Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Facultad de Ingeniería Universidad Central de Venezuela
Print version ISSN 0798-4065
Rev. Fac. Ing. UCV vol.24 no.1 Caracas Mar. 2009
Characterization of polypropylene/cassava starch compounds obtained in an internal mixer
Jeanette González1, Carmen Albano2,3, Marianella Hernández1, Miren Ichazo1, Mary Lavady3
1 Universidad Simón Bolívar, Departamento de Mecánica, Caracas 1080A, Venezuela
2 Universidad Central de Venezuela, Facultad de Ingeniería, Escuela de Ingeniería Química, Caracas, Venezuela
3 Instituto de Investigaciones Científicas (IVIC), Caracas, Venezuela.
email: jjgonza@usb.ve, calbano@ivic.ve
ABSTRACT
The following work presents a broad characterization of Polypropylene and Cassava Starch blends (PP/CS) through the analysis of their thermal, thermodegradative, tensile, rheological and morphological properties, as well as the study of the water absorption behavior by FT-IR and the degradability on an agricultural soil. It was found that the addition of CS to PP increases its thermal stability, the effect being greater when the CS is plasticized (PCS) and when PP-g-MAH is added. The CS acts as a nucleating agent increasing the crystallization temperature and the degree of crystallinity. The Youngs modulus and the tensile strength of the PP increase with 10 phr of CS and start to decrease at higher contents. However, when PP-g-MAH is added to the PP/CS blends, the mentioned properties decrease. Finally, the degradability of the PP/CS blend in an agricultural soil significantly affects the Youngs modulus, whose values decrease from 1841 to 834 MPa.
Keywords: Cassava starch, PP/cassava starch blends, Thermal behavior, Mechanical behavior, Biodegradability.
Caracterización de compuestos de polipropileno/almidón de yuca obtenidos en un mezclador interno
RESUMEN
En este trabajo se presenta una amplia caracterización de mezclas de polipropileno con almidón de yuca a través del análisis de sus propiedades térmicas, termodegradativas, tensiles, reológicas y morfológicas, así como el estudio del comportamiento de la absorción de agua con el tiempo a través de FTIR y el efecto de la degradabilidad en suelos con nutrientes. Se obtuvo que la adición de almidón de yuca al PP incrementa la estabilidad térmica de la mezcla, siendo este efecto más notable en las mezclas donde se utilizó almidón plastificado y cuando se agregó PP-f-MAH. El almidón de yuca actúa como agente nucleante del PP incrementando la temperatura de cristalización y la cristalinidad. El módulo de Young y la resistencia a la tracción del PP incrementan al añadirle 10 ppc de almidón de yuca y comienzan a disminuir a mayores contenidos. Sin embargo, la adición de PP-g-MAH a la mezcla produce una disminución de las propiedades mencionadas. Finalmente, la degradabilidad de la mezcla en suelos fértiles afecta significativamente el módulo de Young, cuyos valores disminuyen de 1841 a 834 MPa.
Palabras clave: Almidón de yuca, PP/almidón de yuca, Comportamiento térmico, Comportamiento mecánico, Biodegradabilidad.
Recibido: mayo de 2008 Recibido en forma final revisado: febrero de 2009
INTRODUCTION
Starch sources more worldwide employed are cereals (corn, wheat and rice) and roots (cassava and potato). However, in our country, there are many cassava plantations that have started to develop industrially; so a growing interest on finding applications for cassava starch has appeared. In general, cassava starch is conformed by two phases; 83% of amylopectin and 17% of amylose (Linden et al. 1996).
On the other hand, polyolefins are the polymers more widely known and employed in the plastics industry. The search of diversity of new blends and applications with these materials conducted to evaluating the starch, a degradable
polymer, as filler for Polypropylene (PP).The use of starch as filler in plastic materials has been of interest during the last 40 years. The main advantages of employing starch in thermoplastic resins are its high biodegradability, its great availability and low costs (Danjaji
et al. 2002; Mali et al. 2005; Pedrosa & Rosa, 2005; Rosa et al. 2007). The first investigations in this area were made by Dosmann & Steel (1961) whom studied the behavior of the starch as filler in polyurethane foams, whereas Westhoff et al. (1974) used it in PVC.Otey
et al. (1977, 1980, 1987) employed extrusion processes for obtaining films from blends of starch with poly (ethylene-co-acrylic acid) (EAA) resulting compounds with 50% content of plasticized starch. These films presented similar characteristics in appearance to those of a conventional polyethylene.The starch, due to its hygroscopic nature, must be treated in a way so that it becomes more compatible with non-polar polymers such as polyolefins. With the purpose of reducing the hydrophobic nature of the polyolefin or the hydrophilic character of the starch, several attempts have been made to modify one or other. For this reason, Evangelista
et al. (1991) added to Polyethylene (PE) 25% of starch previously modified with octenil succinate. These blends showed better mechanical properties than the unmodified polyethylene-starch blends. On the other hand, Jane et al. (1991) incorporated oxidized polyethylene as a compatibilizer in blends of polyethylene and starch. Nevertheless, the modification was quite expensive, and the compounds still presented poor mechanical properties.St. Pierre
et al. (1997) studied the mechanical properties of low density polyethylene (LDPE)/thermoplastic starch blends and of linear low density polyethylene (LLDPE)/thermoplastic starch. These compounds had better processability and mechanical properties than those with the untreated starch. Nevertheless, for high percentages of thermoplastic starch, there was a drastic reduction in the mechanical properties. Therefore, Matzinos et al. (2001) studied the effect of incorporating low density polyethylene functionalized with maleic anhydride (LDPE-g-MAH) as a compatibilizer with 30% of natural corn starch, emphasizing that the biodegradation of the compatible blends was only slightly lower than that of the non compatible compounds.Sailaja & Chanda (2001) analyzed blends of high density polyethylene (HDPE)-starch and HDPE-thermoplasticized starch using HDPE functionalized with maleic anhydride (HDPE-g-MAH) as compatibilizer.
Among other studies on blends of polyolefins with starch, one can mention Zuchowska
et al. (1998), whom analyzed the physical structure after ageing of the blends of PP with 40-50% of starch, plasticized with glycerol.Since almost all the investigations up to date have used polyethylene as the polymeric matrix and the mixing method in almost all of them has been by extrusion, the present work has the objective of characterizing blends of polypropylene (PP) with different concentrations of cassava starch obtained in an internal mixer. Moreover, the plasticization of the starch with glycerol and water and the use of a polypropylene functionalized with maleic anhydride (PP-g-MAH) as compatibilizer are also studied with the purpose of obtaining a blend with mechanical properties comparable to the pure polymer, but with the possibility of having a biodegradable behavior.
EXPERIMENTAL
Commercial Polypropylene (PP11H01A) Propilco with a melt flow index (MFI) of 11 dg/min (190 /2.16) was employed. The cassava starch (CS) came from plantations on Miranda, Venezuela. Functionalized polypropylene with maleic anhydride (PP-g-MAH) from Sigma Aldrich (MFI: 11.5 dg/min at 190/2.16) was also used. The cassava starch was dried in a vacuum oven at 90ºC during 24 h, to guarantee the maximum water extraction (15-20%).
The thermoplastic starch (PCS) was prepared in an internal mixer, varying the water and glycerol content, where the optimum composition chosen was 33% water, 19% glycerol and 48% cassava starch (Rodríguez
et al. 2003). The mixing conditions of the starch, glycerol and water blend were 70ºC and 50 rpm. Mixing time was reduced to the minimum where the blend could be homogeneous. The PCS obtained was dried during 24 hours in a convection oven at 90 °C. Then, it was passed through a mortar and milled until the minimum particle size was obtained.The compounds of PP with natural (CS) and plasticized (PCS) cassava starch and with PP-g-MAH were prepared in an internal mixer (Haake Rheomix) with a maximum capacity of 60 cc., at a temperature of 200 ºC, rotor speed of 30 rpm and during 10 min. Immediately after finalizing the mixing step, one proceeded to the preparation of laminates of 1mm of thickness in a Carver hydraulic press, with the purpose of avoiding water absorption by the blends. The test specimens were cut according to the specifications of ASTM D-638 procedure. The tensile properties of the blends were evaluated using a universal testing machine Lloyd Instruments, model EZ 20, at a speed of 50 mm/min.
Thermal measurements were performed using a Differential Scanning Calorimeter (DSC) Mettler Toledo Star System. The specimens were heated up to 200 °C in order to erase any thermal history. They were maintained at that temperature for 5 min and then cooled up to room temperature so the crystallization temperature could be determined. A second heating was performed up to 200 °C for determining the melting temperature and the crystallinity degree. Tests were run at a scan rate of 10 °C/min.
The temperature of initiation of the degradation process (T
id) and the activation energy were determined from the thermograms obtained in a thermogravimetric analyzer (TGA) Mettler Toledo Star System, according to the following conditions: heating rate of 10 ºC/min in nitrogen atmosphere and within a temperature range from 20 to 650 ºC. The average weight of the samples was 10 mg.Morphological studies of cryogenically fractured samples of PP/CS were carried out using a Hitachi S-2400 scanning electron microscope (SEM). In addition, a morphological analysis of the non-plasticized and plasticized starch was made through SEM. Starch particles were also covered with platinum-palladium.
With the purpose of measuring the degradability of the blends due to the presence of starch, samples with 10 phr of starch were previously weighed with an error of ± 0.001 g and then were buried in an agricultural soil rich on minerals and natural degradable components, with an approximate depth of 30 cm in vertical position and with a separation between samples of 5 cm. Once the fixed time passed, all samples were unburied; the weight loss was measured followed by tensile testing.
In order to determine the water uptake of the blends, plaques of approximately 6.35 x 1.27 x 0.27 cm
3 were taken to constant weight, placing them in an oven at 80 ºC. Afterwards, they were immersed in distilled water. At regular time intervals, the specimens were taken out of the water, wiped with tissue paper to remove surface water, reweighed and immediately put back into water. At least three specimens for each sample were used. The water uptake was determined gravimetrically from the weight difference of the sample at a given immersion time and the initial weight (before immersion in water) enter the initial weight.FTIR spectra were taken from compression-molded films of the blends. These spectra were recorded in a Nicolet Magna-IR 560 ESP Spectrometer after 32 scans and at 2 cm
-1 of resolution.RESULTS AND DISCUSSION
Influence of cassava starch content on PP properties
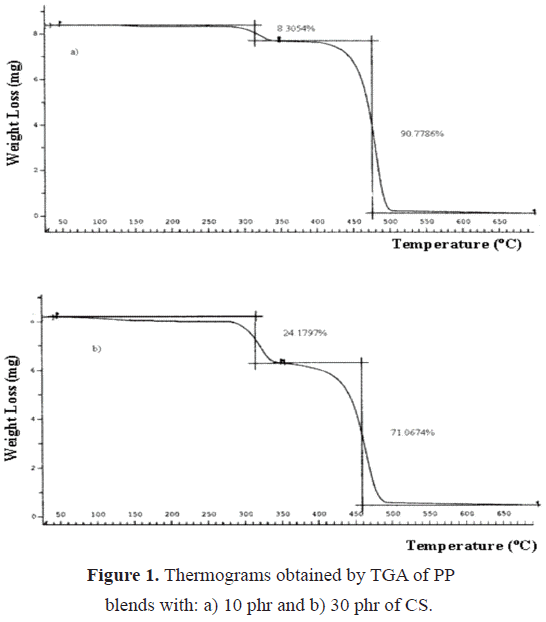
Figure 1 shows the thermograms obtained for the PP/starch blends with 10 and 30 phr. As it can be seen from these thermograms, there exists an initial loss between 100 and 180 ºC corresponding to the humidity present in the starch, which is difficult to eliminate; this loss is markedly evidenced as starch content in blends is increased (Table 1). In the interval between 260-350 °C, the starch decomposition occurs. According to Bastioli (1995) and Griffin (1994), starchs T
id is around 230 °C. Finally, in the 350-550 °C range, PP degrades, leaving a residue that varies as function of the starch content.Table 1. Weight loss, initial decomposition temperature of CS (Tid(CS)) and initial decomposition temperature of PP (Tid(PP)) for studied compounds.
| Samples | Weight loss (%) | Tid(CS) | Tid(PP) | |||
| Humidity | Starch | PP | Other | (ºC) | (ºC) | |
| PP | --- | --- | 99.00 | --- | 290 | 370 |
| PP/CS (10phr) | 1.05 | 7.50 | 91.55 | 0.95 | 290 | 420 |
| PP/CS (20phr) | 1.28 | 16.15 | 79.37 | 4.48 | 290 | 420 |
| PP/CS (30phr) | 3.33 | 24.18 | 71.07 | 4.75 | 290 | 430 |
| PP/PCS* | 2.14 | 4.28 | 89.51 | 4.06 | 290 | 430 |
| PP/CS/PP-g-MAH* | 1.17 | 6.99 | 88.41 | 3.44 | 290 | 430 |
| PP/PCS/PP-g-MAH* | 2.35 | 4.15 | 90.01 | 3.49 | 290 | 430 |
* Blends with 10 phr of CS
In Table 1 one can also see that the beginning of the decomposition process of the starch does not vary, since it maintains around 290 ºC. However, the T
id of the PP increases when starch is added, since the temperature varies from 370 ºC for pure PP to 420-430 ºC for PP within the blends. This fact induces to think in a retardant effect by means of the starch during the degradative process of the PP, since cassava starch not only does not accelerate PP oxidation, but rather acts like an anti-oxidant agent. Thomason (1993) reported that the introduction of fillers (such as rice hush) into polyolefins results in an increase in the thermal stability of the polyolefins. Incorporated fillers cause a reduction in chain mobility in the adsorption and boundary layers. This leads to a decrease in tension induced to the carbon-carbon chain by thermal energy and since the majority of bond braking takes place via this mode, less degradation will occur.It is important to specify that the degradative process of the starch is highly complex, where the starch structure is destroyed and the amylose and amylopectin chains degrade. Starch is more sensible to degradation than PP, since it posses carbon-oxygen bonds, which are more labile when faced to a temperature rise if compared to the carbon-carbon bonds of PP.
On the other hand, in Table 1 one can see a residue that does not correspond to PP since the latter degrades via chain rupture. So, one can infer that this residue probably corresponds to a crosslinked structure that is present in the starch granules, since it is impossible to eliminate all the water present due to its highly hygroscopic nature. This water permits the increase in granules size and their swelling. When swollen, the starch molecules separate into the two phases that conform it. The amylose migrates from the granules, since its lineal structure allows it; while the amylopectin, due to its branching that restricts the mobility, maintains in the granule. Some of these granules can contain more amylose, so they start to collapse because with the heating some chemical reactions occur that originate hydrogen bonds in the structure and so the viscosity increases since the amylopectin couples the amylose chains, forming link points (St. Pierre et al. 1997; Griffin, 1994; Fechner et al. 2005), hence increasing the residue with starch content.
From the thermal studies done to the PP blends with different contents of CS, it can be seen in Table 2 that the crystallization temperature (T
c) increases when CS is incorporated, thus implying a nucleating effect, which increases slightly the crystallinity of PP. The observed change in Tc was, however, quite small. Similar results were obtained by Bagheri (1999) in his studies of PP with corn starch, indicating that the filler particles exert a nucleating action that influences the growth of the PP´s spherullites.Table 2. Crystallization (Tc) and fusion (Tm) temperatures, fusion enthalpy (ΔHm) and crystallinity (Xc) of studied compounds.
| Compounds | T c ± 1 ºC | ΔH m ( J/g) | T m ± 1 ºC | X c (%) |
| PP | 110 | 104 | 163 | 47 |
| PP/ CS (10 phr) | 114 | 111 | 164 | 50 |
| PP/ CS (20 phr) | 114 | 117 | 162 | 52 |
| PP/ CS (30 phr) | 114 | 108 | 162 | 49 |
| PP/PCS* | 113 | 102 | 162 | 46 |
| PP/CS/PP-g-MAH* | 113 | 105 | 163 | 46 |
| PP/PCS/PP-g-MAH* | 112 | 100 | 163 | 45 |
* Blends with 10 phr of CS
Ahmad
et al. (1995) observed a rise in the melting heat of PP in their studies with rice husk ash. Amash & Zugenmaier (1998) found a remarkable increase in the Tc of the polymer matrix in PP compounds with three types of cellulose at 30 wt% and the increase in crystallinity was of 10%, higher that the one obtained in the present work (5%). From the crystallinity values obtained one can infer that the moisture content present in cassava starch can be exerting a plastifying effect, which inhibits a substantial increase in crystallinity.Contradictory results with respect to T
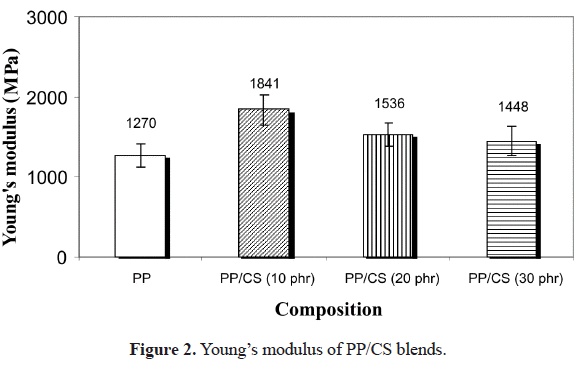
c and crystallinity were obtained by Lui et al. (2004) in compounds of PP with starch of amaranth, rice and corn. This discrepancy could be due to the fact that the surface properties of the starches are different since they affect negatively the crystallization process of PP. Kowaleski & Galeski (1986) showed that a poor interfacial adhesion is better since it does not affect the propagation velocity of the crystallization front. On the other hand, Chartterjee & Price (1974, 1975) studied the effect of various substrates on the crystallization of crystalline polymers, concluding that the surface energy of the substrate was not indicative of its nucleating ability. With respect to the melting temperature (Tm), it can be said that no variations were found in the samples analyzed.Figure 2 shows the influence of cassava starch on the Youngs modulus of the PP/CS blends. As it can be seen, there occurs a pronounced increase (45%) when the content of CS is 10 phr with respect to pure PP. However, when the starch content increases from 20 to 30 phr, the rise on modulus is less significant. This is due to the fact that lower proportions of CS are better dispersed and since during the mixing process all agglomerates tend to break up, there is greater amount of small particles that highly improve the modulus value. Nonetheless, when CS content is 20 and 30 phr, the agglomerates proportion increases and, even though these can be separated during mixing, a significant amount of the agglomerates does not separate in small particles, thus the reinforcing effect of the filler decreases, observing the abovementioned behavior.
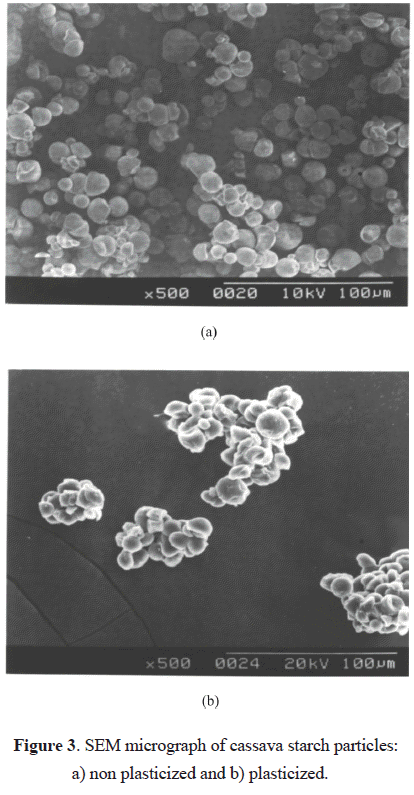
The micrograph presented in Figure 3 shows the particles of the non plasticized and plasticized starch, where one can prove the existence of CS agglomerates. On the other hand, the moisture content that was not possible to eliminate from the CS particles can also be affecting the behavior described for the Youngs modulus at concentrations greater than 10 phr. Kirby
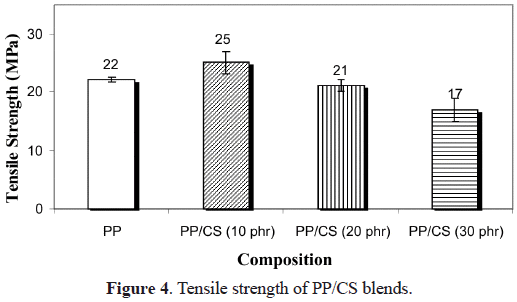
et al. (1993) analyzed the effect of moisture on the stress-strain curves of the starch, indicating that as the water content in starch increases, it experiments a fracture process slower with time, which is reflected on the displacement of the curve towards the strain axis, with a possible absence of fracture. This means that moisture exerts a plastifying effect that permits a better displacement of the filler with respect to the polymer chains. This is the same reason why a diminution of the tensile strength of the compounds when CS content increases is obtained (Figure 4).With respect to the values of elongation at break (ε
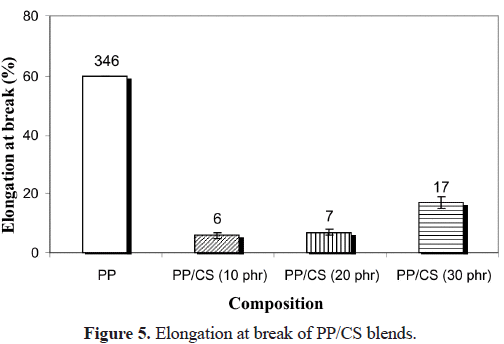
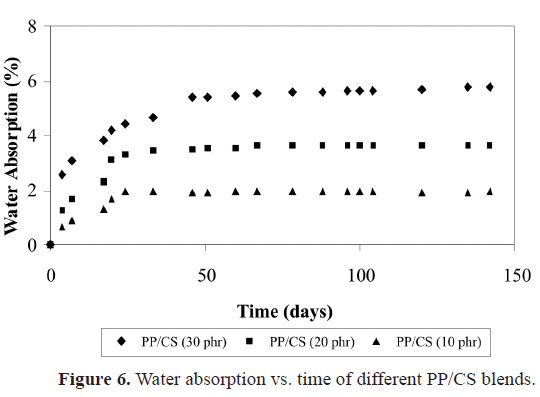
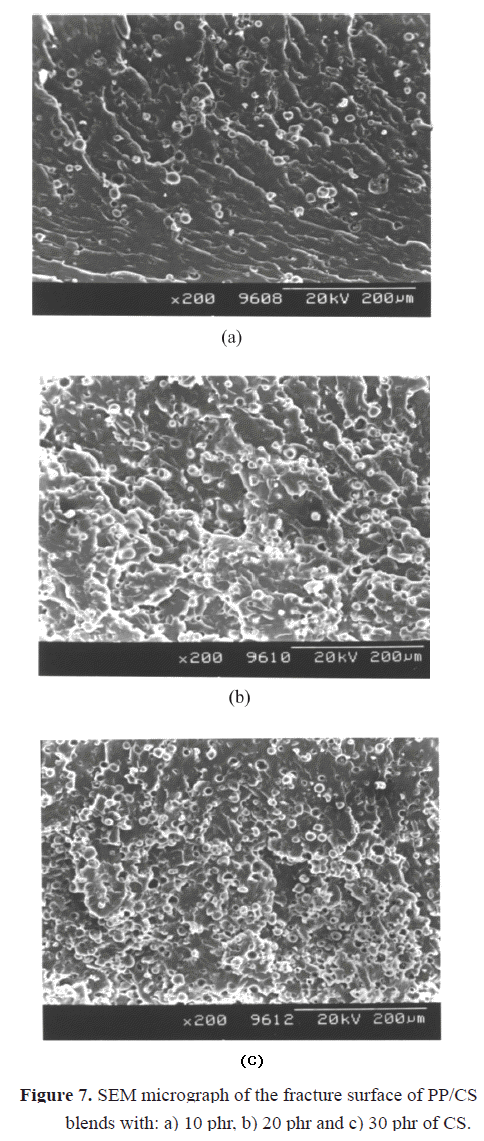
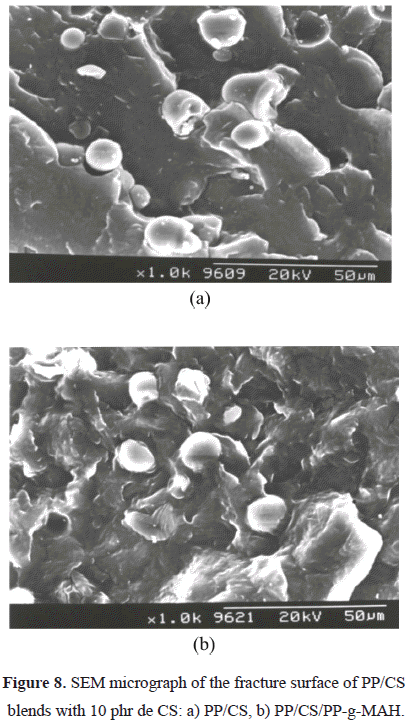
b) of the blends (Figure 5), a considerable diminution is observed when CS is incorporated. The lower decrease in the compound with 30% must be probably due to the plasticizing effect already indicated, and it can be corroborated by the analysis of the water absorption curves of PP with different contents of CS shown in Figure 6. It is shown that the water absorption (WA) increases with the increase in the CS concentration in the compound. This is completely logical since PP is hydrophobic and CS is hydrophilic. When the amount of CS is increased in the blend, its polar character increases and hence the water retention increases. The equilibrium values of WA are reached quicker for systems with lower filler content, when the systems are held in water for 30 days. Similar results were obtained by Ichazo et al. (2001) in PP/wood flour compounds. Concerning the effect with respect to PP, any rigid filler in a polymeric matrix restricts its deformation, thus reducing the elongation at break. The morphological studies done on these blends also confirm the tensile behavior described.Figure 7 shows the micrographs of the PP blends with different CS contents. One can clearly observe the presence of the two phases, which do not adhere due to the non compatibility existent between the polar particles of the cassava starch and the non polar nature of the PP. This lack of adhesion is evidenced by the presence of cavities that are originated by the expulsion of the CS particles at the moment of fracture (Figure 8a). Another important factor that affects the described tensile behavior is the frequency of the different particle sizes. Representative zones were observed and the existent number of particles was counted for each range selected, then this value was divided by the total number of particles analyzed. So, the sum of each of the frequencies obtained for each range and for each blend is equal to the unity.
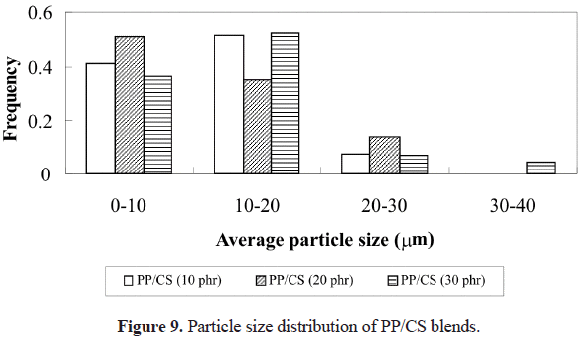
Figure 9 shows that the distribution of particle sizes is present basically in the range of 0-20 mm (these values were obtained from the micrographs of the samples). Nonetheless, when cassava starch content increases to 30 phr, a widening in the distribution of particle sizes is observed, since particles in the range of 30-40 mm also appear, as well as a predominance in the 10-20 mm frequency. This indicates that there is a greater amount of agglomerates in the compounds with higher CS content. In addition, the average size obtained for each blend is shown in Table 3. From the values shown, a slight increase is observed as starch content increases. Also, it can be seen that the average value for all blends is in the range of 10-20 mm, which is in concordance with the value reported on the literature (Thomason, 1993) for this type of filler.
Table 3. Agglomerate average sizes of PP/CS blends.
| Sample | Agglomerate average size (mm) |
| PP/ CS (10 phr) | 12.3 |
| PP/ CS (20 phr) | 12.9 |
| PP/ CS (30 phr) | 13.6 |
| PP/PCS* | 19.7 |
| PP/CS/PP-g-MAH* | 9.1 |
| PP/PCS/PP-g-MAH* | 10.0 |
Influence of cassava starch plasticization and PP-g-MAH addition on PP properties
On the basis of the results obtained for the tensile properties, that is to say, the greater increase in Youngs modulus with 10 phr of CS, it was decided that this composition was the appropriate one to make additional modifications on the compounds. The modifications selected were: plasticization of the starch with water and glycerol (PCS) and the addition of a compatibilizer, polypropylene functionalized with maleic anhydride (PP-g-MAH), to the blends with plasticized and non plasticized starch.
Table 1 shows that the Tid of the PP increases 10 ºC, the value being equal to 430 ºC for the modified compounds, independently of the compatibilizing agent addition and of the CS treatment. These results indicate that the changes made in order to enhance the polymer-filler interaction only slightly affect the behavior of the Tid. Also, one can see that the residue, as well as the humidity content, increases in greater proportion for all compounds when compared with the non-modified compound, this effect being more pronounced in the compound with PCS. This behavior is attributed to the swelling of the starch when being heated up with water and glycerol, giving origin to a paste, which would be the final residue. On the other hand, the addition of PP-g-MAH does not produce significant changes on the different parameters analyzed in the thermograms of the PP/CS and PP/PCS compounds, with a few exceptions.
In Table 2 one can observe neither that, nor the plasticization of the starch with glycerol and water nor the addition of PP-g-MAH to the PP/CS and PP/PCS compounds significantly affect the Tc and Tm values. On the other hand, in the PP/CS blend with PP-g-MAH a decrease in crystallinity is observed since a better polymer-filler adhesion is produced (Figure 8b), due to the action of the functionalized PP. The interaction could be a consequence of the reactions between the starch and the anhydride groups in the PP-g-MAH and also to the branched molecules that are produced by the reaction of the compatibilizer and the CS (Bikiaris & Panayiotou, 1998). These two factors originate a slight decrease in the crystallinity, of approximately 5%.
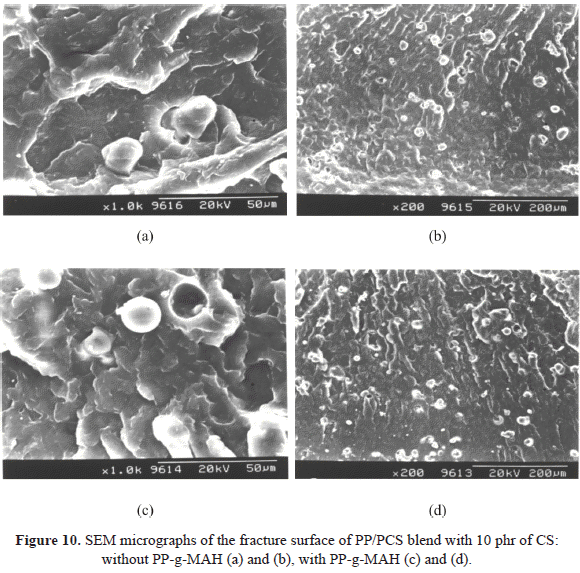
Table 4 reports the values of the tensile properties of the PP blends with natural starch (CS) and with plasticized starch (PCS). When comparing the Youngs modulus of both blends, a considerable decrease is observed for the blend with plasticized starch. This behavior can be attributed to the fact that the PCS has a less reinforcing effect, possibly due to the water content and the glycerol used in the gelification process that can exert a plastifying effect, as well as to the greater size of the agglomerates when the starch is plasticized. This behavior is also observed when comparing the fracture surface of the compounds (Figures 8a and 10a).
Table 4. Mechanical properties of PP/CS blends with 10 phr of CS with and without functionalized PP and the buried compounds after 1 and 3 months.
| Samples | Youngs modulus (MPa) | Tensile strength (MPa) | Elongation at break (%) |
| PP | 1102 ± 73 | 32.4 ± 1.9 | 10 ± 1 |
| PP/CS | 1954 ± 86 | 24.7 ± 1.9 | 6 ± 1 |
| PP/CS/PP-g-MAH | 1543 ± 87 | 28.8 ± 2.7 | 4 ± 1 |
| PP/PCS | 1350 ± 82 | 25.6 ± 1.7 | 5 ± 1 |
| PP/PCS/PP-g-MAH | 1336 ± 74 | 23.6 ± 2.1 | 5 ± 1 |
| PP/CS 1 month | 1422 ± 39 | 24.1 ± 1.2 | 7 ± 1 |
| PP/CS 3 months | 834 ± 96 | 22.3 ± 1.3 | 12 ± 3 |
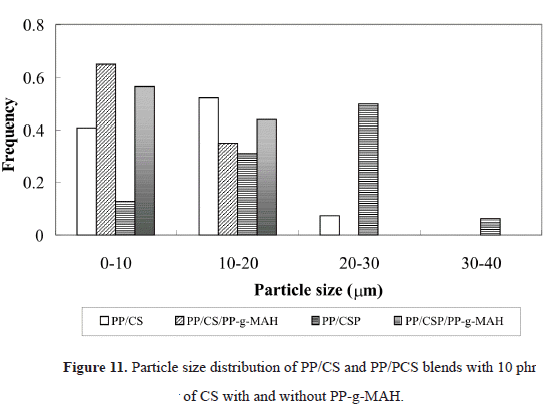
The compatibilized compounds with 10 % of PP-g-MAH have a different behavior. The Youngs modulus decreases when incorporating the PP-g-MAH to the PP/CS blend, while the tensile strength increases. It can be attributed to the use of the compatibilizer that produces a greater amount of small size CS particles (Figure 11), but not small enough to increase the modulus. In addition, in the micrograph shown in Figure 8b, one can observe that the interfacial adhesion between PP and CS was improved with the presence of PP-g-MAH, fact that can be attributed to the chemical reactions between groups in starch and anhydride groups in PP-g-MAH. On the other hand, another contribution to this behavior of the tensile properties is the presence of branched macromolecules that are produced by the reaction of the compatibilizer and the CS. These macromolecules have higher tensile strength compared to the linear ones, but lower elongation at break. Their presence could also explain, in part, why the increase in elongation at break was not as evident as in the tensile strength of the compounds (Bikiaris & Panayiotou, 1998).
With respect to the tensile behavior of the PP/PCS/PP-g-MAH compound, one can see that the modulus as well as the strength and the elongation at break remain approximately constant, with respect to the non compatibilized compound, even though the addition of PP-g-MAH originates a diminution on the size range of PCS agglomerates in the
compound, as observed in Figure 11. This is the reason why it could be said that the effect of the compatibilizer on the PP/PCS compound was not the same as on the PP/CS compound, fact that can be corroborated in Figure 10c, where one can see that there is no polymer-filler interaction, since cavities are formed due to the expulsion of the PCS particles at the moment of fracture.So, it could be inferred that the glycerol did not improve the interfacial properties between the PCS and the PP, because no interaction between the PP and the glycerol was obtained. The interfacial adhesion between PCS and PP could be improved with the addition of PP-g-MAH, however this was not the case for the compound under study. So, one could say that the glycerol is avoiding the interaction that could be achieved by the use of a compatibilizer, and that in this PP/PCS it only produces starch agglomerates of smaller particle size (Figures 10 and 11).
From the values shown in Figure 11, the average sizes of the particle agglomerates were determined, as seen in Table 3. From these results, one can confirm that the compatibilizer does decrease the average size of the particle agglomerates, specifically in the case of the PP/PCS/PP-g-MAH compound, but once again with no improvement in the polymer-filler interaction.
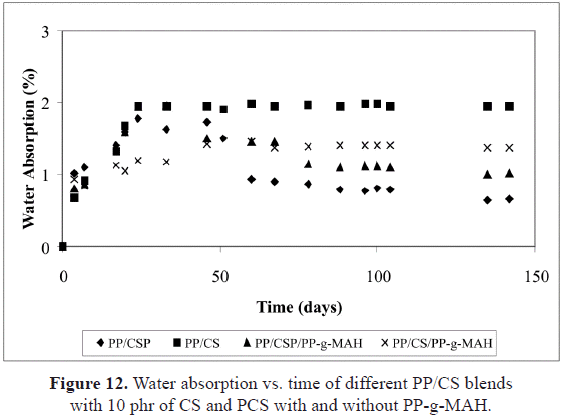
Figure 12 shows the variation of water absorption with respect to the plasticization of the starch and to the presence of a compatibilizer. It can be noticed that the value for the PP/CS/PP-g-MAH compound is lower than the one corresponding to PP with 10 phr of CS. Similar behavior can be observed when the absorption curves of PP/PCS/PP-g-MAH are compared. This can be attributed to the lower amount of free OH in the cassava starch because some of them could be interacting with the succinic anhydride.
On the other hand, the absorption curves of the PP/PCS compounds with and without PP-g-MAH, after 50 days of immersion in water, present a decrease in the quantity of water absorbed. As previously mentioned, the starch is a biopolymer formed by amylose, a linear polymer, and amylopectin, a highly branched polymer. The amylose can be found forming semi-crystalline particles, while the amylopectin forms the amorphous regions. The functionality of the starch depends on the average molecular weight of the constitutive polymers, as well as on the structural organization of these polysaccharides inside the granule. When heat is applied, the starch can suffer a depolymerization, and under these conditions, it becomes a thermoplastic. This is due to the destructuration of the granules in presence of plasticizers (water, glycerol, etc.) (De Graaf
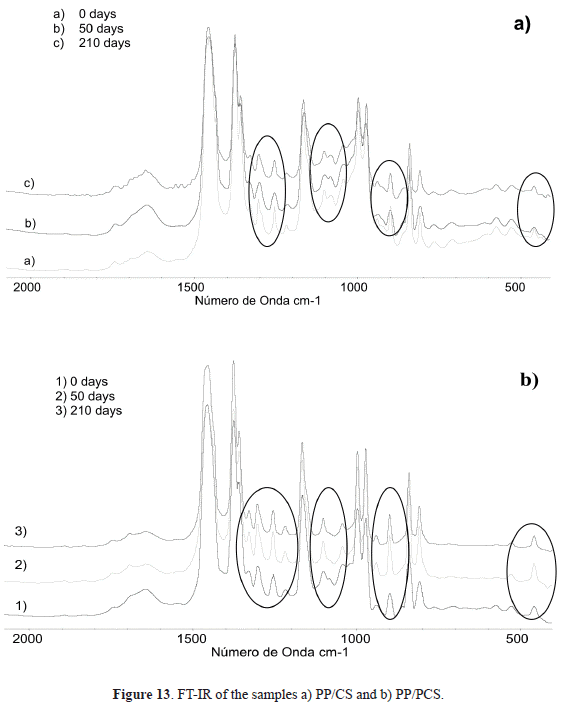
et al. 2003; Acosta et al. 2006). In the thermoplasticized starch, the structure and morphology are determined by the level of disruption (disentanglements and dissociation of both biopolymers) and by the fusion of the granule with the plasticizer, besides the ageing process. This process is related to the retrodegradation, which consists on the reorganization of the biopolymer, giving place to a phase separation; so, the amylose and amylopectin could reorganize their helix and crystallinity. This process increases with the slow cooling and with the differences between the heating and cooling temperatures.Several methods have been used to analyze the retrodegradation of the starch, such as DSC, DRX, RMN, viscosity, as well as TEM. A useful technique is FT-IR, where one can observe the conformational changes produced on the amylose-amylopectin system. In order to study the retrodegradation, the spectra regions of 480, 910 and 1000-1700 cm
-1, corresponding to the skeletal mode of the pyranose ring and to the C-H stretching modes, were analyzed. Based on this, Figure 13 shows the spectra of the PP/CS and PP/PCS compounds, where one can observe the modifications on the mentioned regions. Figure 13b shows that these signals duplicate at 50 days and then start to delay. These changes on intensity manifest the conformational variations of the starch and the possibility of its degradation giving origin to the water desorption.In the IR spectra of the PP/CS blend, after 50 and 210 days of water immersion, no significant changes are observed at those wave lengths (Figure 13a), while in the PP/PCS spectra (Figure 13b), the bands describe a rise on intensity in the regions associated to the skeletal mode of the C-H bond (480 cm-1). This corroborates that the amylopectin has a crystalline nature, and so with time the system formed does not have adsorptive properties. This fact explains the observed desorption for the PP blends that contain plasticized starch after 50 days, whether or not they have PP-g-MAH.
Finally, with respect to the degradability of the compounds with 10 phr of CS, one can notice that the weight increased after 1 and 3 months of being buried, 1.0 ± 0.1% and 2.2 ± 0.1% respectively, due to the absorption of the soil water by the starch of the compound. It was awaited that the weight diminished, since the starch should be consumed by the soil micro-organisms, however, this was not the case.
The tensile properties of the buried compound are shown in Table 4. It can be observed that the Youngs modulus decreased abruptly with time, and that the elongation at break increased slightly. These results indicate that during the burial time, the sample absorbed water from the soil and it acted as a plasticizer in the compound.
CONCLUSIONS
From the thermodegradative studies of the PP/CS blends, one can infer that the CS content induces a retardant effect on the degradative process of the PP, since it increases the initial decomposition temperature of the blend, acting like an anti-oxidant agent, the effect being slightly higher when the CS is thermoplasticized, as well as when PP-g-MAH is added. Also, the CS acts as a nucleating agent, increasing the crystallization temperature as well as the crystallinity from 110 to 114 °C and from 47 to 52%, respectively.
The Youngs modulus and tensile strength increase when CS content is 10 phr, and start to decrease at higher CS contents due to the existence of agglomerates and higher water content reflected on a rise on the elongation at break. However, when PP-g-MAH is added to the PP/CS blends the abovementioned properties decrease. This behavior is not observed on the PP/PCS blends, due to the plasticizing effect of the water on the thermoplasticization stage.
Regarding the water absorption process of the PP/PCS blends with and without PP-g-MAH after 50 days of water immersion, there seems to occur a desorption process that depends on the reactions produced in the PCS, reflected on the changes on the 480, 910 and 1000-1700 cm-1 wave lengths of the FT-IR spectra.
Finally, the degradability of the PP/CS blend in an agricultural soil affects the mechanical properties, decreasing dramatically the Youngs modulus from 1841 to 834 MPa.
ACKNOWLEDGEMENTS
To Fonacit, USB, UCV, IVIC by the financial support and to the MSc. M. Lavady and MSc. Y. Sánchez for their collaboration on the FTIR analysis.
REFERENCES
1. Ah
mad, M.Y., Mustafah, J., Manson, M.S., Mohd, Z.A., Mohd, A.K. (1995). Thermal properties of polypropylene/rice husk ash composites. Polym. Int., 38: 33-43. [ Links ]2. Amash, A. & Zugenmaier, P. (1998). Study on cellulose and xylan filled polypropylene composites. Polymer Bulletin, 40: 251-258. [ Links ]
3. Bagheri, R. (1999). Effect of processing on the melt degradation of starch-filled polypropylene. Polym. Int., 48: 1257-1263. [ Links ]
4. Bastioli, C. (1995). Degradable Polymer, Principles and Applications. Ed. By Scott G. and Gilead D., Chapman and Hall, London, p.115. [ Links ]
5. Bikiaris, D. & Panayiotou, C. (1998). LDPE/starch blends compatibilized with PE-g-MA copolymers. J. Appl. Polym. Sci., 70: 1503-1521. [ Links ]
6. Chartterjee, A.M., Price, F.P., Newman, S. (1975). Heterogeneous nucleation of crystallization of high polymers from the melt. I. Substrate induced morphologies. J. Polym. Sci. Polym. Phys. Ed., 13: 2369-2383. [ Links ]
7. Chartterjee, A.M. (1974). PhD. Thesis University of Massachusetts. [ Links ]
8. Danjaji, I.D., Nawang, R., Ishiaki, U.S., Ismail, H., Moholishak, Z.A. (2002). Degradation studies and moisture uptake of sagro-starch filled low density polyethylene. Polymer Testing, 21: 75-81. [ Links ]
9. De Graaf, R.A., Karman, A.P., Janssen, L.B. (2003). Material properties and glass a thermoplastic starches after extrusion processing. Starch/Stärke, 55: 80-86. [ Links ]
10. Griffin, G.J.L. (1994). Chemistry and Technology of Biodegradable Polymers. Blackie Academic and Professional, Glasgow. [ Links ]
11. Dosmann, L.P., Steel, R.N. (1961). US. Patent 3,334,934. [ Links ]
12. Evangelista, R. L., Sung, W., Jane, J.L., Gelina, R.J., Nikolov, Z.L. (1991). Effect of compounding and starch modification on properties of starch-filled low-density polyethylene. Ind. Eng. Chem. Prod. Rev, 30: 1841-1846. [ Links ]
13. Fechner, P.M., Wartewing, S., Kleinebudder, P., Neubert, R.H.H. (2005). Studies of the retrogradation process for various starch gels using Raman spectroscopy. Carbohydrate Research, 340, 2563-2568. [ Links ]
14. Griffin, G.J.L. (1994). Chemistry and Technology of Biodegradable Polymers. Blackie Academic and Professional, Glasgow. [ Links ]
15. Ichazo, M.N., Albano, C., Gonzalez, J., Perera, R., Candal, M.V. (2001). Polypropylene/Wood flour composites: treatments and properties. Comp. Struct., 54: 207-214. [ Links ]
16. Jane, J.L., Gelina, R. J., Nikolov, Z., Evangelista, R.L. (1991). US Patent 5,059,642. [ Links ]
17. Kirby, A.R., Clark, S.A., Parker, A., Smith, A.C. (1993). The deformation and failure behaviour of wheat starch plasticized with water and polyols. J. Mater. Sci., 28: 5937-5942. [ Links ]
18. Kowalewski, T. & Galeski, A. (1986). Influence of chalk and its surface treatment on crystallization of filled polypropylene. J. Appl. Polym. Sci., 32: 2912-2934. [ Links ]
19. Linden, G. & Lorient, D. (1996). Bioquímica Agroindustrial. Ed. Acribia, S.A. Zaragoza, España. [ Links ]
20. Liu, W., Wang, Y.J., Zun, Z. (2004). Crystallization behavior of starch-filled polypropylene. J. Appl. Polym. Sci., 92: 484-492. [ Links ]
21. Mali, S., Sakanaka, L.S., Yamashita, F., Grossman, M.V.F. (2005). Water sortion and mechanical prperties of cassava starch films and their relation of plastisizing effect. Water Carbohydrate Polym. 60(3): 283-289. [ Links ]
22. Matzinos, P., Bikiaris, D., Kokkou, S., Panayiotou C. (2001). Processing and characterization of LDPE/starch products. J. Appl. Polym. Sci., 79: 2548-2557. [ Links ]
23. Morikawa, K. & Nishinari, K. (2002). Effects of granule size and size distribution on rheological behaviour of chemically modified potato starch. J. Food Sci., 67: 1388-1392. [ Links ]
24. Otey, F.H., Westhoff, R.P., Russell, C.R. 1977. Biodegradable Films from Starch and Ethylene-Acrylic Acid Copolymer. Ind. Eng. Chem. Prod. Res. Dev, 16(4): 305-309. [ Links ]
25. Otey, F.H., Westhoff, R.P., Doane, W.M. (1980). Starch-Based Blown Films Ind. Eng. Chem. Prod. Res. Dev, 19(4): 592-595. [ Links ]
26. Otey, F.H., Westhoff, R.P., Doane, W.M. (1987). Starch-based blown films. 2. Ind. Eng. Chem. Prod. Res, 26: 1659-1663. [ Links ]
27. Pedrosa, A.G. & Rosa, D.S. (2005). Mechanical and morphological characterization of recycled LDPE/corn starch blends. Carbohydrate Polymer, 59: 1-9. [ Links ]
28. Rahman, W.A.W.A., Ali, R.R., Zakaria, N. (2006). Proceedings of the 1st International Conference on Natural Resorurces Engineering of Technology. Putrajaya, Malaysia, 434-444. [ Links ]
29. Rodriguez, F.J. & Ramsay, B.A. (2003). High performance LDPE/thermoplastic starch blends: a sustainable alternative to pure polyethylene. Polymer, 44(5): 1517-1526. [ Links ]
30. Rosa, D.S., Guedes, C.G.F., Carvalho, C.L. (2007). Processing and thermal, mechanical and morphological characterization of post-consumer polyolefins/thermoplastic starch blends. J. Mater. Sci., 42: JJ1-JJ7. [ Links ]
31. Sailaja, R.R.N. & Chanda, M. (2001). Use of maleic anhydride-grafted polyethylene as compatibilizer for HDPE-tapioca starch blends: Effects on mechanical properties J. Appl. Polym. Sci., 80: 863-872. [ Links ]
32. St. Pperre, N., Favis, B.D. Ramsay, J.A., Vergoogt, H. (1997). Processing and characterization of starch/polyethylene blends. Polymer, 38: 647-656. [ Links ]
33. Thomason, J.L. (1993). Fiber induced acceleration of matrix degradation in thermoplastic composites. Plast. Rubber Comp. Process Appl., 20: 265-270. [ Links ]
34. Westhoff, R.P., Otey, F.H., Mehltretter, C.L., Russell, C.R. (1974). Starch-Filled Polyvinyl Chloride Plastics-Preparation and Evaluation Ind. Eng. Chem. Prod. Res Dev., 13: 123-125. [ Links ]
35. Zuchowska, D., Hlavata, D., Steller, R., Adamiak, W., Meissner, W. (1999). Structure and properties of degradable polyolefin-starch blends. Polym. Deg. Stab., 60: 471-480. [ Links ]