, and the presence of degenerated cells with electron-dense cytoplasm [10].
It has been reported that Mn is capable of inducing an increase in the permeability of the mitochondrial membrane thus altering the function of this organelle. It has been proven that Mn has the property of inducing the accumulation of calcium in the mitochondria and can therefore inhibit the transport of the calcium depending membrane, originating activation of a permeable transition pore, allowing the Mn to pass freely thus presumably causing the mitochondrial alterations seen in this study [36].
Studies carried out in the caudate nucleus, substancia nigra and the fronto-parietal cortex show mitochondrial destruction of the astrocytes, oligodendrocytes and microglia [35]. The principal ultrastructural alterations produced by the neurotoxic effect of Mn in different nervous tissue are the elongation of the cytosolic compartment, the increase in the number of altered mitochondria and the presence of a swollen rough endoplasmatic reticulum [24]. These ultrastructural changes have been described previously in brain tumors, trauma, congenital malformations and brain edema [14, 29, 37].
Several research studies provide evidence that the Mn neurotoxicity produces greater injuries and the highest dysfunction in the astrocytes [24, 35]. These cells accumulate the metal due to their high affinity, high capacity and due to the existence of specific transportation which facilitates the entry and increase within the mitochondria causing the disruption of oxidative phosphorylation [33, 36, 38]. When it binds to the benzodiazepine receptor Mn provokes alterations of the oxidative metabolism, mitochondrial proliferation and neurosteroid synthesis [23].
Based on the obtained results, it appears that Mn produced ultrastructural alterations of neurons and neuroglia of the hypothalamus. These changes incremented in severity as the time to exposure increased. The main structures affected by Mn were myelinated fibers, rough endoplasmatic reticulum, mitochondria and Golgi apparatus. During the first stages damages to the cellular storage and transportation systems were seen, while during the last stages the damage pointed specially to the neuronal transmission due to the changes in the myelin covers.
CONCLUSION
The obtained results indicate that ultrastructural alterations in the nervous cells of the hypothalamus of mice take place when the time of treatment with Mn increases. Since the hypothalamus is one of the most important brain regions, due to its regulatory functions and its facilitation of numerous physiological responses, it is probable that the alterations provoked by Mn in this region caused a severe neurological damage affecting not only the correct functioning of the hypothalamus but also that of the other organs connected to the hypothalamus.
BIBLIOGRAPHIC REFERENCES
1. AGENCIA PARA SUSTANCIAS TÓXICAS Y REGISTRO DE ENFERMEDADES (ATSDR) ToxFAQS para manganeso. Manganeso CAS# 7439-96-5. División de Toxicología ToxFAQs. Febrero 2001. En linea: //www.cvs.saude. sp.gob.br/pdf/toxfaq128.pdf. 2 de marzo de 2009. [ Links ]
2. ASCHNER, M.; GUILARTE, T.R.; SCHNEIDER, J.S.; ZHENG, W. Manganese: Recent advances in understanding its transport and neurotoxicity. Toxicol. and Appl. Pharmacol. 221:131-147. 2007. [ Links ]
3. BARBEAU, A. Manganese and extrapyramidal disorders. Neurotoxicol. 5:13-36. 1984. [ Links ]
4. BHARGAVA, H.N. Effect of repeat administration of manganese on striatal cholinergic and dopaminergic receptors in the rat. Toxicol. Lett. 37:135-141. 1987. [ Links ]
5. BIKASHVILI, T.Z.; SHUKAKIDZE, A.A.; KIKNADZE, G.I. Changes in the rat cerebral cortex ultrastructure following peroral adminitration of manganese chloride. Morfolog. 118(5):14 18. 2000. [ Links ]
6. BONILLA, E. Flameless Atomic Absorption Spectrophotometric determination of manganese in rat brain and other tissues. Clin. Chem. 24(3):471-474. 1978. [ Links ]
7. BONILLA, E. L-Tyrosine Hydroxylase Activity in the rats brains after chronic oral administration of manganese chloride. Neurobehav. Toxicol. 2(1):37-41. 1980. [ Links ]
8. BONILLA, E. El Manganeso y su importancia biomédica. 1ª Ed. Astro Data S.A, Maracaibo, Venezuela, 153pp. 1987. [ Links ]
9. BONILLA, E.; ARRIETA, A.; CASTRO, F.; DÁVILA, J.O.; QUIROZ, I. Manganese toxicity: Free amino acids in striatum and olfactory bulb of the mouse. Invest. Clin. 35:175-181. 1994. [ Links ]
10. BONILLA, E.; VILLALOBOS, V.; CASTELLANO, A.; CASPERSEN, R.; GIRALDOTH, D. Ultrastructural changes of the olfactory bulb in manganese treated mice. 35th Annual Meeting Society for Neuroscience. 11/11-16, Programa No. 106.6. Viewer/Itinerary. Planner. Washington, DC: Society for Neuroscience. Abstract. 2005.
11. CANAVAN, M.M.; COBB, S.; DRINKER, CK. Chronic manganese poisoning. Report of a case with autopsy. Arch. Neuro. Psychiat. 32:501-512. 1934. [ Links ]
12. CARDOZO, J.; BONILLA, E. The neuropathology of experimental chronic manganese poisoning in rat. A preliminary report. Invest. Clin. 26: 117-124. 1985. [ Links ]
13. CASTEJÓN, O.J. Manual de técnicas básicas para microscopía electrónica. Facultad de Medicína. 1ª Ed. Universidad del Zulia, Maracaibo, Venezuela, 168pp. 1975.
14. CASTEJÓN, O.J. Electron microscopic study of central axons degeneration in traumatic human brain edema. J. Submicrosc. Cytol. 17:703-718. 1985. [ Links ]
15. CHANDRA, S.V.; SHUKLA, G. Effect of manganese on synthesis of brain catecholamines in growing rats. Acta Pharmacol. Toxicol. 48:349-354. 1981. [ Links ]
16. CHANDRA, S.V.; MALHATRA, K.M.; SHUKLA, G. GABAergic neurochemistry in manganese exponed rats. Acta Pharmacol. Toxicol. 51:456-458. 1982. [ Links ]
17. COOPER, W.C. The health implications of increased manganese in the environment resulting from the combustion of fuel additives a review of the literature. J. Toxicol. Environ. Health. 14:23-46. 1984. [ Links ]
18. CORREALE, J. Esclerosis Múltiple: Antiguas observaciones, nuevas hipótesis, futuras perspectivas. Med. (Buenos Aires). 65:175-180. 2005. [ Links ]
19. DÍAZ, L.; GÓMEZ, A. Neurobiología de la intoxicación por manganeso. Rev. Chil. Neuro-Psiquiat. 31:351-358. 1993. [ Links ]
20. DONALDSON, J.; LA BELLA, FS. The effects of manganese on the cholinergic receptor in vivo and in vitro may be mediated through modulation of free radicals. Neurotoxicol. 5:105-112. 1984. [ Links ]
21. GLOWINSKI, J.; IVERSEN, L. Regional studies of catecholamines in rat brain. I. The disposition of 3H-dopamine and 3H-dopa in various regions of the brain. J. Neurochem. 13:655-669. 1966. [ Links ]
22. GUPTA, S.K.; MURTHY, R.C.; CHANDRA, S.V. Neuromelanin in manganese-exposed primate. Toxicol. Lett. 6:17-20. 1980. [ Links ]
23. HAZELL, A. Astrocytes and manganese neurotoxicity. Neurochem. Intern. 41: 271–277. 2002. [ Links ]
24. HAZELL, A.S.; NORMANDIN, L.; NORENBERG, M.D.; KENNEDY, G.; YI, J. Alzheimer type II astrocytic changes following sub-acute exposure to manganese and its prevention by antioxidant treatment. Neurosc. Lett. 396:167–17. 2006. [ Links ]
25. JOHANSEN, J.E.; FESTISSOV, S.O.; BERGSTRÖM, U.; NILSSON, I.; FA, C.; RANSCHT, B.; HÖKFELT, T.; SCHALLING, M. Evidence for hypothalamic dysregulation in mouse models of anorexia as well as in humans. Physiol. & Behav. 92:278-282. 2007. [ Links ]
26. KUMAR, R.; SRIVASTAVA, S.; ASHOK, K.; SETH, P. Alteration in some membranes in rat brain following exposure to manganese. Pharmacol. Toxicol. 79:47-48. 1996. [ Links ]
27. LAI, JC.; MINSKI, M.J.; CHAN, A.W.; LEUNG, T.K.; LIM, L. Manganese mineral interactions in brain. Neurotoxicol. 20:433-444. 1999. [ Links ]
28. LEUNG, TKC.; LAI, JKC.; LIM, L. (3H)- Quinuclidil benzilate binding in striatal membranes from rats chronically treated with manganese chloride throughout development and for over year. Gen. Pharmacol. 17:121-123. 1986. [ Links ]
29. LONG, DM. Capillary Ultrastructure and Blood-Brain Barrier in human malignant brain tumors. J. Neuros. 32:127-143. 1970. [ Links ]
30. MARTÍNEZ, H.; CARABALLO, F.; ARRIETA, A.; BONILLA, E. Actividad Motora Espontanea y Catecolaminas en cerebro de Ratones Negros C57BL/6 y albinos tratados con Manganeso. Inv. Clin. 45(1):3-15. 2004. [ Links ]
31. MORELLO, M.; ZATTA, P.; ZAMBENEDETTI, P.; MARTORANA, A.; DANGELO, V.; MELCHIORRI, G.; BERNARDI, G.; SANCESARIO, G. Manganese intoxication decreases the expression of manganoproteins in the rat basal ganglia: An immunohistochemical study. Brain Res. Bull. 74:406-415. 2007. [ Links ]
32. MORENO, C.M.; BONILLA, E. Disminución de la captación de H3–GABA de cerebelo de ratas intoxicadas con manganeso. Invest. Clín. 25:193-197. 1984. [ Links ]
33. NORMANDIN, L.; HAZELL A.S. Manganese neurotoxicity: an update of pathophysiologic mechanisms. Metab. Brain Dis. 17(4):375-387. 2002. [ Links ]
34. OJEDA, J.L.; ICARDO, J.M. Neuroanatomía humana: Aspectos funcionales y clínicos. Capítulo 23. Anatomia funcional del lóbulo limbico y del hipotálamo: Bases morfologícas de las emociones, memoria y control vegetativo. Control de los ritmos biologicos. 1ª Ed. Elsevier-Masson. España. Pp 324. 2004. [ Links ]
35. RAMA, K.V.; REDDY, P.V.B.; HAZELL, A.S.; NORENBERG, M.D. Manganese induces cell swelling in cultured astrocytes. NeuroToxicol. 28:807-812. 2007. [ Links ]
36. ROTH, J. Homeostatic and toxic mechanisms regulating manganese uptake, retention, and elimination. Biol. Res. 39:45-57. 2006. [ Links ]
37. SHIBATA, S. Ultrastructure of blood-brain barrier in human brain tumor. No-Shinkei-Geka. 20:1135-1147. 1989. [ Links ]
38. SHUKAKIDZE, A.A.; LAZRIEV, I.L.; KHETSURIANI, R.G.; BIKASHVILI, T.Z. Changes in the ultrastructure of neuroglia in some regions of the rat brain in manganese chloride poisoning. Morfolog. 120: 35-41. 2001. [ Links ]
39. Suzuki, H.; Wadaa, O.; Inoueb, K.; Tosakab, H.; Onoa, T. Role of brain lysosomes in the development of manganese toxicity in mice. Toxicol. and Appl. Pharmacol. 71(3): 422-429. 1983. [ Links ]
40. TALAVERA, J.E.; ARCAYA, J.L.; GIRALDOTH, D.; SUÁREZ, J.; BONILLA, E. Decrease in spontaneous motor activity and in brain lipid peroxidation in manganese y melatonin treated mice. Neurochem. Res. 24(5):705-708. 1999. [ Links ]
41. TAKEDA, A.; KODAMA, Y.; ISHIWATARI, S.; OKADA, S. Manganese transport in the neural circuit of rat CNS. Brain Res. Bull. 45:149-152. 1998. [ Links ]
42. TOWLER, M.C.; PRESCOTT, A.R.; JAMES, J.; LUCOCQ, J.M.; PONNAMBALAM, S. The manganese cation disrupts membrane dynamics along the secretory pathway. Exp. Cell. Res. 259: 167-179. 2000. [ Links ]
43. VILLALOBOS, V.; CASTRO, F.; BONILLA, E.; ESTÉVEZ, J.; DÁVILA, J.O. Manganeso Toxicity: Muscarinic receptor binding in the Mouse brain. J. Toxicol. Environ. Health. 42: 185-191. 1994. [ Links ]
44. VILLALOBOS, V.; SUÁREZ, J.; ESTÉVEZ, J.; NOVO, E.; BONILLA, E. Effect of chronic manganeso treatment on adenosine tissue levels and adenosine A2a receptor binding in diverse regions of mouse brain. Neurochem. Res. 26:1157-1161. 2001. [ Links ]
45. VILLALOBOS, V.; ESTÉVEZ, J.; NOVO, E.; BONILLA, E. Efecto del tratamiento crónico con manganeso sobre los parámetros de fijación del espiroperindol (3H) en cerebro de ratón: estudios IN VIVO E IN VITRO. Rev. Científ. FCV-LUZ. XI(4):306-313. 2001. [ Links ]
46. YAMADA, M.; OHNO, S.; OKAYASU, Y.; OKEDA, R.; HATAKEYAMA, S.; WATANABE, H.; USHIO, K.; TSU, K.H. Chronic manganese poisoning :A neuropathological study with determination of manganese distribution in the barin. Acta Neuropathol. 70:273-278. 1986. [ Links ]












 uBio
uBio