Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Facultad de Ingeniería Universidad Central de Venezuela
Print version ISSN 0798-4065
Rev. Fac. Ing. UCV vol.27 no.2 Caracas June 2012
Chemical modification of cassava starch by carboxymethylation reactions using sodium monochloro acetate as modifying agent
Simón E. Barrios1, Jesús M. Contreras1, Francisco López-Carrasquero1, Alejandro J. Müller2.
1 Grupo de Polímeros ULA, Departamento de Química, Facultad de Ciencias, Universidad de Los Andes, Mérida 5101A, Venezuela.
2 Grupo de Polímeros USB, Departamento de Ciencia de los Materiales, Universidad Simón Bolívar, Apartado 89000, Caracas 1080A, Venezuela.
Corresponding authors Jesús M. Contreras (jeco@ula.ve).
Recibido: marzo 2011 Recibido en forma final revisado: junio 2012
ABSTRACT
Carboxymethylation of cassava starch was carried out in three different reaction media: iso-propanol (IP), dimethylsulfoxide (DMSO) and water. Monochloroacetic acid (MCAA) and sodium monochloroacetate (SMCA) were employed as modifying agents. With the objective of obtaining the best compromise between a high degree of substitution (DS) and a low degradation of the starting polymer (indicated by a high zero-shear viscosity of the carboxymethyl starch (CMS) in aqueous solution), the following reaction conditions were systematically varied: temperature, reaction time and reactants ratios. The carboxymethylation reactions carried out using DMSO or water yielded water soluble derivatives while those performed in IP were insoluble. When SMCA was used as a modifying agent, CMS with higher DS and viscosities were produced as compared to those obtained with MCAA. On the other hand, factors such as temperature and time increased the DS but reduced viscosity values. From all the studied conditions, those which produced the best results were: water as a solvent at
Keywords: Cassava starch, carboxymethylation, carboxymethyl starch, rheology, biopolymers.
Modificación química del almidón de yuca mediante reacciones de carboximetilación usando monocloro acetato de sodio como agente modificante
RESUMEN
En este trabajo se llevó a cabo la carboximetilación de almidón de yuca en tres medios de reacción diferentes: isopropanol (IP), dimetilsulfóxido (DMSO) y agua, usando ácido monocloroacético (AMCA) y monocloroacetato de sodio (SMCA) como agentes modificantes. A fin de obtener un almidón modificado con el mayor grado de sustitución (DS) y con la menor degradación posible, se realizaron varias reacciones donde se variaron sistemáticamente la temperatura, tiempo de reacción y la proporción de los reactivos empleados. Las reacciones de carboximetilación llevadas a cabo utilizando DMSO o agua produjeron derivados solubles en agua, mientras que los obtenidos en IP resultaron insolubles. Cuando se empleó el SMCA como agente modificante se obtuvieron productos con grados de sustitución y viscosidades mayores que cuando se utilizó el AMCA. Por otro lado, aunque la temperatura y el tiempo de reacción favorecen el aumento del DS, disminuyen la viscosidad de las soluciones ya que ambos promueven la degradación del almidón. Los mejores resultados se encontraron cuando se usó agua como solvente a una temperatura de 60 º C, con un tiempo de reacción de 6 horas y una proporción almidón / SMCA de 1:3(mol:mol). En estas condiciones el derivado producido exhibió una DS de 0,32 y una viscosidad de corte cero (ηo) de 0,073 Pas. (para disoluciones de 2% de CMS en agua). Cuando se empleó DMSO se obtuvieron valores de DS de hasta 0,47 pero con valores de ηo tan bajos como 0.006 Pas (para disoluciones de 2% de CMS en agua).
Palabras Clave: Almidón de yuca, carboximetilación, almidón carboximetilado, reología, biopolímeros.
INTRODUCTION
Cassava (Manihot Esculenta, sin. M. Utilissima) known also as manioc or yucca is a tropical plant and represents one of the most important crops in some tropical countries. Cassava is an important starch source. The content of amylose in cassava starch usually lies within the range of about 20-30% by weight. Industrial uses of cassava starch have been mainly oriented to textile and food industries. This fact is based fundamentally in some of the natural properties of starch such as its granular structure, low solubility in cold water, colloidal dispersion during heating, adhesiveness and thickening and texturing capacities (Jaspreet et al. 2007). Chemical modification of starch and other polysaccharides is one of most important alternatives for the production of biodegradable polymers. In the particular case of starch these modifications involve changes in its granular structure yielding products with different degrees of gelatinization, swelling and solubility behavior as compared to native starch. Hydrophilic or hydrophobic starches can be synthesized depending on the nature of the modifying agent; these chemical modifications increase the potential industrial applications of starch (Jaspreet et al. 2007; Heinz & Koschell. 2005).
The carboxymethylation reaction as a tool for the chemical modification of starch has been studied in the last few decades since it is a simple way to obtain derivatives with interesting industrial properties [Lazi et al. 2002; Zhang, 2001; Kittipongpatana et al. 2006; Zhou et al. 2007; Jie et al. 2004; Volkert et al. 2004; Moorthy et al. 2006). This modification is in essence an etherification reaction whose aim is to insert a hydrophilic group in the anhydroglucose unit (AGU) of starch in order to stabilize it in aqueous media and prevent its retrogradation at low temperatures. Carboxymethylation involves two successive steps in agreement with the Williamson etherification reaction shown in Scheme 1. For simplicity, in this scheme the substitution is shown only in the C6 position of the AGU, but it can also occur in the C2 and C3 positions.
Scheme 1. Carboxymethylation reaction.
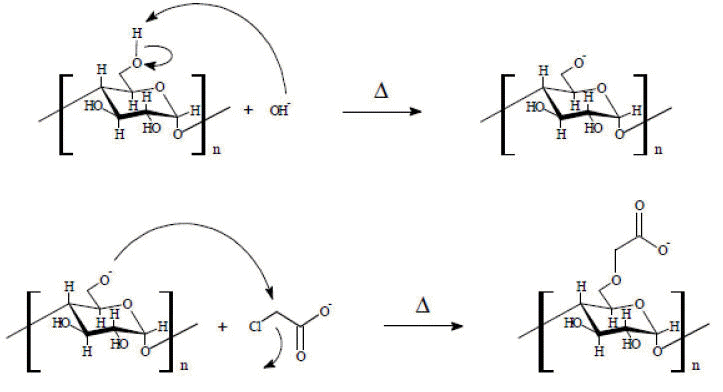
The degree of substitution (DS) and the yield of the reaction obtained in the carboxymethylation process, as in other modifications of starch, depend on its source (Jie et al. 2004; Volkert et al. 2004; Moorthy et al. 2006). During the last fifty years, the starch has been used as feedstock for the development of polymer additives in the field of oil industry. In this regard, many studies based on the preparation and properties of etherified starches for use in the oil field had been doing, in particular in the preparation of highly substituted derivatives (Zhang, 2001). However, there is a huge gap in terms of rheological studies relating the structure and modification of the CMS with behaviors that can present their solutions to be subjected to cutting processes (Lapasin et al. 1992; Sangseethong et al. 2005).
Therefore, the objective of the present work is the optimization of the carboxymethylation reaction in order to obtain a relatively high degree of substitution with the lowest possible degradation and good rheological
properties. The degradation was monitored by determining the low shear rate viscosity of aqueous solutions of CMS, since these are the main basis for studying the application of these products as possible substitutes for other additives currently used in drilling mud formulations. Here were explored different reaction media such as isopropanol (IP), dimethylsulfoxide (DMSO) and water and two modifying agents: monochloroacetic acid (MCAA) and sodium monochloroacetate (SMCA). There were employed different starch/modifying agent molar ratios, temperatures and reaction times in order to determine the best conditions to generate a derivative with the highest DS and highest low shear viscosity (ηo) values in aqueous solutions.
EXPERIMENTAL TECHNIQUES
MATERIALS
Cassava starch which contains 30% amylose was kindly supplied by Agroindustriales Mandioca S.A.,
STARCH MODIFICATION
In a three neck flask the base is dissolved in the selected solvent (NaOH in DMSO or water and KOH in IP) then, under magnetic stirring the starch is added and dispersed in the solvent (about 2%w/v). The mixture is heated to reach the reaction temperature and maintained during the required time for the starch activation. Thereafter, the modifying agent, monochloroacetic acid (MCAA) or its sodium salt (SMCA) is added under stirring at a preset temperature, the reaction is left to progress during a selected time. Finally, the product is precipitated with MeOH and the solution is refrigerated overnight to allow the precipitation of the entire product. The modified starch is filtered and dried in vacuum desiccators or in an oven at
ANALYTICAL METHODS
Infrared spectra were registered on a Perkin-Elmer 2000 FTIR instrument employing KBr discs. The values of DS were estimated by the titration method previously described by Stojanovic (Stojanovic et al. 2005). Rheological measurements were carried out in a Rheometric Scientifics ARES (902-30004) rheometer under strain rate controlled conditions. The solutions were prepared in distilled water at a concentration of 2% w/w of the modified starch (CMS). A double wall couette fixture was employed with a temperature control set at 25.00 ±
RESULTS AND DISCUSSION
As previously discussed, the aim of this work is to determine the best reaction conditions to perform the carboxymethylation of cassava starch in order to obtain CMS derivatives with the highest degrees of substitution (DS) and solution viscosities. Care should be exercised since the variables that usually enhance the DS of the derivatives also produce starch degradation, with the concomitant reduction in the solution viscosity (Jie et al. 2004; Sangseethong et al. 2005; Qiu &, He, 1999). With this aim in mind, the following strategy was employed: First, it was studied the influence of the solvent starting with experimental conditions previously reported in the literature (Heinz & Koschell., 2005; Mollega et al. 2011; Flores, 2006). Then, when the best reaction media was selected, it was studied the influence of employing MCAA or SMCA as modifying agents mainly because they have been widely employed in previous literature (Heinz & Koschell, 2005; Jie et al. 2004; Sangseethong et al. 2005; Stojanovic et al. 2005, Qiu & He, 1999; Mollega et al. 2011, Flores, 2006). Finally once the best conditions to obtain maximum DS and solution viscosities were selected, the effects of reaction time and temperature were studied. The results obtained are reported below.
EFFECT OF THE SOLVENT ON THE MODIFICATION
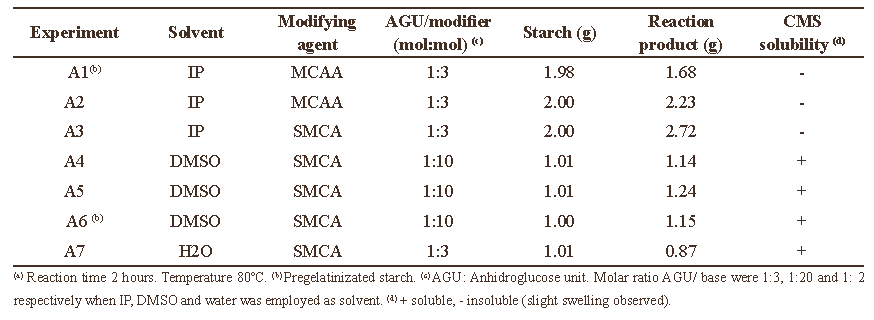
A number of experimental studies have been undertaken to determine the influence of the solvent on the carboxymethylation of Cassava Starch. In this regard, different water-alcohols mixtures (Jie et al. 2004; Sangseethong et al. 2005; Qiu & He, 1999), water (Flores, 2006, Hofreiter, 1986) and organic solvents (Tijsen et al. 2001) has been used as a reaction medium. In the first stage of this work the carboxymethylation reaction was performed using three different solvents: IP, DMSO and water. The AGU/(modifying agent) molar ratios employed were based on those previously reported in the literature (Heinz & Koschell A, 2005; Flores, 2006), and they are listed in Table
When the reaction was carried out in water the reaction mixture was completely soluble. In DMSO, the starch granules were swollen and partially dissolved, while in IP the cassava starch was only dispersed and a heterogeneous media was obtained. After the reaction time elapsed, the derivatives were purified by MeOH addition and the derivatives obtained in water and DMSO precipitated from solution, while the products obtained in IP remain always insoluble throughout the reaction.
After the derivatives were purified and dried, they were placed in vials to try to dissolve them in distilled water. The derivatives obtained in water and DMSO, were soluble and transparent solutions were successfully prepared. This solubility was not only due to the alkalization treatment of native starch, but also would indicate the introduction of negatively charged carboxymethyl groups into native starch. The derivative obtained in IP was only swollen in water and a cloudy solution/dispersion resulted with very limited solubility. This fact suggests that the modification of cassava starch in IP only occurs on the surface of the granular material and the partial solubility in pure water is a consequence of the activation in the alkali medium of the external OH groups of the AGU, together with a low insertion of carboxymethyl groups on the surface of the starch particles. The reaction conditions and the obtained results at this stage are shown in Table 1.
The results reported in Table 1 were reproducible for each solvent and no appreciable differences were observed when pregelatinized or initially native materials were employed.
The FTIR spectra of the obtained CMS are shown in Figure 1 and they are in agreement with the expected structure of the modified starches (Qiu & He, 1999; Mollega et al. 2011). As may be appreciated in Figure 1, the main difference between the spectra of modified and native starch is the broadening of the signal that appears at 1645 cm-1 which is related to the water content associated with the starch. In the CMS this signal is overlapped with the asymmetric stretching vibration of the C=O bond of the carboxylate group which appears near 1600 cm-1. The overlapping of these signals was previously reported by us in a work which included a study with deconvolutionated IR spectra with analogous CMS´s (Mollega et al. 2011). Furthermore, these spectra also show another band near 1420 cm-1 attributed to the symmetric stretching of the carboxylate group. All spectra of the modified starches do not showed SMCA bands at 1248 and 673 cm-1, which indicate that all of the obtained CMS were free of modifying agent residues.
The FTIR spectra, in addition to the fact that the modified starches were ultimately soluble in water, indicate that the carboxymethylation reaction was effective in water and DMSO.
On the other hand, the poor solubility of the derivatives obtained in IP strongly suggests that the modification in this solvent (even though it is also documented in Figure 1) is only superficial since the reaction was carried out in a heterogeneous media. Therefore, the granular structure of native starch was not substantially destroyed hindering the modification within the particles. This is consistent with the results reported by other authors, whereby the addition of water to isopropanol result in a significative improvement of starch particles swelling which facilitates mass transfer of the reactant into starch particles, and consequently an increase in the degree of substitution (Jie et al. 2004; Sangseethong et al. 2005).
Table 1. Experimental conditions and results of the carboxymethylation of cassava starch carried out in IP, DMSO and water(a).
EFFECT OF THE MODIFYING AGENT
In order to study the effect of the modifying agent, a series of assays were carried out in water as a solvent, since it provides a homogeneous reaction media. For these experiments all the reactions were performed at
Figure 1. FTIR spectra of: (A) native cassava starch and (B) CMS prepared in water (The IR spectra of CMS´s obtained in DMSO and IP were similar to the derivative obtained in water).
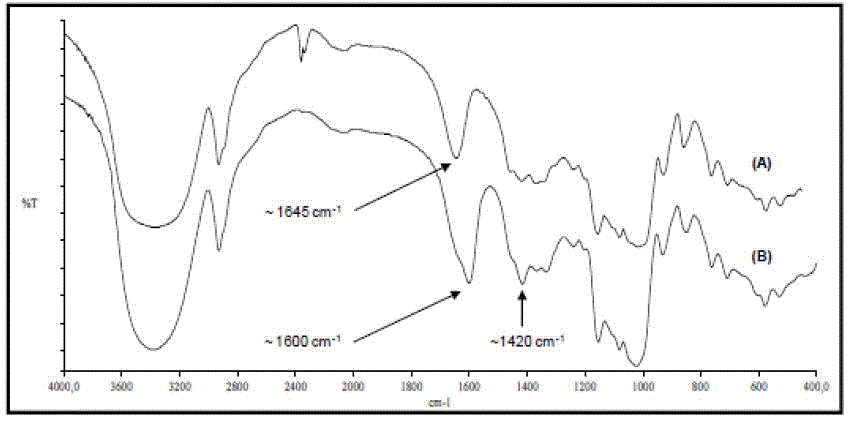
Table 2. Experimental conditions and results obtained for carboxymethylation of cassava starch using water as solvent(a).
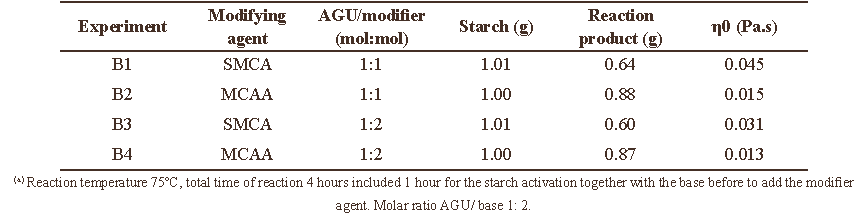
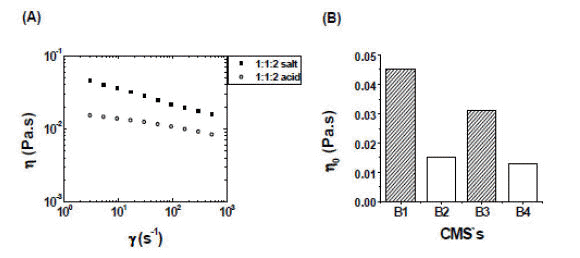
Figure 2A shows the results of simple shear rheological measurements for two chosen derivatives. Both samples exhibit a shear thinning behavior, which is typical of solutions of high molecular weight polysaccharides at moderate concentrations. In this paper we will refer to the viscosity determined at the lowest possible shear rate as a nominal zero shear rate viscosity or η0 (even though the viscosity value may not be strictly extrapolated to zero shear rate since in most cases we do not observe a low shear rate plateau value, therefore, the values reported as η0 were measured at shear rates in the range of 1 - 3 s-1). As can be seen in Figure 2A the starch solutions display viscosities that are at least one and a half orders of magnitudes higher than that of water (the viscosity of water at
Figure 2. (A) Shear viscosity as a function of shear rate for two derivatives chosen as examples from Table 2 with a starch:modifier:NaOH molar ratio of 1:1:2. (B) Zero-shear viscosities of the derivatives obtained in water with the experimental conditions shown in Table 2. All rheological measurements were performed at
EFFECT OF THE TEMPERATURE AND REACTION TIME
According to the previously described results, the carboxymethylation of cassava starch can be performed satisfactorily in water or in DMSO, since water soluble products are obtained that display viscosities at least 10 times higher than water. We also obtained better results (higher solution viscosities) with SMCA instead of MCAA. Therefore, a new series of experiments were performed in water and DMSO employing SMCA as modifying agent in order to study the effects of AGU:SMCA molar ratio, temperature and reaction time while the AGU:NaOH molar ratio was fixed at 1:2 (Mollega et al. 2011). Table 3 provides a summary of the results obtained.
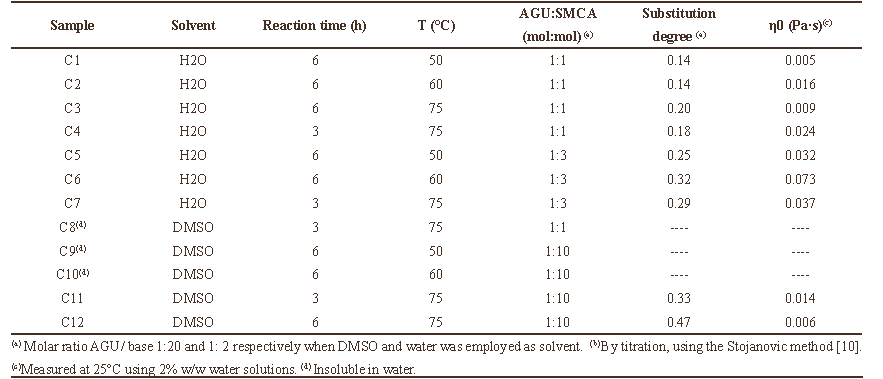
As may be appreciated in Table 3, all studied variables have a large influence on the DS and on the solution viscosity values. The CMS´s prepared in water employing an AGU: SMCA molar ratio of 1:3 exhibit higher DS values than those prepared with a 1:1 molar ratio. This can also be appreciated by comparing Figure 3A and Figure 3B.
Table 3. Results of the carboxymethylation of cassava starch varying molar ratio, temperature and reaction time, using water and DMSO as solvents.
In the case of DMSO as solvent, the reaction only took place when an AGU: SMCA molar ratio of 1:10 was employed at
Further analysis of Table 3 and Figure 3 allow us to establish that high DS values and large solution viscosities of the derivatives prepared in water are favored by a moderate temperature (
When the modification of starch was performed in DMSO (Figure
These results indicate that an increase in reaction temperature and the concentration of modifying agent, results in an increase in the reaction rate, in line with expectations in a SN2 reaction (scheme 1) (Smith & March , 2001).
A COMPARISON OF THE ZERO SHEAR VISCOSITIES OF SOLUTIONS OF CMS AND ACTIVATED STARCH.
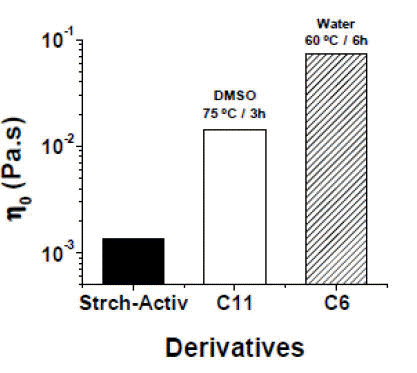
With the aim to establish a comparison between native starch and CMS´s, the former was activated with the following conditions: AGU:NaOH molar ratio of 1:2,
The activated native starch exhibits very low viscosity values (see Figure 4) which are similar to the viscosity of water (i.e., 0.001 Pa.s). This sample is compared in Figure 4 with the best CMS´s obtained in DMSO and water (samples C11 and C6 in Table 3). A logarithmic scale was used to clearly evidence the striking differences. In the case of the CMS prepared in water at
Figure 3. Values of degrees of substitution (DS) and initial viscosities of the modified starches (see Table 3): (A) derivatives prepared in water using an AGU:SMCA molar ratio of 1:1; (B) derivatives prepared in water using an AGU:SMCA molar ratio of 1:3 and (C) derivatives prepared in DMSO using an AGU:SMCA molar ratio of 1:10.
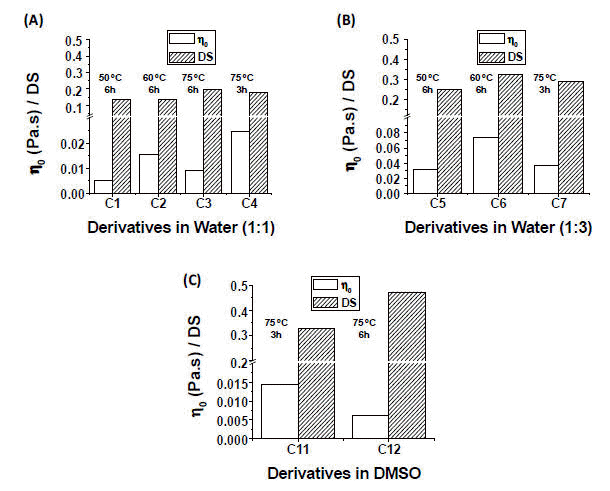
Figure 4. Zero shear viscosity values for the indicated aqueous solutions of: activated native cassava starch, and samples C11 and C6 from Table 3.
CONCLUSIONS
It is possible to perform the carboxymethylation reaction of cassava starch employing IP, DMSO or water as solvents, as previously reported in the literature. Nevertheless, when IP is employed, the process appears to be confined to the surface of the starch particles and the products obtained in this work were not water-soluble.
Under the experimental conditions employed in this work, the best modifying agent was SMCA as compared to MCAA.
Under the same experimental conditions, the modifications carried out in water generate starch derivatives which exhibit higher solution viscosities than those obtained in DMSO, even though those obtained in DMSO may exhibit higher degrees of substitution. This fact indicates that DMSO apparently promotes higher degradation reactions during carboxymethylation than water.
An increase in the temperature of the carboxymethylation reaction favors the increase of the substitution degree but decreases solution viscosity. The increment in the SMCA:starch molar ratio increases both DS and solution viscosity values. The results also suggest that it is not necessary to perform a pregelatinization procedure before the carboxymethylation reaction is carried out.
Finally the CMS that exhibited the highest value of solution viscosity (at a fixed concentration of 2% w/w) was obtained with water as solvent, a starch: SMCA molar ratio of 1:3, a temperature of
It is important to monitor the degradation of the starch during the carboxymethylation reaction (in this case, we employed solution viscosity as a qualitative measure of degradation), since certain reaction conditions may promote higher DS but at the expense of degrading the starch in such an extent that it may be rendered useless for certain specific applications.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the financial support of this work by FONACIT (grant G2005000776) and CDCHT-ULA (grants C-1744-11-08-A and C-1745-11-08-Ed).
REFERENCES
1.Flores, R(2006). Desarrollo de almidones funcionarizados y evaluación de las propiedades reológicas para su aplicación en la industria petrolera. Undergraduate Thesis,
2.Heinz T., Koschell A. (2005). Carboxymethyl ethers of cellulose and starch-A review. Macromol. Symp. 223, 13-39.
3.Hofreiter B.T. (1986). Miscellaneous modifications, in modified starches: Properties and uses. (Ed. O. B. Wurzburg) CRC Press,
4.JaspreetS., LovedeepK., Mccarthy O.J. (2007). Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications-A review. Food Hydrocolloids. 21, 1-22.
5.JieY.,
6.KittipongpatanaO., SirithunyalugJ., Laenger R. (2006). Preparation and physicochemical properties of sodium carboxymethyl mungbean starches. Carbohydr. Polym. 63, 105-112.
7.Lapasin R., Prici S., Tracanelli P. (1992). Carboxymethyl starch: A rheological study. J. Appl. Polym. Sci. 46, 1713-1722.
8.Lazi, W., Heinz T., PfeiffeK., Albrech G., MischnickP. (2002). Starch derivatives of a high degree of functionalization. VI. Multistep carboxymethylation. J. Appl. Polym. Sci. 86, 743-752.
9.MollegaS., Barrios S.E., Feijoo J.L., Contreras J.M., Müller A.J., López-Carrasquero F. (2011). Modificación química de almidón de yuca nativo mediante la reacción de carboximetilación en medio acuoso. Revista de
10.Moorthy S., Anderssonb L., Eliassonc A-C., Santacruzb S., RualesdJ. (2006). Determination of amylose content in different starches using modulated differential scanning calorimetry. Starch/Stärke. 58, 209-214.
11.Qiu H., HeL. (1999). Synthesis and properties study of carboxymethyl cassava starch. Polym. Adv. Technol. 10, 468-472.
12.SangseethongK., KetsilpS., Sriroth K. (2005). The role of reaction parameters on the preparation and properties of carboxymethyl cassava Starch. Starch/Stärke. 57, 84- 93.
13.Smith M., March J. (2001). March´s Advanced Organic Chemistry. Reactions, Mechanisms and Structure. Fifth Edition, A Wiley-Interscience Publication, John Wiley and Sons, Inc.,
14.Stojanovic Z., Jeremic K., Jovanovic S., Dieter M. (2005). A comparison of some methods for the determination of the degree of substitution of carboxymethyl starch. Starch/Stärke. 57, 79-83.
15.Tijsen C.J., KolkH.J., Stamhuis E.J., Beenackers A.A. (2001). An experimental study on the carboxymethylation of granular potato starch in non-aqueous media. Carbohydr. Polym. 45, 29-226.
16.VolkertB., Loth F., LazikW., EngelhardtJ. (2004). Highly substituted carboxymethyl starch. Starch/Stärke. 56, 307-314.
17.ZhangL-M. (2001). A Review of starches and their derivatives for oilfield applications in
18.Zhou X., YangJ., Qu G. (2007). Study on synthesis and properties of modified starch binder for foundry. J. Mater. Process.Tech. 183, 407-411. [ Links ]