Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Zootecnia Tropical
versión impresa ISSN 0798-7269
Zootecnia Trop. vol.30 no.1 Maracay mar. 2012
Parasitic interactions between nosema spp. and varroa destructor in apis mellifera colonies
Fernando Mariani1, Matias Maggi* 2, 3, Martin Porrini2, 3, Sandra Fuselli2, 4, Gustavo Caraballo5, Constanza Brasesco2, Carlos Barrios6, Judith Principal6, Eguaras Martin2, 3
1 Consultor Apícola. Victoria. Entre Ríos. Argentina.
*2 Universidad Nacional de Mar del Plata, Facultad de Ciencias Exactas y Naturales, Laboratorio de Artrópodos. Funes, 3350. 7600. Mar del Plata. Argentina. Email: biomaggi@gmail.com
3 CONICET, Consejo Nacional de Investigaciones Cientícas y Técnicas. Rivadavia, 1917. C1033AJ Buenos Aires. Argentina.
4 Comisión de Investigaciones Cientícas (CIC), Calle 526 e/10 y 11. 1900. La Plata, Argentina.
5 Gobierno de Entre Ríos. Jefe Programa Apicultura. Entre Ríos. Argentina.
6 Universidad Centroccidental Lisandro Alvarado, Decanato de Ciencias Veterinarias. Estación de Apicultura. Lara, Venezuela.
ABSTRACT
The European honey bee Apis mellifera is affected by many parasites and pathogens that modify its immune system being the most destructive ectoparasitc mite Varroa destructor. The parasitic disease caused by this mite results in high mortality levels in honeybee colonies without acaricide treatment. In addition, the microsporidium Nosema apis and Nosema ceranae produce serious damages to the colonies. Taking into account that the sporulation dynamics of the microsporidium can be affected by several factors the objective of this investigation was to analyze if there are parasitic interactions between V. destructor and Nosema spp. when both parasites co-infect A. mellifera colonies. Studies were carried out in an apiary in the Entre Ríos province, Argentina. The apiary was sampled for a 10 month period. Parameters recorded per hive in eld examination were: (a) adult bee population (estimated as number of combs covered with adult bees); (b) brood area (estimated as number of combs covered by at least a 50 % of brood cells); (c) number of honey combs; (d) the V. destructor presence (a colony was considered parasitized by V. destructor when phoretic mite infestation was higher than 1 %) and (e) number of Nosema spp. spores (parasite abundance). Abundance of Nosema (ANij) per colony was analyzed using the mixed general additive model (GAM) with variable intercept. The nal data modeling conrmed that Nosema abundance is explained by the time and by the interaction between the month and the V. destructor infestation. Possible causes explaining this ecological relationship between V. destructor and Nosema spp. populations were discussed.
Key Words: Parasite interactions; Varroa destructor; Nosema; Apis mellifera
Interacciones parasiticas entre Nosema spp y Varroa Destructor en Colonias de Apis Meliffera
RESUMEN
La abeja Europea Apis mellifera es afectada por muchos parásitos y patógenos que modican su sistema inmune siendo el más destructivo el ácaro ectoparasítico Varroa destructor. La enfermedad parasitaria causada por este ácaro resulta en altos niveles de mortalidad en colonias de abejas melíferas sin tratamiento acaricida. Adicionalmente, los microsporidium Nosema apis y Nosema ceranae producen serios daños a las colonias. Tomando en consideración que la esporulación dinámica del microsporidium puede ser afectada por diversos factores, el objetivo de esta investigación fue analizar si existe interacción parasítica entre V. destructor y Nosema spp., cuando ambos parásitos co-infectan las colonias de A. mellifera. Los estudios fueron realizados en un apiario de la Provincia de Entre Ríos, Argentina. El apiario fue muestreado en un periodo de 10 meses. Durante cada revisión en campo, los siguientes parámetros por colonia fueron registrados: (a) población adulta de abejas (estimada como el número de panales cubiertos con abejas adultas); (b) área de cría (estimada como el número de panales cubiertos con al menos el 50% de celdas de cría); (c) número de panales de miel; (d) presencia de Varroa destructor (una colonia fue considerada parasitada por Varroa destructor cuando la infestación de ácaros foréticos era mayor del 1%) y el número de esporas de Nosema spp. (abundancia de parásitos). La abundancia de Nosema (ANij) por colonia fue analizada usando el Modelo estadístico Mixed General Additive model (GAM) con intercepto variable. Los datos nales modelados conrmaron que la abundancia de Nosema es explicada por el tiempo y por la interacción entre el mes y la infestación de V. destructor. Posibles causas que podrían explicar esta relación ecológica entre las poblaciones de V. destructor and Nosema spp. fueron estudiadas.
Palabras clave: interacciones de parásitos, Varroa destructor, Nosema, Apis mellifera
Recibido: 31/05/11 Aprobado: 26/09/12
INTRODUCTION
The European honey bee Apis mellifera is affected by many parasites and pathogens that modify its immune system (Gregory et al., 2005; Evans et al., 2006; Antúnez et al., 2009). From all of them, the most destructive is the ectoparasitc mite Varroa destructor. Parasitosis caused by the mite results in high mortality levels in honeybee colonies without acaricide treatment (Murilhas, 2002). Varroa destructor feeds on the haemolymph of its host producing haemocytes and protein content reduction (Tewarson, 1983; Smirnov, 1978), which leads to a decrease of the immune response of the parasited bees (Weinberg and Madel, 1985; Garedew et al., 2004, Gregory et al., 2005).
Microsporidia were classied as fungi highly specialized, adapted to parasitism (Sina et al., 2005). Among them, Nosema apis and Nosema ceranae constitute the etiological agent of the nosemosis, a disease affecting the A. mellifera intestinal epithelium, causing serious damages to colonies (Fries et al., 1984; Higes et al., 2008). The sporulation dynamics of the microsporidium can be affected by environmental factors such as temperature and rainfalls (Dyess and Wilson, 1978), or by the apiary management system (Bailey and Ball, 1991).
Besides, microsporidia reproduction is helped by hemocyte destruction (Gilliam and Shimanuki, 1967) and by the catabolism acceleration of bee fats (Cornejo and Rossi, 1974). There are few previous studies analyzing if the parasitism caused by the V. destructor mite in bee colonies is connected by nosemosis development. Orantes Bermejo and García Fernández (1997) had tested the hypothesis that N. apis breeding is helped by the bee stress caused by the V. destructor parasitism. However, these authors could not conrm parasite interactions between both species and state that climate conditions under which they conducted studies may have inuenced their results. This was also postulated by Bailey and Ball (1991). Other studies suggested that recent colony losses observed in Europe and the United States may be due to synergist effects between N. ceranae and V. destructor (Anderson and East, 2008; Cox-Foster et al., 2007; Higes et al., 2008; Ribiere et al., 2008), but those observations are not entirely proved.
Gregory et al. (2005) showed that V. destructor causes the inmunosupression of the parasited bee decreasing the production of antimicrobial peptides like abaecin and defensin. Indeed it has been studied that these peptides participate in the inmuno-defense of the bees against N. ceranae and N. apis infections (Antúnez et al., 2009). These authors observed that only N. ceranae was able to inmunosupress the bee and they tested the hypothesis that this phenomena could explain the greater pathogen virulence compared with its nonspecic organism. Few previous studies were found on this subject, consequently, the aim of this work was to analyze if there were parasite interactions between V. destructor and Nosema spp. when both parasites co-infected A. mellifera colonies.
MATERIALS AND METHODS
Sampling zone and apiary characterization Studies were carried out in an apiary in the Entre Ríos province. The apiary was sampled during 10 month (from June 2008 to March 2009). The environmental variables total rainfalls and average temperature were monthly registered from data provided by the National Weather Service. Previous to, and during studies, colonies did not receive parasitic control treatment against Nosema. With regard to V. destructor control the apiary did not receive synthetic acaricide treatments.
Sampling
Samplings were performed at monthly intervals and colonies were examined to detect presence of Paenibacillus larvae and Melisococcus plutonium bacteria or Ascophaera apis fungi. 15 bee colonies placed in Langstroth beehives were selected from an apiary of a total of 50 colonies. Only those with high bee population (from six to ten combs covered by adult bees) were chosen for sampling. During each eld examination the following parameters were recorded per hive: (a) adult bee population (estimated as number of combs covered with adult bees); (b) brood area (estimated as number of combs covered by at least a 50 percent of brood cells); (c) number of honey combs; (d) the V. destructor presence (a colony was considered parasitized by V. destructor when phoretic mite infestation was higher than 1 %) and (e) number of Nosema spp. spores (parasite abundance).
To estimate Nosema spore abundance in each beehive, samples of foraging bees (n=100) were taken from the hive entrance. Sampling was always carried out at midday. The entrance of every hive was closed with a foam rubber so that later on, bees piled up on it could be collected, making them fall into a ask with water. In the laboratory, the abdomens of 60 bees of each sample were obtained in 60 ml of distilled water (Fries et al., 1984). Later on, one drop of the homogenized solution was taken and put in a hemocytometer to quantify the number of spores in the sample using an optical microscope according to the protocol described by Cantwell (1970).
Varroa destructor prevalence per beehive was estimated in phoretic state following the protocol stated by De Jong (2005). The V. destructor prevalence per hive was calculated as:
% V. destructor= (Number of phoretic mites/ number of adult bees) x 100 Data modeling and statistical analysis Previous to data modeling, exploration analyses according to Zuur et al. (2009) were carried out in order to identify: (a) extreme data able to inuence the analysis result; (b) collinearity among the explanatory variables and (c) interaction among the explanatory variables. In agreement with Montgomery and Peck (1992), a high collinearity between variables results in great variances for the regression coefcients. Thus, in the presence of collinearity between variables it is necessary to choose only one of them to be included in the nal model.
After the initial exploration, data of abundance of Nosema (ANij) per colony were analyzed using the mixed general additive model (GAM) with variable intercept. All the generated models followed a Gaussian distribution and an Identity Link function (Zuur et al., 2009). The Identity Link function assure that μi = η (Xi1,. . ., Xiq) and the Gaussian distribution uses the following average and variance:
ANij ~ Normal (μij, σ2)
E (ANij ) = μij y var(ANij ) = σ2
where μij= α + Varroaij + f1(Nbeesij) + f2(Nhoneyij) + f3(Abroodij) + f4(Monthj) + (Month): Varroa + αi+ εij
The α term is the xed intercept: αi is the variable intercept and represented the abundance variation of Nosema attributed by the hive variable and it was assumed that it had a Normal distribution with 0 average and d2 variance. The f1, ,f5 terms were the smoothers for the explanatory variables V. destructor presence (= Varroa), Adult bee population per hive (=N bees), Number of honey combs per hive (=Nhoney), Bee brood area (=A brood) and Month (Splines of cubic regression were used for the smoothers). The : symbol denotes the interaction between the month and the V. destructor prevalence. The sub indexes ij that go with each variable represented data registered for them in the j month and the i hive. As regards the εij wastes it was assumed that they follow a Normal distribution with 0 average and d2 variance.
Calculations were done with the mgcv (Wood, 2006) and nlme (Pinheiro et al., 2009) packages of the free R software (R Development Core Team, 2009.
RESULTS AND DISCUSSION
The presence of A. apis, M. plutonium or P. larvae could not be visually determined in neither colony studied. The initial data exploration demonstrated that the Adult bee population per hive, Number of honey combs per hive and Bee brood area variables presented a high correlation among them.
The same relationship was observed among Month, Temperature and Rainfalls variables. Due collinearity the following model was chosen:
Log (Abundance Nosema spp.) ~ Month : Varroa + Abrood, random= list (fhive=~1)
Where the term random= list (fhive=~1) is the statistically notation to denote the Nosema abundance variability per beehive.
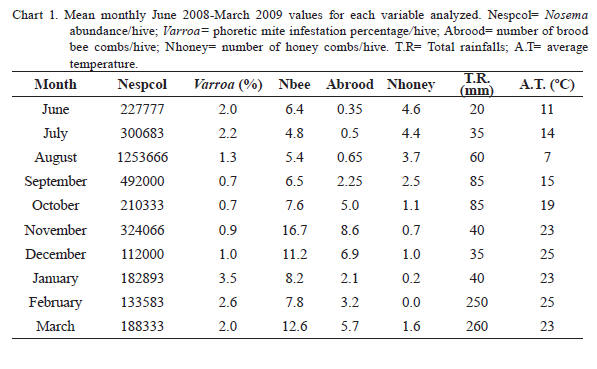
The average monthly values of the variables studied for the apiary are shown in Chart 1 (including environmental variables). The presence of V. destructor in the apiary was detected in every month, with values of mite infestations per bee colony that vary between 0 to 10.2%
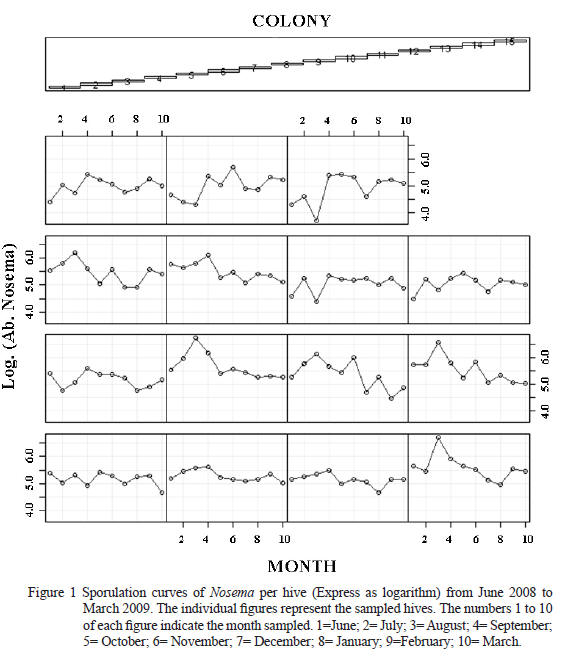
Figure 1 depicts the Nosema sporulation curve for every sampled hive along the time. It can be observed a non linear relationship in the Microsporidia abundance along the time justifying the mixed GAM model application. Each colony shows a different pattern of sporulation of Nosema spp. having an average peak during the colder months of the year (July- August).
However, some colonies had sporulation peaks during spring months (October- November). These results demonstrated the sporulation variability of the microsporidia within an a apiary.
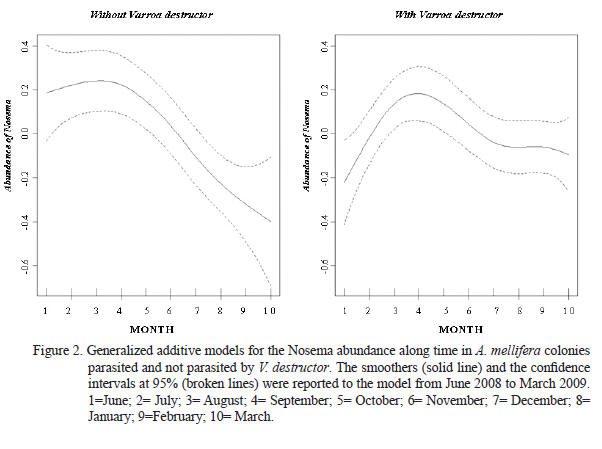
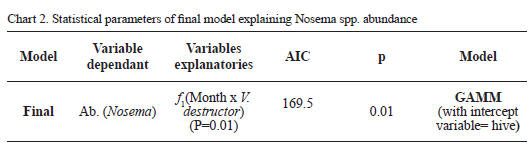
The nal data modeling conrmed that Nosema abundance is explained by the time = rainfalls = temperature variables (variables highly correlated) and by the interaction between the month and the V. destructor infestation. Smoothers obtained for the Nosema abundance along time in the bee hives with and without Varroa presence were represented in Figure 2. Thus, the nal model established that the microparasite abundance uctuates along time showing a maximal sporulation peak between July and September. In addition, Figure 2 shows that the colonies parasited by V. destructor remained with greater loads of Nosema after the Microsporidia sporulation peak took place, compared with free mite colonies. Taking into account that the gures show a curve that touch negative values, means the reduction of the individual spores load on the bees and inside the colony, which means that the samples were done after the sporulation peak (In general after September month). Model parameters explaining the Nosema abundance were shown in Chart 2.
In this study, the initial exploration of data demonstrated high colinearity among bee brood, adult bees and honey combs per beehive. High colinearity was also registered among time, temperature and rainfalls. In agreement with Montgomery and Peck (1992), high colinearity among variables causes great variances for the regression coefcients and authors suggested to choose only one of them to be included in the nal model. Thus, the registered high colinearity among the variables mentioned must be taken into account in future eld researches optimizing the sampling effort.
The nal modeling of data demonstrated that the Nosema abundance is explained by the interaction between time and parasitary loads of V. destructor. Previous studies reported that N. apis and V. destructor could co-exist in the same bee colony, even if their populations grow independently one from the other (Orantes Bermejo and García Fernández, 1997). These authors set the hypothesis that their observations were the result of determined environmental conditions under which they performed their studies and that different climate situations could have resulted in opposite observations (association between Varroa and Nosema). Our results, obtained from a geographical zone of temperate climate, demonstrated that the parasitism caused by V. destructor is able to modify the Nosema development along the annual cycle.
Differences with the cited authors may be also attributable to sampling method. Meana et al. (2010) demonstrated a high variability between Nosema spore counts depending on sampling time and bees age. In the present study, sampling was made always at midday on foraging bees, unlike the composite samples of bees with different age used by Orantes Bermejo and García Fernández (1997). In this way, errors in the statistical conclusions were minimized given the sampling homogeneity. Chart 1. Mean monthly June 2008-March 2009 values for each variable analyzed. Nespcol= Nosema abundance/ hive; Varroa= phoretic mite infestation percentage/ hive; Abrood= number of brood bee combs/hive; Nhoney= number of honey combs/hive. T.R= Total rainfalls; A.T= average temperature
As regards the environmental variables, Nosema development was affected by temperature and rainfalls. When increasing the values of these variables, the parasite abundance decreased. These results are in accordance with previous studies, which determined that the environmental conditions can modify Microsporidia development, both at natural and laboratory conditions (Dyess and Wilson, 1978; Malone and Giacon, 1996; Sarlo et al., 2006; Martín- Hernández et al., 2009).
The N. ceranae presence in Argentina was conrmed in 2008 (Sarlo et al, 2008; Plischuk et al., 2008) despite of having registered alterations in the annual sporular dynamics since 2004 (Sarlo et al., 2006). These reports, obtained from Buenos Aires apiaries demonstrated two abundance peaks, unlike the natural Nosema curve obtained in this study. Our data evidenced only one sporular peak between July and September, which is coincident with the seasonal trend of a typical N. Apis infection. It shows low levels during the summer, small peaks in fall and a rapidly increase in the spring (Furgala and Mussen, 1978). Colonies developed in temperate climates during fall and winter seasons restricted their activity due to the scarce nectar ow, increasing dejections at the inner part of them (Fries, 1993; Fries et al., 2003).
An increasing in the infecting mass, due to dejections inside the colony as well as the longer lifetime of bees, are the reasons why during the cold months great microsporidia loads are developed (Lhenert and Shimanuki, 1979). When air temperature increases, bees prepare the brood nest to the nectar collecting and an increase of activity takes place at the colony, which diminishes the lifetime of adult bees and increases the possibilities of making cleansing ights. This situation could explain why the Microsporidia abundance decreases when the temperature increases. The present study also demonstrates that bee population amount was not correlated with microsporidian abundance. Therefore, we could consider that there are no dilution effects on abundance rate caused by population increase on warmer months.
Compared with colonies not infested by Varroa, parasitized colonies showed higher Nosema counts after the abundance peak. A previous study carried out by Higes et al. (2010) showed that the illnesses produced by these pathogens, produce severe problems at the level of both the colony and the individual honey bee. Mite infestation could contribute to Nosema development. Other studies suggested that recent losses of colonies observed in Europe and in USA may be caused by synergist effects between N. ceranae and V. destructor (Anderson and East, 2008; Cox-Foster et al., 2007; Higes et al., 2008; Ribiere et al., 2008). This interesting behavior could be the result of the action of stressors generated by Varroa that affect the peritrophic membrane of the bee, a physical barrier against microsporidia infections. The histological picture of the ventriculus undergoes changes as a result of the action of stressors such as toxic substances (Bielenin and Ibek, 1980), incorrect nutrition (Szymaś, 1976; 1994) and bacterial infections (Gregorc and Bowen, 2000). Immunosupression caused by Varroa (Amdam et al., 2004; Gregory et al., 2005; Yang and Cox-Foster, 2007) predispose workers to many bacterial, fungal and viral infections. Anyway, at the present there is still little or no evidence to prove that invertebrate hosts have an immune response that can suppress a microsporidia infection once it is established (Agnew et al., 2002). Also, deciencies on vitellogenin levels (Vg) of winter bees (Amdam et al., 2004), is a cause of disequilibrium of population, being another possible stressor that facilitates infection by Nosema. Studies carried out by Antúnez et al. (2009) showed that unlike N. apis, N. ceranae infections partially suppress humoral and cellular defense mechanisms and reduce expression of Vg. As Varroa destructor mite also produces immune suppression and a decrease in Vg levels, simultaneous infection with both pathogens (N. ceranae and V. destructor) has been suggested as devastating for honeybee colonies. In the present study, colonies affected by both parasites survived as well as colonies without Varroa. Nevertheless, our studies were performed during only 10 months.
Future studies should consider more time of sampling to detect bee mortality. In this study, a correlation between Nosema development and honey reserves could not be found.
Previous studies reported that N. apis parasitism negatively affected the reserve production of colonies (Fries et al., 1984). In the present work, Nosema abundances were relatively low (<3 x exp6/bee/ month). The lack of evidence of the Nosema parasitism effects on the honey reserves of the colonies in these apiaries could be due to a low virulence of the mite or due to the short time of sampling. Higes et al. (2008) reported times signicantly higher than those of this study to observe colony collapse of bees naturally infected by N. ceranae.
The low sporular loads of the apiary studied, compared with other studies, resulted to be of interest. Taking into account that the Microsporidia species were not identied in the apiary studied and that the presence of N. ceranae was reported from bee populations sampled in other zones of Argentina (Plischuk 2008; Sarlo et al., 2008), these different dynamics of the populations could be explained by the fact that there was N. apis microsporidia infections in the apiary studied. Other studies reported N. apis abundance values similar to those registered in this research (Fries, 1988; Orantes Bermejo and García Fernández, 1997; Pohorecka and Skubida, 2004). In addition to this, previous studies suggested that the reproduction rate of N. apis is lower than that of N. ceranae (Higes et al., 2007; Chen et al., 2009).
CONCLUSION
In view of the aforementioned, at the study conditions tested, it could be concluded that: (a) it was conrmed the existence of parasitary interactions between V. destructor and Nosema, (b) the average temperatures would be were inversely correlated with microsporidia development and (c) it is possible that both microsporidia species causing nosemosis in Argentina (N. apis and N. ceranae) are differently distributed in the country. Future DNA studies in Nosema populations affecting A. mellifera colonies in the country would be of great importance to understand the epidemiology of these Microsporidia and to make solid conclusions in studies performed in the eld.
REFERENCES
1. Agnew P, J. J. Becnel, D. Ebert, Y. Michalakis. 2002 Symbiosis of microsporidia and insects. In Insect Symbiosis (ed. Bourtzis K.), pp. 145–163.
2. CRC Press LLC, Florida. Amdam, G. V., K. Hartfelder, K. Norberg, A. Hagen, S. W. Omholt. 2004 Altered Physiology in Worker Honey Bees (Hymenoptera: Apidae) Infested with the Mite Varroa destructor (Acari: Varroidae): A Factor in Colony Loss During Overwintering? J. Econ Entomol 97(3): 741-747.
3. Anderson, D.L., I. J. East. 2008. The latest buzz about colony collapse disorder. Science 319: 724–725. [ Links ]
4. Antúnez, K., R. Martin-Hernandez, L. Prieto, A. Meana, P. Zunino, M. Higes. 2009. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ Microbiol doi:10.1111/ j.1462-2920.2009.01953x
5. Bailey, L, B. V. Ball. 1991 Honey Bee Pathology (2nd ed.). Academic Press, London. Bailey, 1955 Bielenin, I., Z. Ibek. 1980. Wpływ uorku sodu na nabłonek jelita środkowego pszczoły miodnej, Apis mellifera L. (Apidae, Hymenoptera). Zesz Nauk AR Krak 159 (20): 49-67.
6. Cantwell, G. E. 1970. Standard methods for counting Nosema spores. Am Bee J 110: 222-223.
7. Chen, Y., J. D. Evans, L. Zhou, H. Boncristiani, K. Kimura, T. Xiao, A. M. Litkowski, J. S. Pettis. 2009. Asymmetrical coexistence of Nosema ceranae and Nosema apis in honey bees. J Invertebr Pathol 101(3):204-9.
8. Cornejo, L. G., C. O. Rossi. 1974. Enfermedades de las abejas, su prolaxis y prevención. Ediciones Hemisferio Sur. Buenos Aires, Argentina. 238. p.
9. Cox-Foster, D. L., S. Conlan, E. C. Holmes, G. Palacios, J. D. Evans, N. A. Moran, P. L. Quan, T. Briese, M. Hornig, D. M. Geiser, V. Martinson, D. Vanengelsdorp, A. L. Kalkstein, A. Drysdale, J. Hui, J. H. Zhai, L. W. Cui, S. K. Hutchison, J. F. Simons, M. Egholm, J. S. Pettis, W. I. Lipkin. 2007. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318: 283–287.
10. De Jong, D. 2005. Workshop sobre Control de la Varroosis en Climas Subtropicales. 27 y 28 de junio, Salta Argentina.
11. Dyess, E. G., C. A. Wilson. 1978. A study of the seasonal variations of Nosema apis Zander of honey bee in Mississippi. Am Bee J. 118: 33–35.
12. Evans, J. D., K. Aronstein, Y. P. Chen, C. Hetru, J. L. Imler, H. Jiang, M. Kanost, G. J. Thompson, Z. Zou, D. Hultmark. 2006. Immune pathways and defense mechanisms in honey bees Apis mellifera. Insect Mol Biol 15: 645-656.
13. Fries, I. 1988. Comb replacement and Nosema disease (Nosema apis Z.) in honey bee colonies. Apidologie 19: 343–354.
14. Fries, I. 1993. Nosema apis – a parasite in the honeybee colony. Bee World 74: 5-19.
15. Fries, I., G. Ekbohm, E. Villumstad. 1984. Nosema apis, sampling techniques and honey yield. J Apicult Res 23: 102–105.
16. Fries, I., H. Hansen, A. Imdorf, P. Rosenkranz. 2003. Swarming in honey bees (Apis mellifera) and Varroa destructor population development in Sweden. Apidologie 34: 389–398.
17. Furgala, B., E. Mussen. 1978. Protozoa. In Honey bee pests, predators and diseases (ed. Morse RA). New York: Comstock Publishing Associates, Cornell University Press. pp. 63-73
18. Garedew, A., E. Schmolz, I. Lamprecht. 2004. The energy and nutritional demand of the parasitic life of the mite Varroa destructor. Apidologie 35: 419–430.
19. Gilliam, M., H. Shimanuki. 1967. In vitro phagocytosis of Nosema apis spores by honey-bee hemocytes. J Invert Pathol 9: 387–389.
20. Gregorc, A., I. D. Bowen. 2000. Histochemical characterization of cell death in honeybee larvae midgut after treatment with Paenibacillus larvae, amitraz and oxytetracycline. Cell Biol Inter 24 (5): 319-324.
21. Gregory, P.G., J. D. Evans, T. Rinderer, L. De Guzman. 2005. Conditional immune-gene suppression of honeybees parasitized by Varroa mites. J Insect Sci 5: 7-11.
22. Higes, M., P. García-Palencia, C. Botías, A. Meana, R. Martín-Hernández. 2010. The differential development of microsporidia infecting worker honey bee (Apis mellifera) at increasing incubation temperature. Env Microbiol 2: 745- 748.
23. Higes, M., R. Martin-Hernandez, C. Botias, E. G. Bailon, A. V. Gonzalez-Porto, L. Barrios, M. J. Del Nozal, J. L. Bernal, J. J. Jimenez, P. G. Palencia, A. Meana. 2008. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ Microbiol 10: 2659–2669.
24. Higes, M., P. Garcia-Palencia, R. Martin-Hernandez, A. Meana. 2007. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 94, 211– 217.
25. Lhenert, T., H. Shimanuki. 1979. Population change and Nosema spore levels in colonies started with package bees. Apidologie 10: 17-21.
26. Malone, L.A., H. A. Giacon. 1996. Effects of Nosema apis Zander on inbred New Zealand honey bees (Apis mellifera ligustica L.). Apidologie 27: 479–486.
27. Martín-Hernández, R., A. Meana, P. García-Palencia, P. Marín, C. Botías, E. Garrido- Bailón, L. Barrios, M. Higes. 2009. Effect of temperature on biotic potential of honeybee microsporidia. Appl Environ Microbiol 75 (8): 2554-2557.
28. Meana, A., R. Martín-Hernández, M. Higes. 2010. Reliability of spore counts to diagnose Nosema ceranae infection in honey bees. J Apic Res 49: 212-214
29. Montgomery, D. C., E. A. Peck. 1992. Introduction to Linear Regression Analysis. Editorial John Wiley & Sons, New York USA. 527. p.
30. Murilhas, A. M. 2002. Varroa destructor infestation impact on Apis mellifera carnica capped worker brood production, bee population and honey storage in a Mediterranean climate. Apidologie 33: 271-281.
31. Bermejo, O. F., P. García Fernandez. 1997. Nosema disease in the honey bee (Apis mellifera L) infested with Varroa mites in southern Spain. Apidologie 28: 105-112
32. Pinheiro, J., D. Bates, S. Debroy, D. Sarkar, R. Core Team. 2009. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-96
33. Plischuk, S., R. Martin-Hernandez, A. Meana, C. E. Lange, M. Higes. 2008. First report of Nosema ceranae in depopulated honey bee colonies in Argentina. 3rd European Conference of Apidology (EurBee3), Belfast, UK. Belfast. 57. p.
34. Pohorecka, K., P. Skubida. 2004. Healthfulness of honeybee colonies (Apis mellifera L.) wintering on the stores with addition of honeydew honey. Bull Vet Inst Pulawy 48: 409-413.
35. Development, R., Core Team. 2009. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL: http://www.Rproject.org
36. Ribiere, M., V. Olivier, P. Blanchard, F. Schurr, O. Celle, P. Drajnudel, J. P. Faucon, R. Thiery, M. P. Chauzat. 2008. The collapse of bee colonies: the CCD case (Colony collapse disorder) and the IAPV virus (Israeli acute paralysis virus). Virologie 12: 319–322.
37. Sarlo, E., S. K. Medici, M. Braunstein, M. Eguaras. 2008. Presencia y distribución de Nosema ceranae en la región sudeste de la Provincia de Buenos Aires. II Congreso Argentino de Apicultura. Mar del Plata, Buenos Aires, Argentina. 47. p.
38. Sarlo, E., S. Medici, M. P. Porrini, M. Eguaras. 2006. Dinámica de esporulación de Nosema apis Z. en colmenas de Apis mellifera L. ubicadas en el sudeste de la provincia de Buenos aires, Argentina; determinación de factores predisponentes. Workshop Malattie delle api e resiui nei prodotti dell´ alveare. Roma, Italia. 124. p.
39. Sina, M., G. Alastair, M. Farmer, R. Andersen, O. Anderson, J. Barta, S. Bowser, G. Brugerolle, R. Fensome, S. Fredericq, T. James, S. Karpov, P. Kugrens, J. Krug, C. Lane, L. Lewis, J. Lodge, D. Lynn, D. Mann, R. Mccourt, L. Mendoza, O. Moestrup, S. Mozley-Standridge, T. Nerad, C. Shearer, A. Smirnov, F. Spiegel, M. Taylor. 2005. The new higher level classication of Eukaryotes with emphasis on the taxonomy of Protists. J Eukar Microbiol 52: 399–451.
40. Smirnov, A. M. 1978. Progrès actuels de la science en Union Soviétique dans létude de léthiologie, de la pathogénie, de lépizootologie, du diagnostic et de la lutte contre la varroase des abeilles. Apiacta 13: 149-162.
41. Szymaś, B. 1976. Histologiczna ocena zmian nabłonka jelita środkowego pszczół miodnych Ŝywionych namiastkami pyłku. Rocz AR Pozn 88 (22): 141-147.
42. Szymaś, B. 1994 Ocena wartości odŜywczej surogatów pyłku kwiatowego dla pszczoły miodnej (Apis mellifera L.). Rocz AR Pozn Rozpr Nauk 256: 1-68
43. Tewarson, N. C. 1983. Nutrition and reproduction in the ectoparasitic honeybee (Apis sp.) mite, Varroa jacobsoni. Dissertation zur Erlangung des Grades eines Doktors der Naturwissenschaften, Eberhard-Karls-Universität Tübingen, German Federal Republic 98. p.
44. Weinberg, K. P., G. Madel. 1985. The inuence of the mite Varroa jacobsoni Oud on the protein concentration and the hemolymph volume of the brood of worker bees and drones of the honey bee Apis mellifera L. Apidologie 16: 421-435.
45. Wood, S. N. , F. R. G. Hannover. 2006. Generalized additive models: An introduction with R. Chapman and Hall/CBR. England, UK. Pp. 410.
46. Yang, X., D. Cox-Foster. 2007. Effects of parasitization by Varroa destructor on survivorship and physiological traits of Apis mellifera in correlation with viral incidence and microbial challenge. Parasitology 134: 405–412.
47. Zuur, A., E. Ieno, N. Walker, A. Saveliev, A. Smith. 2009. Mixed effects models and extensions in ecology with R. Ed. Springer. England, UK. 575. p.













