Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Facultad de Ingeniería Universidad Central de Venezuela
versión impresa ISSN 0798-4065
Rev. Fac. Ing. UCV vol.30 no.1 Caracas mar. 2015
High density polyethylene - hydroxyapatite composites synthetized by in situ ethylene polymerization
Vanessa Hermán1*, Arquimedes Karam1, Carmen Albano2, Gema González3
1 Centro de Química, Laboratorio de Polímeros, Instituto Venezolano de Investigaciones Científicas (IVIC). Caracas 1020, Venezuela. Email: vherman@ivic.gob.ve, akaram@ivic.gob.ve Phone: +58212-5041832
2 Universidad Central de Venezuela, Facultad de Ingeniería, Escuela de Ingeniería Química, Zip Code 1020, Caracas, Venezuela. Email: carmen.albano@ucv.ve
3 Centro Ing. Materiales y Nanotecnología, Laboratorio de Materiales, IVIC. Caracas 1020, Venezuela. Email: gemagonz@ivic.gob.ve Phone: +58212-5041430
*Corresponding author
ABSTRACT
A novel synthesis method of obtaining polyethylene - hydroxyapatite (HDPE-HA) composites is proposed using in situ ethylene polymerization, employing Cp2ZrCl2/MAO as catalytic system. In this work, the influence of different polymerization conditions on the HA dispersion was evaluated. The parameters studied were: stirring velocity (600-2000 rpm) and temperature (10-75°C). It was found that, combining high stirring velocities (2000 rpm) and low temperatures (10 °C), it was possible to reach good filler dispersion with the HA nanocrystals interconnected in a network without the presence of agglomerates. Thermal degradation of HDPE-HA composites involved chemical degradation reaction of first order, accompanied by formation of the gas phase inside the melt polymer.
Keywords: Synthesis, polyethylene, Composites, Hydroxiapatite, Thermal degradation.
Compuestos de polietileno de alta densidad hidroxiapatita (PEAD-HA) sintetizados mediante la polimerizacion in situ de etileno
RESUMEN
Los materiales compuestos de polietileno de alta densidad – hidroxiapatita (PEAD-HA) fueron sintetizados mediante la polimerización in situ de etileno, empleando Cp2ZrCl2/MAO como sistema catalítico. En el presente trabajo se estudió la influencia de diferentes condiciones de polimerización en la dispersión de la HA. Los parámetros estudiados fueron: velocidad de agitación (600-2000 rpm) y temperatura (10-75°C). Al combinar altas velocidades de agitación (2000 rpm) con bajas temperaturas (10 °C) fue posible obtener una buena dispersión de las nanopartículas de HA interconectadas entre sí formando una red sin la presencia de aglomerados. La degradación de los materiales compuestos PEAD-HA ocurre mediante una reacción de primer orden, acompañado por la formación de gas dentro del polímero fundido.
Palabras clave: Síntesis, Polietileno, compuestos, Hidroxiapatita, Degradación térmica
Recibido: diciembre 2013 Recibido en forma final revisado: diciembre 2014
INTRODUCTION
High density polyethylene (HDPE) is a polyolefin that is widely used in diverse fields, including biomedicine, mainly due to its bioinert characteristics (Billmeyer, 1984; Park, 2003). Nevertheless, its application in biomedicine has been restricted due to poor mechanical properties, such as low resistance (Billmeyer, 1984; Bonfield et al., 1981; Park, 2003; Wang et al., 1994; Tanner et al., 1994). To overcome these problems, HDPE has been used in composites, in which the polyolefin acts as a matrix, and different fillers have been incorporated to improve mechanical properties. Some of the fillers include silica (Woo et al., 1995, 1999) clays (Yano A et al., 1998), carbon nanotubes (Bonduel et al., 2005; Tong et al., 2004), and hydroxyapatite (HA) (Bonfield et al., 1981). The last one is a biocompatible inorganic salt that has been used in implant manufacture, because of its structural and physicochemical similarity with human bone (Bonfield, 1981; Park, 2003).
Diverse methods have been employed to obtain HDPE-HA composites, including chemical solutions and melt mixing. However, these methods did not succeed in obtaining a good dispersion of HA, resulting in agglomerates formation.
These agglomerates decrease the torsional and tensile modulus of the HDPE-HA composite produced, since filler-rich sites promote fracture (Unwin et al., 2001; Albano et al., 2006a and 2006b; Wang. et al., 2002; Shahbazi et al., 2006; Zhang et al., 2008).
Recent studies have reported that using in situ polymerization gives better filler dispersion especially at higher filler contents than simple melt compounding. In situ polymerization of monomers in the presence of nanofillers is a promising approach for a more homogeneous distribution, due to the close contact of polymer and filler during synthesis( Kaminsky et al., 2006).
In situ ethylene polymerization using HA as filler has not been reported up to now, to the best of our knowledge. Due to the important potential applications of this composite and to the possible beneficial effects of nanohydroxyapatite in biomedical applications, in the present work the synthesis of HDPE-HA composites using in situ ethylene polymerization with Cp2ZrCl2/MAO as catalytic system was studied by varying different reactions parameters in order to improve HA dispersability into HDPE matrix.
EXPERIMENTAL TECHNIQUES
Materials
Calcium hydroxide and di-amonium hydrogen phosphate were supplied by Fisher Chemicals. Ethylene 5.0 grade of purity (Boc Gases). Toluene (Riedel de Haën, p.a.). Cp2ZrCl2 (Sigma Aldrich). MAO with 12.77% aluminum (Akzo Chemicals).
Synthesis of HA
A wet chemical precipitation reaction was used to synthesize HA with equimolar solution of calcium hydroxide and di-amonium hydrogen phosphate (Koutsopoulos, 2002). The resulting suspension was washed with de-ionized water and centrifuged several times until neutral pH was achieved. Afterwards, HA was dried at 80 °C for 48 h to remove water absorbed and then pulverized and sieved.
Synthesis of HDPE-HA composites
HDPE-HA composites were synthesized by in situ ethylene polymerization, previously dried toluene was used as solvent, (Aemarego, 2003) and Cp2ZrCl2/MAO as the catalytic system. All manipulations were carried out under nitrogen atmosphere using standard Schlenck techniques and dry box (Shiver, 1986). In situ ethylene polymerization was carried out at different stirring velocities (600-2000 rpm) and temperatures (10-75 ºC), with a constant filler percentage (15wt% ~ 0.8720 g). HA was suspended in toluene and transferred to a Büchi reactor by Schlenck techniques. After 10 min of stirring, toluene catalyst solution (1μmol/ml) was transferred into the reactor. Polymerization was carried out at a constant ethylene pressure (1 bar) for 30 min. The reaction was quenched by addition of an acidic solution (10 % HCl in ethanol). The polymer obtained was washed several times with ethanol and dried in vacuum at 60 ºC for 12 h.
Characterization of HA and HDPE-HA composites
Fourier transformed infrared spectroscopy (FTIR, Nicolet iS10) was used to determinate characteristic functional groups of HA, using KBr and 64 scans. Transmission electron microscopy was carried out in a JEOL 1220, operating at 100 KeV to study morphology, size, and dispersion of HA nanoparticles into the polymeric matrix. Samples were prepared by suspension in water: ethanol (70:30).
Differential scanning calorimetric (DSC) analyses were carried out in a Mettler-Toledo DSC 822e. Samples (9 10 mg) were heated up to 170 °C and subsequently kept for about 3 min in order to erase the previous thermal history. This initial heating was performed at a rate of 20 °C/min. Then, the samples were cooled to room temperature and subsequently heated up to 170 °C both at a rate of 10 °C/min. The values of melting temperature (Tm), crystallization temperature (Tc), and crystallinity degree (c) were determined from the thermograms of the cooling and second heating. Crystallinity degree was calculated using equation (1):
![]()
where cc is the crystallinity degree, ΔHmexp is the experimental melting enthalpy and ΔHmtheo corresponds to 100% of crystalline PE, 293 J/g (Bandrup, 1999).
Thermogravimetric analysis (TGA) was performed in order to elucidate the thermal stability of the synthetized composites. Samples (9-10 mg) were heated from room temperature up to 700 °C at 10 °C/min in a Mettler-ToledoTGA/STDA analyzer. Coasts-Redfern kinetic model (Coats et al., 1964) was used to determine the reaction order (n) and E2 Function model (Chen et al., 2004) to calculate kinetics parameters (Ea and A). The equation used for this calculation is the following:
![]()
where α is the degree of conversion, G(α) the integral conversion function (reaction mechanism), β the constant heating rate, T the temperature in Kelvin, R the universal gas constant, Ea the activation energy for the decomposition process and A the pre-exponential factor.
RESULTS AND DISCUSSION
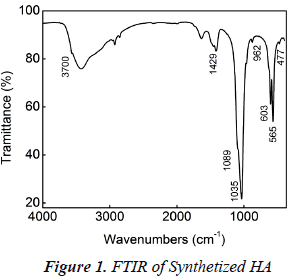
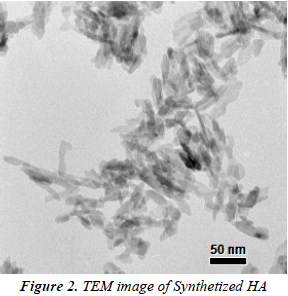
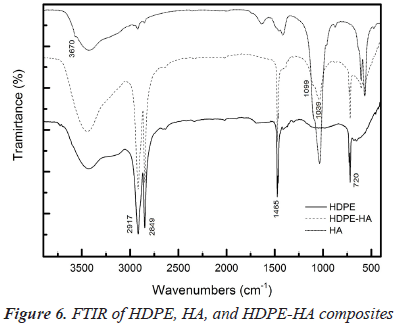
Hydroxyapatite (HA) synthesis was extremely effective, resulting in a high yield (99%). Characterization by FTIR showed the characteristic HA functional groups bands: OH- (3700 and 1600 cm-1), PO4-3 (1089-1035, 962, 603-565, and 477 cm-1) (figure 1) (Rehman I et al., 1997). HA nanocrystals have needle-like morphology with an average length around (AL) of (55 ± 9) nm and average diameter (AD) of (10 ± 1) nm as can be seen in figure 2 (Koutsopoulos, 2002).
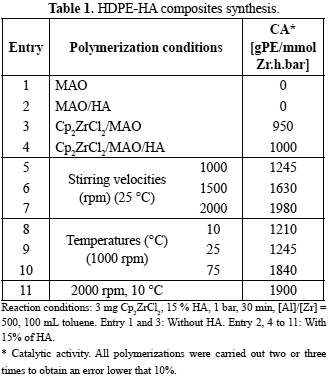
In order to standardize the polymerization reaction MAO, MAO/HA, and Cp2ZrCl2/MAO were tested on high density polyethylene production (HDPE). The ethylene polymerizations were carried out under the conditions presented on table 1 (entry 1-4). It was found that MAO or MAO/HA was not active on the ethylene polymerization. Cp2ZrClx/MAO was active producing HDPE as expected (thermal properties presented on table 2 are similar to the theoretical thermal properties previously reported) (Bandrup et al., 1999). The HDPE mass obtained was used as reference to calculate the equivalent mass of 15 wt% HA, that was employed in the synthesis of HDPE-HA composites. HA effect on the catalytic activity was studied (reaction 3 versus 4, table 1) it can be observed that HDPE production was not affected by the incorporation of the filler into polymerization medium. In general, it has been reported that supports that contain hydroxyl surface groups (i.e. zeolites, clays, others) show a decay in the catalytic activity due to block of active sites for polymerization (Hlatky, 2000). In the particular case of HA, a few OH groups are at the surface (Koutsopoulos, 2002), therefore, that the catalytic would decay, however, this effect was not observed in these experiments. Once it was confirm that HA has no negative effect on catalytic activity and polymer production, then the influence of stirring velocity and temperature on the filler dispersion was evaluated.
The stirring velocity was evaluated at first from 600 to 2000 rpm (table 1, entry 4-7). As can be seen at 2000 rpm (table 1, entry 7) the amount of polymer obtained was almost twice the amount when 600 rpm was used (table 1, entry 4). This is attributed to a better dispersion of the catalyst in the reaction medium at higher stirring velocities and also to a better dispersion of the synthesized polymer (Quijada et al., 1998).
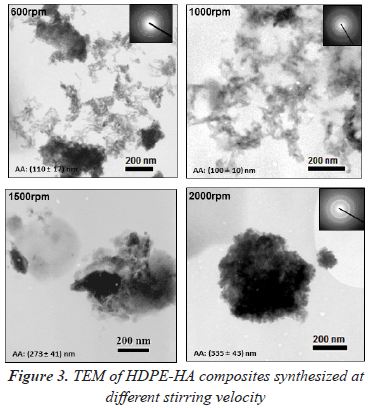
TEM images of HDPE-HA composites obtained at different stirring velocities are shown in figure 3. At higher stirring velocities (1500 and 2000 rpm) formation of HA agglomerates were obtained. This could be a consequence of the higher amount of polymer formed impeding a good dispersion of the filler. To corroborate these observations several measures of different images were carried out, obtaining the following AA sizes: (273 ± 41) nm for 1500 rpm and (345 ± 43) nm for 2000 rpm. When the synthesis was carried out at lower stirring velocities (600 rpm) few agglomerates can be observed presenting an AA of (110 ± 17) nm. The best HA dispersion was obtained at 1000 rpm with the lowest AA size of (100 ± 10) nm, this result indicated that at room temperature the better dispersion is achieved when intermediate stirring velocities are used.
The effect of temperature (10 -75 °C) was evaluated at 1000 rpm because under this stirring velocity the best HA nanocrystals dispersion was achieved. No significant differences on the catalytic activity were obtained at 10 and 25 °C (entry 8-9). However, when the synthesis was carried out at 75 °C, an increase of approximately 20 % on HDPE production was obtained. This increasing reaction rate with temperature is caused by the increased molecular collisions frequency, as a consequence olefin molecules travels faster and react more often with the active site, which is stable under these polymerization conditions. Similar results have been reported in the synthesis on high density polyethylene-carbon nanotube composites (Bonduel et al., 2007; Kao, 2006; Quijada et al., 1998; Kaminsky et al., 2006).
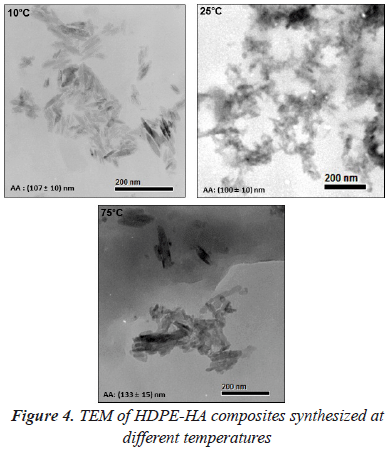
HA nanocrystals dispersion was studied by TEM images (figure 4). Composites synthetized at 10 and 25 °C did not present significant differences in AA sizes (107 ± 10 nm and 100 ± 10nm, respectively). However, at 10 °C it was found a better dispersion being possible to differentiate each single HA nanocrystal. Meanwhile, small agglomerates were observed at 25 °C. When the temperature was raised to 75 °C there was an increase in the formation agglomerates. This could be a consequence of the higher amount of polymer formed impeding a good dispersion of the filler. In the TEM image it was observed how the HA nanocrystals are embedded into the polymeric matrix.
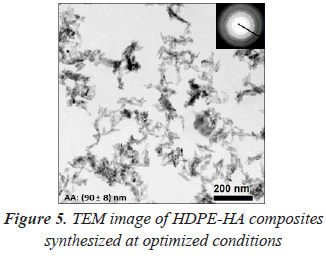
In order to produce higher amount of HDPE and maintain the polymer/filler ratio, HDPE-HA composites were synthetized at 2000 rpm and 10 °C (table 1, entry 11). Combining high stirring velocities and low temperatures HDPE production was increased on a 60% with respect to entry 5 and 8 (table 1). A synergistic effect of these two reactions parameters seems to be present, resulting in the best filler dispersion throughout these studies, with HA nanocrystals interconnected in a network without formation of agglomerates (figure 5). Therefore it can be conclude that in situ ethylene polymerization is an alternative synthesis method for the synthesis of HDPE-HA composites, and the different parameters studied showed that it is possible to obtain optimum conditions able to accomplish a good dispersion, compared to the other synthesis methodologies previously reported (Albano et al., 2006c; Wang et al., 1998; Roeder et al., 2003; Zhang et al., 2003).
HDPE and HDPE-HA composites were characterized by FTIR, composites spectra were very similar, for this reason only the spectrum of HDPE-HA composite synthetized at 2000 rpm and 10ºC is presented (figure 6). The HDPE spectrum shows the signal associated to the vibrational deformation mode of methylene CH groups (CH2)n in the polymer at 720 cm-1, this vibration is characteristic for polymers with more than 4 carbon atoms in the polymer chain. Also, the bands associated to vibrational deformation C-H bond at 1465 cm-1 (Bandrup et al., 1999), were observed. The HDPE-HA composite spectrum presents the bands of the two main components of the composites, when HDPE-HA spectrum is compared to the HA spectrum significance displacement of the signals associated to filler functional groups were observed, this behavior was observed in all the synthetized composites.
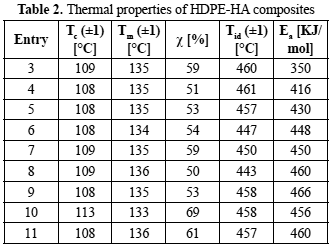
Additionally, the thermal behavior of HDPE-HA composites was studied by TGA. It was found that HA incorporation into the polymeric matrix did not have a significant effect on polyethylene thermal properties, such as crystallization temperature, melting temperature, or crystalline percentage (table 2) (Albano et al., 2006a, 2006b; Minkova et al., 1993). However, in some cases there are some significant changes in this thermal properties, it has been reported that this kind of behavior could be attributed to a change in polymer molecular weight, that can be due to the differences synthesis conditions (Cossee et al., 1964; Chien et al., 1993)
Also, a slightly change (0.2-3.7) % of the initial decomposition temperatures of HDPE-HA compounds with respect to the polymerization of ethylene without filler was observed. The activation energy (Ea) of the polymer and each composite was calculated using E2 Function model (table 2), the results obtained presented an increased on this parameter when HA was added into the polymeric matrix, this could indicate that although HA does not change the thermal properties produced an improvement on the thermal stability of the composites, slowing down the degradation processes of the HDPE (Bikiaris, 2011; Chrissafisa et al., 2011; Hermán et al., 2013).
As it is known, polymers degradation is a very complex phenomenon, consequently, the degradation process are represented by a set of functions and not for a single function. Although the E2 Function model allowed obtaining Ea value using the heating rate, the proper kinetic model must be chosen employing a model fitting method. The best reaction mechanism will be that which best fits equation (Eq. 2). In this article, fourteen mechanisms functions were evaluated(Budrugeac et al., 2001; Mamleev et al., 2000). The best fit obtained for all samples was the nth order reaction mechanism with n = 1. The models with the second and third best fits were nucleation and nucleus growth with n = 1/2 and 1/3, respectively. In general, the thermal degradation of polymeric composites is a heterogeneous process ruled by more than one mechanism. Therefore, thermal degradation of HDPE-HA composites evaluated in the present work, can be describe as a physicochemical phenomenon that involves chemical degradation reaction of first order, accompanied by formation of the gas phase inside the melt polymer and by nucleation and nucleus growth kinetics of degraded species in a heterogeneous medium (Budrugeac et al., 2001; Hermán et al., 2013; Mamleev et al., 2000).
Entry 1 and 2 are not in the table because in these syntheses conditions polyethylene was not obtained.
CONCLUSIONS
In situ ethylene polymerization is an alternative synthesis method for the synthesis of HDPE-HA composites, combining high stirring velocities (2000 rpm) and low temperatures (10 °C) was possible to reach good dispersion of HA nanocrystals interconnected in a network without the presence of agglomerates.
A possible interaction between the filler and the polymer is proposed due to a significant displacement of HA functional groups observed in FTIR spectra. Thermal properties of HDPE-HA did not change significantly compared to HDPE alone. Activation energy increased with HA incorporation, indicating that HA improves the thermal stability of the composites. Thermal degradation of HDPE-HA composites involved chemical degradation reaction of first order, accompanied by formation of the gas phase inside the melt polymer.
The use of in situ ethylene polymerization for synthetized these composites can be interesting to improve mechanical properties for biomedical applications; these properties are under current investigations.
ABREVATIONS
CA: Catalytic activity
Cp2ZrCl2: Bis-(cyclopentadienyl) zirconium dichloride
HA: Hydroxyapatite
HDPE: High density polyethylene
FTIR: Fourier transforms infrared spectroscopy
MAO: Metylaluminoxane
TC: Crystallization temperature
TEM: Transmission electron microscopy
Tid: Initial decomposition temperature
Tm: Melting temperature
XRD: X-ray diffraction
REFERENCES
1. Aemarego (2003). Purifaction of Laboratory Chemicals. Woburn: Heinemann-Butterworth. [ Links ]
2. Albano C., Karam A., Dominguez N., Sanchez Y., Perera R., Gonzalez G. (2006a). Optimal condictionting for preparation of HDPE-HA composite in an internal mixer. Mol. Cryst. Liq. Cryst. 448: 251-259. [ Links ]
3. Albano C., Karam A., Gonzalez G., Domínguez N., Sanchez Y. (2006b). Study of Gamma Radiation Effect on PMMA/HA Composites Prepared by Solution. Mol. Cryst. Liq. Cryst. 448: 845-851. [ Links ]
4. Albano C., Karam A., Perera R., González G., Domínguez N., González J., Sánchez Y. (2006c). HDPE/HA composites obtained in solution: Effect of the gamma radiation. Nucl. Instr. Meth. Phys. Res. B. 247: 331-341. [ Links ]
5. Bandrup J., Immergut E. H., Grulke E. A. (1999). Polymer Handbook. New York: John Wiley & Sons. [ Links ]
6. Bikiaris D. (2011). Can nanoparticles really enhance thermal stability of polymers? Part II: An overview on thermal decomposition of polycondensation polymers. Thermochimica Acta. 523: 25-45. [ Links ]
7. Billmeyer Von F. W. (1984). Textbook of Polymer Science. New York: John Wiley & Sons. [ Links ]
8. Bonfield W., Grynpas M.D., Tully A.E., Bowman J., Abram J. (1981). Hydroxyapatite reinforced polyethylen a mechanically ompatible implant material for bone replacement. Biomaterials. 1-2: 185-186. [ Links ]
9. Bonduel D., Mainil M., Alexandre M., Monteverdeb F., Dubois P. (2005). Supported coordination polymerization: a unique way to potent polyolefin carbon nanotube nanocomposites. Chem. Common. 14(6): 781-783. [ Links ]
10. Bonduel D., Breadeu S., Alexandre M., Monteverde F., Dubois P. (2007). Supported metalloce catalysis as an efficient toll for the preparation of polyhetylene/carbon nanotube nanocomposites: effect of the catalytica system on the coating morphology. J. Mater. Chem. 17: 2359- 2366. [ Links ]
11. Budrugeac P., Segal E. (2001). Some methodological problems concerning nonisothermal kinetic analysis of heterogeneous solid gas reactions. International Int. J. Chem. Kinet. 33(10): 564-573. [ Links ]
12. Chen H., Lai K., Lin Y. (2004). Methods for Determining the Kinetic Parameters from Nonisothermal Thermogravimetry: A Comparison of Reliability. J. Chem. Eng. Japan. 37(9): 1172-1178. [ Links ]
13. Chien J., Woei-Min T. (1993). Zirconocenium cation catalysis of propene polymerization. Makromol. Chem., Macromol. Symp. 66(1): 141-156. [ Links ]
14. Chrissafisa K., Bikiaris D. (2011). Can nanoparticles really enhance thermal stability of polymers? Part I: An overview on thermal decomposition of addition polymers. Thermochimica Acta. 523: 1-24. [ Links ]
15. Coats A.W., Redfern J.P. (1964). Kinetic parameters from thermogravimetric data. Nature. 201:68-78. [ Links ]
16. Cossee P., Arlman E. J. (1964). Ziegler-Natta Catalysis III. Stereospecific Polymerization of Propene with the Catalyst System TiCI3-AlEt3. J. Cat. 3: 99-104. [ Links ]
17. Hermán V., Albano C., Karam A., González G., Covis M. (2013). Thermodegradative study of HDPE–HA nanocomposites: IKP and E2 function. Polym. Bull. 70: 81-96. [ Links ]
18. Hlatky (2000). Heterogeneous Single-site catalysts for olefin polymerization. Chem. Rev. 100: 1347-1376. [ Links ]
19. Kaminsky W., Funck A., Wiemann K. (2006). Nanocomposites by In Situ Polymerization of Olefins with Metallocene Catalysts. Macromol. Symp. 239: 1-6. [ Links ]
20. Kao Kuo-Tseng L. (2006). Nanosized silica-supported metallocene/MAO catalyst for propylene polymerization. J. Appl. Polym. Sci. 101(4): 2573-2580. [ Links ]
21. Koutsopoulos S. (2002). Synthesis anda characterization of hydroxyapatite crystals: A review study in analytical methods. J. Biomed. Mater. Res. 62(4): 600-612. [ Links ]
22. Mamleev V., Bourbigot S., Le Bras M., Dequesne S., Sestak J. (2000). Modelling of nonisothermal kinetics in thermogravimetry. Phys. Chem. Chem. Phys. 2: 4708- 4716. [ Links ]
23. Minkova L., Magagnini P. L. (1993). Crystallization behaviour and thermal stability of HDPE filled during polymerization. Polym. Degra. Stab. 42: 107-115. [ Links ]
24. Park J. (2003). Biomaterials: Principles and Applications. EUA: CRC Press. [ Links ]
25. Quijada R., Rojasa R., Narvaeza A., Alzamorab L., Retuertb J., Rabagliatic F. M. (1998). The effect of reaction parameters on catalytic activity in the polymerization of ethylene using supported and unsupported metallocene catalysts. App. Cat. A: General. 166(1): 207-213. [ Links ]
26. Rehman I., Bonfield W. (1997). Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Scie.: Mater. Med. 8(1): 1-4. [ Links ]
27. Roede R., Sproul M., Turner C. (2003). Hydroxyapatite whiskers provie improved mechanical properties in reinforced polymer composite. J. Biomed. Mater. Res. Part A. 67: 801-812. [ Links ]
28. Shahbazi R., Javadpour J., Khavandi A. R. (2006). Effect of nanosized reinforcement particles on mechanical properties of high density polyethylene-hydroxyapatite composites. Adv. App. Ceram. 105(5): 253-258. [ Links ]
29. Shiver D. F., Drezdozn M. A. (1986). The manipulation of air-sensitive compounds. Wiley Interscience pp. 7-10. [ Links ]
30. Tanner K., Downes R. N., Bonfield W. (1994). Clinical applications of hydroxyapatite-reinforced polyethylene materials. Br. Ceram. Trans. 93: 104-107. [ Links ]
31. Tong X., Liu C., Hui-Ming C., Zhao H., Yang F., Zhang X. (2004). Surface modification of a single wall carbon nanotubes with polyethylene via in situ Ziegler-Natta polymerization J. App. Polym. Sci. 92(92): 3697-3700. [ Links ]
32. Unwin A. P., Ward I. M., Ukleja P., Weng J. (2001). The role of pressure annealing in improving the stiffness of polyehtylene/hydroxyapatite composites. J. Mater. Sci.: Mater.Med. 36: 3165-3177. [ Links ]
33. Wang M., Porter D., Bonfield W. (1994). Processing, characterization, and evaluation of hydroxyapatite reinforced polyethyelene composites. Br. Ceram. Trans. 93(3): 91-95. [ Links ]
34. Wang M., Chandrasekaran M., Bonfield W. (1998). Hydroxyapatite-polyethylene composites for bone substitution: effects of ceramic particle size and morphology. Biomaterials. 19: 2357-2366. [ Links ]
35. Wang M., Joseph R., Bonfield W. (2002). Friction and wear of hydroxyapatite reinforced high density polyethylene againts the stainless steel counterface. J. Mater. Sci.: Mater. Med. 13: 607-611. [ Links ]
36. Woo S., Soo Ko Y., Kyu Han T. (1995). Polymerization of ethylene over metallocenes confined inside the supercage of a NaY zeolite. Macromol. Rapid. Comm. 16(7): 489-494. [ Links ]
37. Woo S. (1999). Process for preparing metallocene catalyst for polyolefin polymerization US 5,869,417 A. [ Links ]
38. Yano A., Morihiko S. (1998) Olefin polymerization catalyst and olefin polymerization process. US 5,830,820. [ Links ]
39. Zhang Y., Tanner K. E. (2003). Impact behavior of hydroxyapatite reinforced polyethylene composites. J. Mater. Sci. Mater. Med. 14: 63-68. [ Links ]
40. Zhang Y., Tanner K. E. (2008). Effect of filler surface morphology on the impact behaviour of hydroxyapatite reinforced high density polyethylene composites. J. Mater. Sci: Mater. Med. 19: 761-766. [ Links ]